Sex Differences in Inferior Parietal Lobule Volume in Schizophrenia
Abstract
OBJECTIVE: The inferior parietal lobule is a heteromodal association cortical region that has been implicated in the pathophysiology of schizophrenia. Inferior parietal lobule gray matter volumes have been shown to differ between healthy male and female subjects, with male subjects having larger left volumes. The authors sought to determine whether these volumetric sex differences also exist in patients with schizophrenia. METHOD: The authors used magnetic resonance imaging to measure inferior parietal lobule volumes of 15 pairs of male and female schizophrenic subjects who were individually matched to each other and to 15 pairs of healthy male and female subjects. RESULTS: Male schizophrenic patients exhibited a reversal of the normal left-greater-than-right male asymmetry in this region and had left inferior parietal lobule gray matter volumes that were significantly smaller than those of healthy male subjects. Female schizophrenic patients did not differ significantly from healthy female subjects in left or right inferior parietal lobule volume or in asymmetry. CONCLUSIONS: This study provides further evidence of brain morphology sex differences in schizophrenia that possibly contribute to the differential clinical disease expression in men and women.
There has been evidence suggesting that several neuropsychological deficits found in schizophrenia may be associated with parietal lobe dysfunction (1–3). These abnormalities are associated with problems in attention, perception, affect recognition, and visuospatial processing (2–5), functions subserved by the inferior parietal lobule as part of a circuit in conjunction with other, particularly frontal, brain regions.
The inferior parietal lobule, also referred to as “posterior parietal cortex” (6), consists of the supramarginal and angular gyri, which correspond approximately to Brodmann’s areas 39 and 40 (7). It is part of the heteromodal association cortex (6, 8, 9), the regions of which are the last to evolve and mature and comprise higher-order cortical circuits that receive and process information from primary motor and sensory areas, as well as other unimodal and polymodal association cortical regions (10, 11). The inferior parietal lobule also has extensive interconnections with the limbic system and hypothalamus (12).
Heteromodal association cortex areas are usually highly lateralized (figure 1), with the region in one hemisphere having greater area or volume than the other (9, 13). These regions also exhibit normal sex differences, especially with regard to asymmetry (9, 13–24). We have previously shown that the left inferior parietal lobule is larger in healthy male subjects than in female comparison subjects (13, 25). As the inferior parietal lobule is involved in visuospatial processing, in which men tend to outperform women (26), such sex-based brain differences may mediate some of the normally observed subtle cognitive differences (27–30).
Several of the cognitive tasks mediated by the parietal lobe appear to be lateralized. For example, the right parietal lobe may be more involved in processing affect (31–33) and relationships between body parts (34). In contrast, the left inferior parietal lobule is more involved in cognitive tasks related to perception and visuospatial processing (35–40).
Several functional neuroimaging studies in schizophrenia have demonstrated that parietal regions exhibit reduced regional cerebral blood flow (rCBF) in resting states (2, 32, 41, 42) and a failure to deactivate rCBF appropriately during cognitive tasks (43, 44). Some of these activation/deactivation patterns appear to lateralize (44) or relate to symptoms (2, 45). These rCBF patterns are often associated with concurrent inappropriate activation/deactivation in other brain regions, particularly frontal areas, which implies that the inferior parietal lobule is part of a functional circuit that is disturbed in patients with schizophrenia (43).
Differential disease expression between the sexes has been observed in patients with schizophrenia (46–55). Both the sex-based brain asymmetries, particularly in heteromodal association cortex regions, seen in healthy subjects and the involvement of the heteromodal association cortex in schizophrenia suggest that disruption of these normal sex-based heteromodal association cortex asymmetries may occur in schizophrenia (9, 16, 56). Most research that has examined the heteromodal association cortex, however, has focused on frontal and temporal lobe structure and function (13, 19, 22, 57–59). By means of a locally developed method for measuring inferior parietal lobule gray matter volume, we previously reported stronger asymmetry in normal male subjects than in normal female subjects (25). Given this stronger male asymmetry, plus the possible disruptions of normal asymmetries seen with schizophrenia, we hypothesized that in patients with schizophrenia, the normal leftward male inferior parietal lobule asymmetry would be disturbed.
METHOD
Subjects
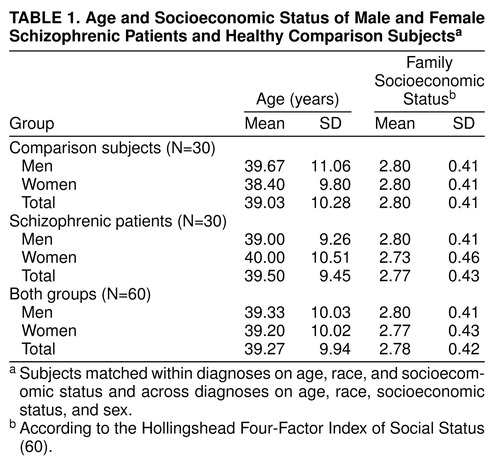
Figure 2 depicts the individual matching design between subjects. We examined a total of 60 subjects in which 15 pairs of normal male and female subjects, individually matched to each other on age (plus or minus 5 years), race, and parental socioeconomic status (60), were also individually matched on the same variables as well as by sex to 15 pairs of similarly matched male and female subjects with DSM-III-R schizophrenia (as assessed by the SCID [61]). In terms of diagnostic reliability, all patients continue to be followed by the investigators, and there have been no diagnostic changes. All 60 subjects were right-handed as assessed by the Chapman inventory (62). Table 1 outlines group demographic data.
Comparison and schizophrenic subjects were recruited from the community through advertisements (N=18) or had been reached through a random telephone digit dialing protocol as part of a community-based aging study (N=4). Additional subjects were taken from the Epidemiologic Catchment Area study (63) (N=6) and the Maryland Epidemiologic Sample (64) (N=10) or were employees of Johns Hopkins Hospital (N=3). In addition, nine schizophrenic and 10 comparison subjects were derived from a parallel study by a collaborator in London (T.S.). Exclusion criteria included history or magnetic resonance imaging (MRI) evidence of overt brain disease as determined by radiologist interpretation; lifetime history of substance abuse/dependence; or any medical illnesses known to affect the brain, e.g., severe head injury with loss of consciousness for more than 1 hour, severe hypertension (e.g., requiring treatment with two or more medications), or clinically significant cardiovascular disease that required medical or surgical treatment. Additional exclusionary criteria for comparison subjects included lifetime history of significant psychopathology or history of major mental illness in a first-degree relative as assessed by a locally developed questionnaire. After complete description of the study to the subjects, written informed consent was obtained from all subjects in accordance with institutional internal review board standards.
MRI Protocol
Coronal MRI scans from throughout the entire brain were obtained by means of high-resolution contiguous 1.5-mm thick spoiled gradient recall acquisition in the steady state (TE=5 msec, TR=35 msec, field of view=20 or 24 cm, image matrix=256 × 256 pixels, flip angle of 45°) on one of three Signa 1.5-T units that used the same General Electric software. An approximately equal number of patients and comparison subjects were imaged on each scanner. Scans were then transferred from the MRI archive and stored on CD-ROMs.
Image Processing and Measurement
All raters were blind to subject sex and diagnosis. By using the software developed in our laboratory (Measure) (65), raters stripped all brains of skull and dura by means of reliable semiautomated techniques (59, 66) and rendered realistic three-dimensional brain images, which enabled easy visualization of sulcal-gyral patterns. All brains were aligned along the anterior-posterior commissural line and the interhemispheric fissure.
By means of a “paint and point-counting” method described elsewhere (25, 59), the inferior parietal lobule was first delineated by painting the sulcal and gyral landmarks on a three-dimensional rendering of the cortical surface. The anterior boundary was the postcentral sulcus and the superior boundary was the intraparietal sulcus. The inferior boundary consisted of 1) the Sylvian fissure from the postcentral sulcus to the posterior lateral edge of the planum temporale, 2) a plane that passed through this posterior lateral edge and the temporo-occipital incisure to the superior temporal (or parallel) sulcus, and 3) the parallel sulcus to its horizontal segment (anterior occipital sulcus) and its connection with the intraparietal sulcus.
The spoiled gradient recall acquisition data set was filtered by using locally developed anisotropic diffusion filtering software (K=1.5 × average sigma value of 10 random values within the caudate nucleus; number of iterations=3) to better visualize the gray-white boundary (67). A three-dimensional grid of points, spaced 4.5 mm apart and yielding approximately 200 points per inferior parietal lobule, was superimposed on the entire volume (25). “Paint” that demarcated the inferior parietal lobule anatomical borders was then superimposed on the filtered image set, and gray matter points lying within the painted borders were selected. This stereological volume estimation method (Cavalieri) has been discussed in previous publications (59, 65). Because of imprecise white matter boundaries for this and other cortical regions, inferior parietal lobule white matter volumes were not obtained. As reported previously (25), intra- and interrater reliability of the inferior parietal lobule volume stereological measurements separately yielded intraclass correlation coefficients of 0.98 in five randomly selected brains.
In addition to total brain volume and volumes of the left and right inferior parietal lobules, asymmetry indexes of inferior parietal lobule volume ([left – right]/0.5[left + right]) and ratios of left and right inferior parietal lobule volumes to total brain volume were calculated for each subject and are presented in table 2.
Statistical Analysis
Given our previous finding of sex differences in the inferior parietal lobule in normal subjects (25), we compared subjects within the same sex group so as not to obscure potentially important disease state findings. In order to retain the greatest statistical power from our individually matched group, we conducted paired-samples t tests for total brain volume, asymmetry index, and ratios of left and right inferior parietal lobule volume to total brain volume within each sex group. As asymmetry indexes were unitless and were derived from the mean inferior parietal lobule volumes, no total brain volume correction was required.
Chi-square analyses or t tests, as appropriate, were used to examine group differences on demographic variables. Analyses of variance (ANOVAs) of total brain volume, left and right inferior parietal lobule volumes, and asymmetry indexes were performed to examine main effects of MRI scanner.
RESULTS
Neither the male versus female nor the schizophrenic versus comparison groups differed significantly on age, race (86.7% of the entire group was Caucasian), or family socioeconomic status (table 1). ANOVA showed no significant main effect of MRI scanner on total brain volume (F=0.04, df=2, 59, p=0.97), left inferior parietal lobule volume (F=0.22, df=2, 59, p=0.81), right inferior parietal lobule volume (F=0.12, df=2, 59, p=0.88), or asymmetry index (F=0.52, df=2, 59, p=0.60).
Results of the statistical analyses for the measurement data are summarized in table 2. Regarding our primary hypothesis, male schizophrenic patients differed significantly from male comparison subjects on asymmetry index and the left inferior parietal lobule to total brain volume ratio, but not on total brain volume or the right inferior parietal lobule to total brain volume ratio. Female schizophrenic patients differed significantly from female comparison subjects only on total brain volume.
DISCUSSION
This structural MRI study provides evidence of inferior parietal lobule abnormality in patients with schizophrenia that appears to be mediated by sex. Previously, we had shown that inferior parietal lobule volume in healthy men exhibited a left-greater-than-right asymmetry and that left inferior parietal lobule gray matter volumes were significantly larger in men than in women (25). Conversely, this study demonstrated that male schizophrenic subjects had significantly smaller left inferior parietal lobule volumes than healthy male comparison subjects, as well as a reversal of the normal left-greater-than-right asymmetry. Whereas female schizophrenic patients had significantly smaller total brain volumes than healthy female comparison subjects, these differences were not accompanied by significant differences in left or right inferior parietal lobule volume or asymmetry. Hence, in patients with schizophrenia, disruption of the normal inferior parietal lobule asymmetry appears to be limited to men only. To our knowledge, this is the first study to demonstrate such a finding. Such sexual dimorphisms may underlie some of the sex-based cognitive and clinical differences observed between male and female schizophrenic patients (46–55).
Given our previous finding in normal subjects of larger left inferior parietal lobule volumes in men versus women (25), it is important when comparing diseased and healthy subjects to analyze data by sex so as not to obscure potentially important sex-based findings. Closer inspection of table 2 reveals that combining male and female inferior parietal lobule data for each diagnostic group may lead to the incorrect conclusion of no inferior parietal lobule volume differences in schizophrenia. While not statistically significant, scrutiny of the data also reveals that female schizophrenic patients not only had larger inferior parietal lobule volumes than female comparison subjects, even when corrected for total brain volume, but that female schizophrenic patients exhibited the greatest degree of inferior parietal lobule rightward asymmetry overall (figure 3). Thus, the inferior parietal lobule may be affected in female schizophrenic patients, and further studies are required.
The inferior parietal lobule is one component of the heteromodal association cortex family of regions that also include the planum temporale, Broca’s area, and the dorsolateral prefrontal cortex (6, 8, 9). Heteromodal association cortex regions normally exhibit asymmetries (9, 13), especially with regard to sex differences (9, 13, 17). In schizophrenia, heteromodal association cortex regions show disruption of these normal asymmetries, as well as volume losses, e.g., in Broca’s area (32, 45) and the planum temporale (13, 68, 69). Functionally, activation abnormalities of rCBF in several heteromodal association cortex regions, including the inferior parietal lobule, suggest impairment of a neural circuit in schizophrenia (43).
Possible deficiencies of this study include lack of white matter volume data and all of the subjects being right-handed. Many asymmetrical brain measures (e.g., planum temporale and radius of gyration) correlate with handedness (18, 70). Thus, left-handed individuals may show variants of the pattern described here. As for white matter data, precise white matter boundaries for the inferior parietal lobule, or other cortical surface regions, do not exist, and appropriate valid and reliable measurement techniques remain to be developed.
As we have noted, the inferior parietal lobule is involved in neurological functions related to attention, perception, and visuospatial processing, with studies demonstrating hemispheric lateralization of these functions. For example, the left inferior parietal lobule appears to mediate visuospatial functions such as mental rotation of three-dimensional figures (38) and judgments of target speed (35) and position (39). Studies have also demonstrated that normal male subjects tend to perform better than normal women on visuospatial tasks (26).
In schizophrenia, male/female comparisons of cognitive functions have provided mixed results. Men with schizophrenia tend to develop the disorder earlier (47) and exhibit more negative symptoms (48, 53, 55), poorer social functioning (46, 49, 50, 52), and more abnormal neurologic signs (51). They may also exhibit more cognitive impairment (46, 71), although other studies do not report this (54, 72–74). While our study was conducted in a very well-matched subject population and used a highly reliable measurement method, the lack of accompanying detailed neuropsychological and clinical data makes direct comparison between brain findings and clinical symptoms speculative. Future studies (e.g., functional neuroimaging studies) that assess activation during cognitive tests specific to parietal regions, with an emphasis on sex differences and lateralization in normal and disease states, would be useful.
Received May 26, 1999; revision received Aug. 17, 1999; accepted Aug. 24, 1999From the Division of Psychiatric Neuro-Imaging, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine; and the Section of Cognitive Psychopharmacology, Institute of Psychiatry, London. Address reprint requests to Dr. Pearlson, Division of Psychiatric Neuro-Imaging, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 600 North Wolfe St., Meyer 3-166, Baltimore, MD 21287; [email protected] (e-mail). Supported in part by grants from NIMH (MH-43775), the National Institute on Aging (AG-11859), the NIH General Clinical Research Center (RR-00722), the Shapiro Foundation, and the Stanley Foundation (Dr. Pearlson).
 |
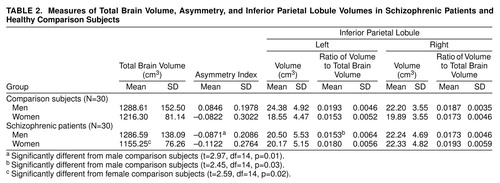 |
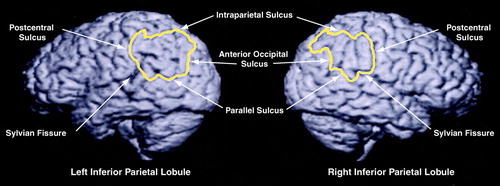
FIGURE 1. Left and Right Inferior Parietal Lobules in a Normal Male Brain a
aSulcal boundaries are labeled. The left-greater-than-right asymmetry typically seen in healthy male subjects is illustrated here (left inferior parietal lobule gray matter volume=26.10 cm3; right inferior parietal lobule gray matter volume=17.30 cm3).
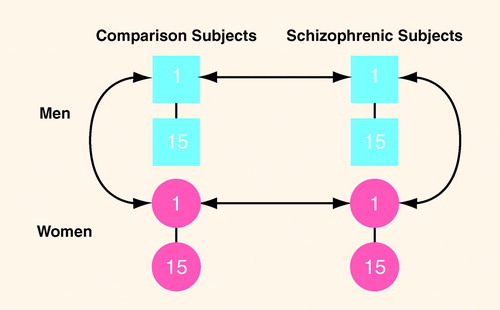
FIGURE 2. Paradigm for Matching Subjects in Study of Inferior Parietal Lobule Volume in Schizophreniaa
aSubjects matched individually within diagnoses on age, race, and parental socioeconomic status, and individually across diagnoses on these variables, as well as sex.
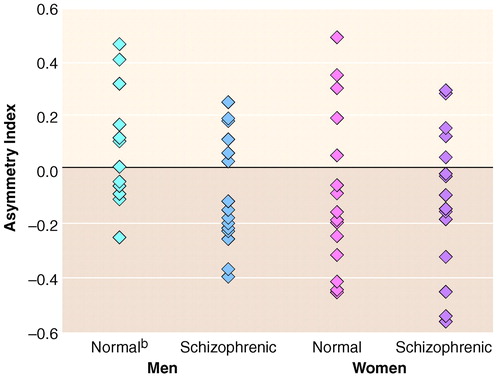
FIGURE 3. Lateral Asymmetry of the Inferior Parietal Lobule in Male and Female Schizophrenic Subjects and Healthy Comparison Subjectsa
aPositive asymmetry values indicate left-greater-than-right asymmetry; negative values indicate right-greater-than-left asymmetry.
bAsymmetry index values of two subjects coincided exactly in three cases.
1. Manschreck TC, Ames D: Neurologic features and psychopathology in schizophrenic disorders. Biol Psychiatry 1984; 19:703–719Medline, Google Scholar
2. Kaplan RD, Szechtman H, Franco S, Szechtman B, Nahmias C, Garnett ES, List S, Cleghorn JM: Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by positron emission tomography (PET). Schizophr Res 1993; 11:47–54Crossref, Medline, Google Scholar
3. Wigal SB, Swanson JM, Potkin SG: Lateralized attentional deficits in drug-free and medicated schizophrenic patients. Neuropsychologia 1997; 35:1519–1525Google Scholar
4. Petersen SE, Robinson DL, Currie JN: Influences of lesions of parietal cortex on visual spatial attention in humans. Exp Brain Res 1989; 76:267–280Crossref, Medline, Google Scholar
5. Heilman KM, Watson RT, Valenstein E, Damasio AR: Localization of lesions in neglect, in Localization in Neuropsychology. Edited by Kertez A. New York, Academic Books, 1993, pp 471–492Google Scholar
6. Mesulam MM: From sensation to cognition. Brain 1998; 121:1013–1052Google Scholar
7. Duvernoy HM: The Human Brain Surface, Three-Dimensional Sectional Anatomy and MRI. New York, Springer-Verlag Wien, 1991, pp 10–11Google Scholar
8. Mesulam MM: Principles of Behavioral Neurology. Philadelphia, FA Davis, 1985Google Scholar
9. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–17Google Scholar
10. Geschwind N: The development of the brain and the evolution of language, in Report of the 15th Annual RTM on Linguistic and Language Studies. Edited by Stuart CIJM. Washington, DC, Georgetown University Press, 1964Google Scholar
11. Mesulam MM, Geschwind N: On the possible role of neocortex and its limbic connections in the process of attention and schizophrenia: clinical cases of inattention in man and experimental anatomy in monkey. J Psychiatr Res 1978; 14:249–259Crossref, Medline, Google Scholar
12. Zec RF, Weinberger DR: Handbook of Schizophrenia, vol 1: The Neurology of Schizophrenia. New York, Elsevier Science, 1986, p 191Google Scholar
13. Honeycutt NA, Frederikse ME: Brain asymmetries in schizophrenia. J Advances in Schizophrenia and Brain Res 1999; 1:98–105Google Scholar
14. Geschwind N, Galaburda AM: Cerebral lateralization: biological mechanisms, associations, and pathology, II: a hypothesis and a program for research. Arch Neurol 1985; 42:521–552Crossref, Medline, Google Scholar
15. Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D: Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849Google Scholar
16. Pearlson GD, Pulver AE: Sex, schizophrenia and the cerebral cortex, in Schizophrenia: Exploring the Spectrum of Psychosis. Edited by Ancill R. New York, John Wiley & Sons, 1994, pp 345–362Google Scholar
17. Marsh L, Casper R: Gender differences in brain morphology and in psychiatric disorders, in Women’s Health: Hormones, Emotions, and Behavior. Edited by Casper RC. New York, Cambridge University Press, 1998, pp 53–82Google Scholar
18. Steinmetz H, Volkmann J, Jancke L, Freund HJ: Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol 1991; 29:315–319Crossref, Medline, Google Scholar
19. Barta PE, Petty RG, McGilchrist I, Lewis RW, Jerram M, Casanova MF, Powers RE, Brill LB, Pearlson GD: Asymmetry of the planum temporale: methodological considerations and clinical associations. Psychiatry Res 1995; 61:137–150Crossref, Medline, Google Scholar
20. Barta PE, Pearlson GD, Brill LB II, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG: Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry 1997; 154:661–667Link, Google Scholar
21. Foundas AL, Leonard CM, Heilman KM: Morphologic cerebral asymmetries and handedness: the pars triangularis and planum temporale. Arch Neurol 1995; 52:501–508Crossref, Medline, Google Scholar
22. Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE: Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiatry 1995; 152:715–721Link, Google Scholar
23. Witelson SF, Kigar DL: Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurol 1992; 323:326–340Crossref, Medline, Google Scholar
24. Kulynych JJ, Vladar K, Jones DW, Weinberger DR: Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cereb Cortex 1994; 4:107–118Crossref, Medline, Google Scholar
25. Frederikse ME, Lu A, Aylward EH, Barta PE, Pearlson GD: Sex differences in the inferior parietal lobule. Cereb Cortex 1999; 9:896–901Crossref, Medline, Google Scholar
26. Halpern DF: Sex Differences in Cognitive Abilities. Hillsdale, NJ, Lawrence Erlbaum Associates, 1986Google Scholar
27. Bakan P, Putnam W: Right-left discrimination and brain lateralization: sex differences. Arch Neurol 1974; 30:334–335Crossref, Medline, Google Scholar
28. Maccoby E, Jacklin C: The Psychology of Sex Differences. Stanford, Calif, Stanford University Press, 1974Google Scholar
29. Benbow CP, Stanley JC: Sex differences in mathematical ability: fact or artifact? Science 1980; 210:1262–1264Google Scholar
30. Gladue BA, Beatty WW, Larson J, Staton RD: Sexual orientation and spatial ability in men and women. Psychobiology 1990; 18:101–108Google Scholar
31. Borod JC, Koff E, Perlman LM, Nicholas M: The expression and perception of facial emotion in brain-damaged patients. Neuropsychologia 1986; 24:169–180Crossref, Medline, Google Scholar
32. Cleghorn JM, Garnett ES, Nahmias C, Firnau G, Brown GM, Kaplan R, Szechtman H, Szechtman B: Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res 1989; 28:119–133Crossref, Medline, Google Scholar
33. Cleghorn JM, Kaplan RD, Nahmias C, Garnett ES, Szechtman H, Szechtman B: Inferior parietal region implicated in neurocognitive impairment in schizophrenia. Arch Gen Psychiatry 1989; 46:758–760Crossref, Medline, Google Scholar
34. Cutting J: Delusional misidentification and the role of the right hemisphere in the appreciation of identity. Br J Psychiatry Suppl 1991; 14:70–75Medline, Google Scholar
35. Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE: Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 1991; 11:2383–2402Google Scholar
36. Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit-Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D: Brain activation induced by estimation of duration: a PET study. Neuroimage 1996; 3:119–126Crossref, Medline, Google Scholar
37. Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS: Functional anatomy of a common semantic system for words and pictures. Nature 1996; 383:254–256Crossref, Medline, Google Scholar
38. Alivisatos B, Petrides M: Functional activation of the human brain during mental rotation. Neuropsychologia 1997; 35:111–118Crossref, Medline, Google Scholar
39. Lacquaniti F, Perani D, Guigon E, Bettinardi V, Carrozzo M, Grassi F, Rossetti Y, Fazio F: Visuomotor transformations for reaching to memorized targets: a PET study. Neuroimage 1997; 5:129–146Crossref, Medline, Google Scholar
40. Winstein CJ, Grafton ST, Pohl PS: Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol 1997; 77:1581–1594Google Scholar
41. Satoh K, Suzuki T, Narita M, Ishikura S, Shibasaki M, Kato T, Takahashi S, Fukuyama H, Ohnishi H, Morita R: Regional cerebral blood flow in catatonic schizophrenia. Psychiatry Res 1993; 50:203–216Crossref, Medline, Google Scholar
42. Kishimoto H, Kuwahara H, Ohno S, Takazu O, Hama Y, Sato C, Ishii T, Nomura Y, Fujita H, Miyauchi T: Three subtypes of chronic schizophrenia identified using 11C-glucose positron emission tomography. Psychiatry Res 1987; 21:285–292Crossref, Medline, Google Scholar
43. Schroeder J, Buchsbaum MS, Siegel BV, Geider FJ, Haier RJ, Lohr J, Wu J, Potkin SG: Patterns of cortical activity in schizophrenia. Psychol Med 1994; 24:947–955Crossref, Medline, Google Scholar
44. Fletcher PC, McKenna PJ, Frith CD, Grasby PM, Friston KJ, Dolan RJ: Brain activations in schizophrenia during a graded memory task studied with functional neuroimaging. Arch Gen Psychiatry 1998; 55:1001–1008Google Scholar
45. McGuire PK, Shah GM, Murray RM: Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet 1993; 342:703–706Crossref, Medline, Google Scholar
46. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
47. Hafner H: Epidemiology of schizophrenia, in Search for the Causes of Schizophrenia. Edited by Hafner H, Gattaz WF, Janzarik W. Berlin, Springer-Verlag, 1987, pp 47–74Google Scholar
48. Goldstein JM, Link BG: Gender and the expression of schizophrenia. J Psychiatr Res 1988; 22:141–155Crossref, Medline, Google Scholar
49. Angermeyer MC, Kuhn L, Goldstein JM: Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull 1990; 16:293–307Crossref, Medline, Google Scholar
50. Eaton WW: Update on the epidemiology of schizophrenia. Epidemiol Rev 1991; 13:320–328Crossref, Medline, Google Scholar
51. Goldstein JM, Seidman LJ, Santangelo S, Knapp PH, Tsuang MT: Are schizophrenic men at higher risk for developmental deficits than schizophrenic women? implications for adult neuropsychological functions. J Psychiatr Res 1994; 28:483–498Crossref, Medline, Google Scholar
52. Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, Chakos M, Koreen A, Jody D, Kane J, Woerner M, Cooper T: Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry 1995; 152:698–703Link, Google Scholar
53. Gur RE, Petty RG, Turetsky BI, Gur RC: Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res 1996; 21:1–12Crossref, Medline, Google Scholar
54. Lewine RRJ, Walker EF, Shurett R, Caudle J, Haden C: Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry 1996; 153:1178–1184Google Scholar
55. Lindamer LA, Lohr JB, Harris MJ, McAdams LA, Jeste DV: Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry 1999; 60:61–67Crossref, Medline, Google Scholar
56. Pearlson GD, Marsh L: Structural brain imaging changes in schizophrenia. Biol Psychiatry 1999; 46:627–649Crossref, Medline, Google Scholar
57. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
58. Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW: Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry 1995; 52:279–288Crossref, Medline, Google Scholar
59. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049–1055Google Scholar
60. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
61. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
62. Chapman LJ, Chapman JP: The measurement of handedness. Brain Cogn 1987; 6:175–183Crossref, Medline, Google Scholar
63. Regier DA, Myers JK, Kramer M, Robins LN, Blazer DG, Hough RL, Eaton WW, Locke BZ: The NIMH Epidemiologic Catchment Area Program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry 1984; 41:934–941Crossref, Medline, Google Scholar
64. Pulver AE, Bale SJ: Availability of schizophrenic patients and their families for genetic linkage studies: findings from the Maryland epidemiology sample. Genet Epidemiol 1989; 6:671–680Crossref, Medline, Google Scholar
65. Barta PE, Dhingra L, Royall R, Schwartz E: Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods 1997; 75:111–118Crossref, Medline, Google Scholar
66. Aylward EH, Augustine A, Li Q, Barta PE, Pearlson GD: Measurement of frontal lobe volume on magnetic resonance imaging scans. Psychiatry Res 1997; 75:23–30Crossref, Medline, Google Scholar
67. Gerig G, Kubler O, Kikinis R, Jolesz F: Nonlinear anisotropic filtering of MRI data. IEEE Trans Med Imaging 1992; 11:221–232Crossref, Medline, Google Scholar
68. DeLisi LE, Hoff AL, Neale C, Kushner M: Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res 1994; 12:19–28Crossref, Medline, Google Scholar
69. Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC: Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Arch Gen Psychiatry 1997; 54:433–440Crossref, Medline, Google Scholar
70. Bullmore E, Brammer M, Harvey I, Murray R, Ron M: Cerebral hemispheric asymmetry revisited: effects of handedness, gender and schizophrenia measured by radius of gyration in magnetic resonance images. Psychol Med 1995; 25:349–363Crossref, Medline, Google Scholar
71. Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT: Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry 1998; 155:1358–1364Google Scholar
72. Goldberg TE, Gold JM, Torrey EF, Weinberger DR: Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry 1995; 152:883–888Link, Google Scholar
73. Perlick D, Mattis S, Stastny P, Teresi J: Gender differences in cognition in schizophrenia. Schizophr Res 1992; 8:69–73Crossref, Medline, Google Scholar
74. Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, Morganville J, Braff DL: Gender differences in schizophrenia. J Nerv Ment Dis 1995; 183:522–528Crossref, Medline, Google Scholar



