Striatal Dopamine Transporter Binding in Neuroleptic-Naive Patients With Schizophrenia Studied With Positron Emission Tomography
Abstract
OBJECTIVE: Recent in vivo imaging studies indicate a dysregulated presynaptic function of the striatal dopaminergic system in patients with schizophrenia. To further explore the basis of this phenomenon, the authors studied brain dopamine transporter binding in vivo in patients with first-episode, never-medicated schizophrenia. METHOD: Nine patients with schizophrenia and nine healthy matched comparison subjects were recruited. Striatal dopamine transporter binding was measured with positron emission tomography and a specific dopamine transporter ligand, [18F]CFT, a radiolabeled form of 2β-carbomethoxy-3β-(4-fluorophenyl)tropane. RESULTS: Average caudate and putamen dopamine transporter binding potentials were almost identical in the patients and comparison subjects, but the patients lacked the right-left asymmetry of the caudate dopamine transporter binding seen in the comparison group. CONCLUSIONS: Average striatal dopamine transporter density is unaltered in neuroleptic-naive patients with schizophrenia. However, patients lack asymmetry in caudate dopamine transporter binding, which conforms with disrupted brain lateralization in this disorder.
Recent in vivo imaging studies have provided direct evidence for altered presynaptic dopamine function in neuroleptic-naive patients with schizophrenia. Presynaptic dopamine synthesis capacity (1, 2) and amphetamine-stimulated dopamine release (3, 4) in the striatum are, on average, enhanced in patients with schizophrenia. In addition, the right-left asymmetry of the dopamine synthesis capacity seen in the caudate of young, healthy comparison subjects is lost in neuroleptic-naive patients with schizophrenia (1, 2). The dopamine transporter is a protein located on the presynaptic membrane of the dopaminergic synapse, and its density has been shown to correlate well with the density of dopaminergic nerve terminals (5). To further examine the basis of altered presynaptic dopamine function in patients with schizophrenia, we studied dopamine transporter binding in nine first-episode, neuroleptic-naive patients and nine matched comparison subjects by means of positron emission tomography (PET) and a radiolabeled form of 2β-carbomethoxy-3β-(4-fluorophenyl)tropane, [18F]CFT (6).
METHOD
This study was approved by the ethics committee of the University of Turku/Turku University Central Hospital, Turku, Finland, and was performed in accordance with the ethical standards of the Declaration of Helsinki. After a complete description of the study to the subjects, written informed consent was obtained.
Nine neuroleptic-naive patients (six men and three women) who met the DSM-III-R criteria for schizophrenia were recruited from Turku City Psychiatric Hospital and Keropudas Psychiatric Hospital (Tornio, Finland). In addition, nine age- and gender-matched healthy volunteers with no history of mental or physical illness or substance abuse were recruited. The mean ages were 30.1 years (SD=7.0) and 29.9 years (SD=5.6) in the patient and comparison groups, respectively. At the time of the PET scan, the patients had a median duration of active illness of 9 months (range=9–39) and a median total duration of illness of 19 months (range=9–120), including prodromal symptoms. The prodromal phase was estimated by using the prodrome criteria from DSM-III-R. The severity of the symptoms and symptom dimensions was derived from the Positive and Negative Syndrome Scale (7). The ratings were carried out by a senior psychiatrist (H.V.) within 1 day of the PET scan. The mean Positive and Negative Syndrome Scale score was 73 (SD=23, range=41–114).
The dopamine transporter density was measured with PET by using [18F]CFT as a tracer (6). PET experiments were performed by using a whole-body PET scanner (ECAT 931/08-12, Computer Technology & Imaging, Knoxville, Tenn.). Injected doses of [18F]CFT were 4.2 mCi (SD=0.7) and 4.3 mCi (SD=0.4) for the comparison and schizophrenic groups, respectively. Each subject also underwent a T1-weighted, 1.5-T magnetic resonance imaging scan (Siemens Magnetom, Iselin, N.J.). Anatomical regions of interest (the caudate, putamen, and cerebellar cortex) were drawn without the knowledge of patient diagnosis on magnetic resonance images resliced according to the PET slices. The tracer uptake was quantified as a mean binding potential ratio 3.5–4.5 hours after the injection (6). To evaluate the asymmetry of striatal binding values, we calculated an asymmetry index, (right – left)/(right + left).
Student’s independent-sample t tests or two-way repeated measures analyses of variance (ANOVAs) were used to compare groups; paired t tests were used for right-left comparisons within groups. Relationships between [18F]CFT binding and symptoms were studied with Pearson’s correlation analysis. The effect of illness duration on [18F]CFT binding was studied with analysis of covariance (ANCOVA) by using age as a covariate; p values of less than 0.05 were considered statistically significant. For power analyses, values of 0.05 and 0.8 were used for alpha and beta, respectively.
RESULTS
The average striatal [18F]CFT binding potentials were almost identical in the comparison and patient groups: mean=4.50 (SD=0.54) and mean=4.56 (SD=0.57), respectively, in the caudate (t=–0.24, df=16, p=0.81, independent-samples t test); and mean=4.81 (SD=0.41) and mean=4.86 (SD=0.59) in the putamen (t=–0.20, df=16, p=0.84, independent-samples t test). However, two-way ANOVAs indicated a significant interaction between group and hemisphere in the caudate (F=10.00, df=1, 16, p=0.01 but not in the putamen (F=1.67, df=1, 16, p=0.21) (figure 1). Post hoc paired t tests revealed significantly higher (6.7%) [18F]CFT binding in the right caudate than in the left in the comparison group: mean=4.64 (SD=0.62) and mean=4.35 (SD=0.48) in the right and left caudate, respectively (t=3.50, df=8, p=0.01). In the schizophrenic group, no asymmetry was found: mean=4.53 (SD=0.58) and mean=4.59 (SD=0.59) in the right and left caudate (t=–0.75, df=8, p=0.48). No asymmetry was seen in the putamen in either group. The total duration of the illness, but not the duration of active illness, correlated with [18F]CFT binding in the putamen (r=–0.77, df=6, p=0.03, ANCOVA, with age as a covariate) but not in the caudate. None of the [18F]CFT uptake parameters correlated with any of the rated symptom dimensions.
DISCUSSION
Our results indicate that average in vivo striatal dopamine transporter binding, an index of dopaminergic innervation density (5), is not altered in neuroleptic-naive patients with schizophrenia. The group in our study was relatively small, but according to the power analysis, it would have been adequate to detect group differences of approximately 15%. Our findings are supported by postmortem studies, which indicate no change in dopamine transporter binding in patients with schizophrenia (5), although neuroleptic-treated patients with chronic schizophrenia in those studies cannot be directly compared to our patients. In contrast to this, increased presynaptic dopamine function is a relatively well-documented phenomenon in schizophrenia at the group level and in neuroleptic-naive patients (1–4). Our report indicates that striatal dopaminergic hyperactivity in schizophrenia is due to the increased activity rather than to the increased density of dopaminergic terminals in the striatum. The negative correlation between the total duration of illness and dopamine transporter density was clearly caused by the three patients with the longest illness durations and must be further explored.
According to our results, young, healthy people have, on average, a higher density of dopamine transporters in their right than left caudate. To our knowledge, this is the first time lateralized dopamine transporter density in normal human brains has been reported. However, no asymmetry was found in the caudate of patients. In the putamen, no asymmetry of dopamine transporter binding was seen in either group. These observations are practically identical to those seen in an independent study of neuroleptic-naive patients with schizophrenia who were studied with 6-[18F]-fluorodopa (1, 2). This indicates both structural and functional impairment of the lateralization of dopaminergic neurons projecting to the caudate. The fact that the disrupted pattern in dopaminergic innervation is seen in the caudate rather than in the putamen is reasonable, since the caudate has extensive interconnections with limbic and cortical regions and preferential involvement in emotional processes and cognition (8).
In conclusion, these results support dysregulation of the striatal presynaptic dopamine system in patients with schizophrenia and indicate the following.
1. Average striatal dopamine transporter binding is unchanged in neuroleptic-naive patients with first-episode schizophrenia, which suggests that the previously reported dysregulation in striatal presynaptic dopamine function in patients with schizophrenia is not due to an altered number of dopaminergic terminals.
2. The asymmetry in dopamine transporter binding in the caudate is lost in about one-half of the patients with schizophrenia. This phenomenon is probably a part of a larger disturbance of structural and functional brain asymmetry in patients with this disorder.
Presented in part at the 27th annual meeting of the Society for Neuroscience, New Orleans, Oct. 25–30, 1997. Received March 16, 1999; revision received June 28, 1999; accepted July 8, 1999. From the Department of Psychiatry and the Department of Pharmacology and Clinical Pharmacology, University of Turku, Turku, Finland; the Keropudas Psychiatric Hospital, Tornio, Finland; the Turku PET Centre; and the Turku City Psychiatric Hospital, Turku, Finland. Address reprint requests to Dr. Hietala, Turku PET Centre, Turku University Central Hospital, Kiinamyllynkatu 4-8, FIN-20520 Turku, Finland; [email protected] (e-mail). Supported by the Academy of Finland, the Finnish Cultural Foundation, the Finnish Medical Foundation, the Lundbeck Foundation, the Pharmacal Foundation, Emil and Blida Maunula’s Foundation, and the Technology Development Centre of Finland. Research Biochemicals International supplied the CFT precursor.
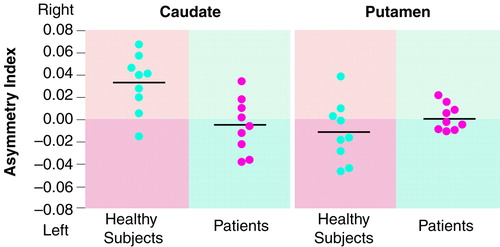
FIGURE 1. Asymmetry of Dopamine Transporter Binding in the Caudate and Putamen of Healthy Comparison Subjects (N=9) and Patients With Schizophrenia (N=9)a
aDopamine transporter binding measured as uptake of [18F]CFT shown by PET. Asymmetry index (right – left/right + left). Black horizontal lines indicate mean values.
1. Hietala J, Syvälahti E, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Kirvelä O, Ruotsalainen U, Salokangas RKR: Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 1995; 346:1130–1131Google Scholar
2. Hietala J, Syvälahti E, Vilkman H, Vuorio K, Räkköläinen V, Bergman J, Haaparanta M, Solin O, Kuoppamäki M, Eronen E, Ruotsalainen U, Salokangas RKR: Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res 1999; 35:41–50Crossref, Medline, Google Scholar
3. Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB: Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93:9235–9240Google Scholar
4. Breier A, Su T-P, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D: Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94:2569–2574Google Scholar
5. Bannon MJ, Granneman JG, Kapatos G: The dopamine transporter: potential involvement in neuropsychiatric disorders, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 179–188Google Scholar
6. Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Hietala J: [18F]CFT ([18F]WIN 35,428), a radioligand to study the dopamine transporter with PET: characterization in human subjects. Synapse 1998; 28:244–250Crossref, Medline, Google Scholar
7. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
8. Parent A: Extrinsic connections of the basal ganglia. Trends Neurosci 1990; 13:254–258Crossref, Medline, Google Scholar



