Novel Neurotransmitters and Their Neuropsychiatric Relevance
Abstract
OBJECTIVE: The purpose of this review is to integrate insights regarding novel neurotransmitters or neuromodulators of neuropsychiatric significance. METHOD: Evolving concepts of neurotransmitter criteria are reviewed in light of the unexpected properties displayed by recently identified transmitters. RESULTS: Classic criteria for transmitters were based on the properties of acetylcholine but were markedly revised with the recognition of the catecholamines, serotonin, γ-aminobutyric acid (GABA), and other amino acid transmitters and neuropeptides. Nitric oxide and carbon monoxide are notably atypical, as they are not stored in synaptic vesicles, are not released by exocytosis, and do not act at postsynaptic membrane receptor proteins. d-Serine, recently appreciated as the endogenous ligand for the glycine site of the glutamate N-methyl-d-aspartate (NMDA) receptor, overturns fundamental axioms of biology as well as those of neuroscience. It is a d-amino acid, and it is synthesized and stored in glia rather than neurons. Released glutamate acts on receptors on the protoplasmic astrocytes closely apposed to the synapse to release d-serine, which coactivates postsynaptic NMDA receptors together with glutamate. d-Serine is formed by serine racemase, which directly converts l-serine to d-serine. Inhibitors of this enzyme should reduce NMDA neurotransmission and might be therapeutic in stroke and other conditions associated with glutamate excitotoxicity. CONCLUSIONS: The diversity of novel neurotransmitters and venues of their activity afford multiple opportunities for therapeutic intervention.
Neurotransmitters, the key information molecules of the brain, mediate the actions of all known psychoactive drugs. We currently know of at least 50 to 100 possible neurotransmitters representing diverse chemical classes, including the biogenic amines, amino acids, peptides, and gases. As recently as the late 1950s, we knew of only two neurotransmitters, acetylcholine and norepinephrine. Acetylcholine was well established as the neurotransmitter that is active at the neuromuscular junction and in the autonomic nervous system. Norepinephrine was appreciated as the neurotransmitter of postganglionic sympathetic neurons. It was assumed that acetylcholine was important in the brain, but its functions in the central nervous system (CNS) were unclear. Norepinephrine had not yet been identified in CNS neurons. During these years, seminal observations were spawning the field of psychopharmacology. Newly developed flourometric techniques permitted the simple and specific measurement of biogenic amines. Soon it was discovered that reserpine, which in the late 1950s had been associated with depression in some patients, depleted the brain of serotonin and norepinephrine, whereas monoamine oxidase inhibitors, which had antidepressant effects, elevated the levels of these biogenic amines. There emerged a simplistic but reasonably accurate idea that norepinephrine and serotonin were important determinants of mood, with low levels causing and elevated levels reversing depression.
What was extraordinary about this early work, besides its prescience, was that this functional/psychiatric insight about biogenic amines preceded any evidence that these chemicals were neurotransmitters or even that they were contained within neurons. Establishing the presence of a substance in neurons and visualizing the specific neuronal populations containing the substance provides a quantum leap in insight. For biogenic amines, this information did not emerge until the mid 1960s, when a histochemical technique for visualizing the amines, developed by the Swedish investigator Nils-Erik Hillarp, was applied to the brain by his students Kjell Fuxe and Annica Dahlstrom. They successfully mapped out the biogenic amine neuronal pathways in the brain (1).
These historical reflections highlight a long-standing question in the neurosciences that is not yet fully resolved: what is a neurotransmitter? Over the years, criteria for defining a neurotransmitter have become so problematic that many neuroscientists gloss over the problem by labeling substances with vague words such as “neuromodulator.” For purposes of classifying neurotransmitters, neurophysiologists have discriminated two principal types of physiologic actions, fast and slow. Fast effects usually involve opening or closing of ion channels that are often part of a receptor protein, such as the sodium channel that is contained within the nicotinic acetylcholine receptor. Slow actions typically arise from metabolic alterations in target cells whose receptor proteins are termed metabotropic. Classic examples include the formation of cyclic GMP, cyclic AMP, or inositol trisphosphate by enzymes that are linked to receptors via GTP binding G-proteins (e.g., the muscarinic receptors for acetylcholine). Neurotransmitters are believed to signal through both fast and slow mechanisms. What else defines a neurotransmitter?
Although most of us have a vague idea of what we expect of a neurotransmitter, some neuroscientists have enunciated concrete criteria for “transmitterhood.” Since acetylcholine was the first neurotransmitter to be identified and characterized, the early criteria assumed that candidate neurotransmitters should be “just like” acetylcholine. For example, it was known that acetylcholine is synthesized by choline acetyltransferase, stored in synaptic vesicles, and released into the synapse after fusion of the vesicles with the plasma membrane in response to elevated calcium through exocytosis. Thus, a candidate transmitter should be localized to neurons, have a special biosynthetic enzyme, and be released in a calcium-dependent fashion when the nerve is depolarized. Of course, the candidate transmitter should mimic the actions of the endogenous transmitter when applied to postsynaptic cells. Acetylcholine, when acting postsynaptically, binds to specialized receptor proteins such as the nicotinic and muscarinic acetylcholine receptors. Accordingly, any new candidate transmitter was also expected to bind to a receptor protein localized to the external surface of the target cell’s plasma membrane. Finally, to mimic acetylcholine, an enzyme near the receptor (i.e., like acetylcholinesterase) should degrade and thereby inactivate the transmitter at the synapse.
Thus, a neurotransmitter has been thought of as a chemical stored in a nerve terminal that is released when the nerve fires to act on adjacent cells, altering their level of excitation. However, there are many challenges to this straightforward conceptualization. For example, neurophysiologists have now identified many unconventional ways that neurons can signal to each other via their cell bodies, dendrites, and portions of the axons other than the nerve terminals. These findings have generated new questions about neurotransmitter functions. Might a neurotransmitter be released from a dendrite rather than a nerve terminal? Can a chemical that acts on the same neuron that released it be considered a neurotransmitter? Neurons account for only a minority of cells in the brain, with glia making up about 85% of total. Although originally characterized as “supporting cells,” glia are now known to display a number of interesting electrical and chemical features. Might a substance that acts on glial cells rather than neurons be considered a neurotransmitter? Can glia release neurotransmitters?
By the early 1960s, difficulties with the acetylcholine-based definition of a neurotransmitter became evident as research moved beyond acetylcholine. In the early characterization of norepinephrine, monoamine oxidase, the only known degrading enzyme, was presumed to provide synaptic inactivation. However, monoamine oxidase inhibitors did not potentiate sympathetic neurotransmission. When Julius Axelrod discovered catechol-O-methyltransferase (COMT), he thought that this enzyme might constitute the synaptic inactivating system. However, COMT inhibitors also failed to potentiate synaptic transmission. Axelrod (2) and his associates then made a key discovery: synaptic inactivation of norepinephrine did not involve enzymes at all but rather a novel reuptake mechanism whereby the presynaptic neuron removed the neurotransmitter from the synapse by transporting it back into the nerve that had released it. If one applied the original, rigid criteria for a transmitter based on the characteristics of acetylcholine, norepinephrine would fail to qualify. Over time, it became evident that reuptake is common. Almost all biogenic amines, including serotonin and dopamine, are inactivated by reuptake (3, 4). Histamine provides an interesting exception to this generalization. Although histamine is formed by a specific enzyme, histidine decarboxylase, and is localized to discrete neuronal populations in the brain, there is no evidence that reuptake accounts for its synaptic inactivation. In addition, the histamine metabolizing enzymes, histamine methyltransferase and diamine oxidase, do not account for histamine’s inactivation. Thus, the question of whether specific synaptic inactivation terminates histamine signaling remains unanswered (5).
In addition to biogenic amines, amino acids were found to have neurotransmitter functions. Signaling by γ-aminobutyric acid (GABA), glutamate, glycine, and other amino acid transmitters, like signaling by the biogenic amines, is terminated by specific reuptake transport proteins. Gradually, neuroscientists accepted that neurotransmitter reuptake is as valid a means of providing synaptic inactivation as enzymatic degradation. In fact, reuptake eventually became appreciated as the rule rather than the exception.
The discovery and characterization of amino acid transmitters in the brain challenged other dogma about what constitutes a neurotransmitter. GABA was readily accepted as a transmitter because it is primarily, if not exclusively, devoted to a neurotransmitter role, and GABA is synthesized by a specific enzyme, glutamic acid decarboxylase. Moreover, the enzyme is localized to discrete neurons, GABA neurons. Glutamate and glycine were far more difficult for the scientific community to accept, since these amino acids are involved in protein synthesis and other metabolic pathways, with only a minor fraction of the total neuronal pool involved in a transmitter role. Glutamate posed a particular challenge, because its total concentration in the brain is extraordinarily high (≈20 mM), and glutamate is crucial in numerous pathways of intermediary metabolism. Even now, it is difficult to identify histochemically exactly which neurons in the brain employ glutamate as a neurotransmitter. Since one cannot identify specific biosynthetic enzymes for glutamate and glycine, one could argue that these molecules do not satisfy classic criteria for a neurotransmitter.
Despite such initial concerns, neuroscientists now accept amino acids as neurotransmitters with widespread functions in the brain. Although exact percentages are hard to pin down, glutamate is likely the neurotransmitter for 50% or more of synapses in the brain and is unquestionably the principal excitatory neurotransmitter. Aspartate chemically resembles glutamate, having just one less methyl group, so the two are difficult to distinguish in terms of transmitter function and localization. However, aspartate is thought to be an important excitatory neurotransmitter in the spinal cord. GABA is the principal inhibitory neurotransmitter in the brain and occupies 25%–40% of synapses, depending on the brain region. In the spinal cord, glycine may be the major inhibitory transmitter, signaling in 25%–30% of synapses. By contrast, dopamine, norepinephrine, and serotonin each are thought to account for only about 1% of brain synapses, and acetylcholine might occupy up to 5%. Thus, amino acids are quantitatively the principal neurotransmitters in the brain.
Like the amino acids, adenosine is involved in multiple metabolic functions and occurs in high concentrations in all tissues. Adenosine is neither a biogenic amine nor an amino acid, although it does possess amine-like properties. Discrete neuronal populations, with uniquely high densities of adenosine, have been identified by immunohistochemistry, suggesting a transmitter role (6). Adenosine is neuroactive, acting on nerve terminals to inhibit the release of most neurotransmitters. Moreover, a robust reuptake system for adenosine exists, and potent, selective adenosine reuptake inhibitors potentiate adenosine effects on neural activity.
By the end of the 1960s, acetylcholine, biogenic amines, and amino acids had been generally accepted as neurotransmitters. The 1970s became the decade of the peptide transmitters (7). Substance P was the first appreciated neuropeptide. It was discovered in the 1930s as an unidentified factor in tissue extracts that caused smooth muscle contraction. Substance P was isolated and sequenced by Susan Leeman (8), culminating her efforts to identify a tissue component that stimulates salivation in rats. Widespread interest in neuropeptides followed the identification of opiate receptors and the discovery of enkephalins as their endogenous ligands (9, 10). Enkephalins are small peptides that contain five amino acids. They are highly localized to the same discrete sites as opiate receptors. Actions of the enkephalins closely mimic those of morphine, which led to rapid acceptance of the enkephalins as neuroactive substances with physiologic roles in the brain. The localization of the enkephalins made use of immunohistochemistry, a technology pioneered through the elegant studies of Tomas Hokfelt and his associates (11), as a tool to characterize neuropeptides. In the late 1970s and early 1980s immunohisochemistry contributed to a veritable explosion in the number of identified neuropeptides. During this time, substance P was found to be enriched in intestinal neurons as well as brain neurons. Indeed, many neuropeptides were first identified in the intestine and related organs such as the pancreas. Examples include vasoactive intestinal polypeptide, cholecystokinin, gastrin, and even insulin (12).
During the time neuropeptides were being discovered in the brain and intestine, one might have argued that the amines and amino acids had “filled up the brain” so that no neuronal populations without transmitters remained. The question of which neurons would use neuropeptides was addressed by Hokfelt and associates (11), who established evidence for cotransmitters. We now know that most neurons contain an amino acid transmitter stored together with a biogenic amine or a peptide. Although any given neuropeptide probably accounts for only 1% or fewer of synapses, the 50 to 100 known neuropeptides may collectively occupy a substantial portion of CNS neurons. It is now generally accepted that that few if any brain neurons contain a single transmitter.
Neuropeptides also challenge the dogmatic criteria for a neurotransmitter. In terms of synaptic inactivation, peptides are presumably hydrolyzed by various peptidases. However, no one has rigorously demonstrated that a specific peptidase accounts for synaptic activation of individual neuropeptides. Most peptides do not elicit neuronal excitation or inhibition, as do acetylcholine and GABA. Rather, neuropeptides appear to have a modulatory action that has been difficult for neurophysiologists to characterize clearly. In some instances, certain actions of serotonin, norepinephrine, and dopamine also appear modulatory. This characteristic has led some to deny these molecules “transmitter status” and relegate them to the muddled role of neuromodulators. Perhaps, if the discovery of neuropeptides had preceded acetylcholine, the latter would have been challenged to fulfill transmitter criteria modeled on neuropeptide function.
By the mid 1980s so many neuropeptides had been identified that most neuroscientists were ready to close the book on chemical classes of neurotransmitters, with completed “chapters” on biogenic amines, amino acids, and neuropeptides. The 1990s have brought us two new major chemical classes of novel neurotransmitters that we will now explore in detail: gases and d-amino acids.
Gases
Nitric Oxide
The physiological role of nitric oxide (NO) was discovered in the 1980s in studies of blood vessels and macrophage activity. In 1980, Robert Furchgott discovered that the ability of acetylcholine to relax blood vessels did not involve direct actions through cholinergic receptors on smooth muscle (13). Instead, acetylcholine’s action required the endothelium to elaborate a vasoactive substance that would enter the smooth muscle to relax the muscle. This endothelial derived relaxing factor (EDRF) was later shown to be NO (14–16). Independently, other workers were trying to explain how activated macrophages kill tumor cells and bacteria. Arginine, later found to be the precursor of NO, was known to be crucial, and NO was identified as the key active molecule (15, 17–19). Garthwaite and colleagues (20) reported evidence for a substance with EDRF activity in the cerebellum. We became curious about a possible role for NO in the brain. Since it was virtually impossible at that time to measure NO gas directly, we decided to focus on the enzyme that makes NO. NO is generated in a single step from the amino acid arginine through the action of an enzyme designated NO synthase (NOS) (Figure 1 and Figure 2). Using NADPH as an electron donor, NOS oxidizes one of the guanidino nitrogens of arginine to form NO, with citrulline as an amino acid coproduct. Despite extensive investigations, a neuroactive role for citrulline has not been found.
To establish whether NO plays a role in neurotransmission in the brain, we took advantage of what was already known about how NO relaxes smooth muscle in blood vessels. By stimulating the activity of soluble guanylyl cyclase, the enzyme that makes cyclic GMP, NO initiates a signaling cascade that involves protein phosphorylation and causes smooth muscle relaxation. The cerebellum contains the highest levels of cyclic GMP in the brain. In addition, it was known that glutamate could trigger a 10-fold augmentation of cyclic GMP levels through activation of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors. As an index of NOS activity, we monitored the conversion of radiolabeled arginine to citrulline in ex vivo preparations of rat cerebellum. After NMDA receptor activation, NOS activity rapidly tripled in parallel with increased cyclic GMP levels (22), an effect also noted by Garthwaite and colleagues (23). We used arginine derivatives that inhibit NOS activity by competing with arginine for the active site in NOS to explore a link between NOS and cyclic GMP. NOS inhibitors specifically blocked the rise in cyclic GMP. Therefore, we could attribute increased cyclic GMP production to NO, establishing a role for NO in mediating actions of glutamate in the brain.
Clearly, NO dynamics are determined by NOS activity. Thus, it was crucial to characterize this enzyme. Several groups had tried to purify the NO-generating protein without success, because most purification processes led to loss of enzyme activity. We guessed that the enzyme itself might not be particularly labile, but that a cofactor might be dissociated during the purification. Knowing that calcium augmented enzyme activity, we hypothesized that the calcium-binding protein, calmodulin, might be the cofactor. When calmodulin was added to partially purified preparations, NOS activity was completely restored (24). Besides permitting purification of the enzyme, this finding explained how glutamate, through NMDA receptor activation, could almost immediately stimulate NO formation: after the NMDA receptor ion channel opens in response to binding glutamate, calcium enters the neuron, where it binds calmodulin to form a calcium-calmodulin complex (CaM) that binds and activates NOS.
Subsequently, we biochemically purified NOS (24) and developed specific antibodies to allow immunohistochemical visualization of NO neurons (25). NOS-containing neurons have very discrete localizations and represent only about 1% of neuronal cells in the brain. However, their axons ramify so extensively that virtually every cell in the brain may encounter a NOS nerve terminal (26, 27). Presumably, glutamate neurons synapse on NOS neurons, enabling NMDA receptor activation to trigger NO formation. As a diatomic gas, NO is freely diffusible and thus can readily enter adjacent neuronal cells or other cells (Figure 1). Once inside target cells, NO binds the iron in heme contained within the active site of soluble guanylyl cyclase, activating the enzyme to form cyclic GMP.
Given what we know about the synthesis and action of NO, does NO fulfill classic criteria for a neurotransmitter? In the brain, NO is formed in neurons in response to calcium influx, reminiscent of calcium dependent exocytic release of other neurotransmitters. However, as a gas, NO cannot be stored in synaptic vesicles or released by exocytosis. Moreover, there are no “receptors” for NO on the postsynaptic membrane of adjacent cells. Instead, NO diffuses from NOS neurons into neighboring cells, where it binds guanylyl cyclase, an “enzyme receptor.”
For most neurotransmitters, only a small percentage of the total store is released with each nerve stimulation, as there is a large storage pool of neurotransmitter in synaptic vesicles. Since NO cannot be stored, NOS must be activated every time a neuron needs to release NO. Accordingly, one might expect NOS enzymatic activity to be exquisitely regulated. Biochemical purification of NOS allowed us to clone the gene encoding it, obtain the full amino acid sequence, and express the recombinant protein in vitro for detailed characterization (27). NOS contains multiple sites for regulation (28). Although most oxidative enzymes use one electron donor, NOS is more complex. NOS possesses tightly bound flavin adenine mononucleotide (FMN) and flavin adenine dinucleotide (FAD), in addition to NADPH. It also utilizes heme and tetrahydrobiopterin (BH4) as electron donors. NOS possesses sites for phosphorylation by the major phosphorylating enzymes, including cyclic AMP-dependent protein kinase (PKA), protein kinase C (PKC), calcium-calmodulin-dependent protein kinase (CaMK), and cyclic GMP-dependent protein kinase (PKG) (28–30). Recently, NOS in endothelial cells has been shown to be phosphorylated by protein kinase B (PKB), also known as Akt, which participates in signaling cascades that affect nuclear function (31–33).
NOS can be regulated by interactions with other proteins. In the brain, NOS is physically linked to the postsynaptic membrane near NMDA receptors through its interaction with postsynaptic density protein-95 (34). By using the yeast 2-hybrid technique, we identified two other NOS binding partners: a protein inhibitor of NOS (PIN) (35) and carboxyl-terminal PDZ ligand of NOS (CAPON) (36). PIN is a phylogenetically conserved small protein that inhibits neuronal NOS (nNOS) by preventing the formation of NOS dimers, the form required for enzyme activity. CAPON appears to be a chaperone or scaffolding protein that links nNOS to other proteins.
Molecular cloning of NOS from brain permitted the identification of genes for distinct NOS isozymes from endothelial cells in blood vessels (37–39) and macrophages (40–42). The form of NOS that we initially purified and cloned was designated nNOS (neuronal NOS), the macrophage form is termed inducible NOS (iNOS), and the endothelial form is called endothelial NOS (eNOS). iNOS is so designated because, under resting conditions, macrophages and other cells display negligible enzyme activity. However, in response to physiologic stimuli, such as exposure to lipopolysaccharide from Gram-negative bacteria, these cells are induced to express iNOS and generate large amounts of NO sufficient to kill bacteria or tumor cells. Such stimuli provoke new synthesis of iNOS enzyme protein in just 1–2 hours. By contrast, nNOS and eNOS proteins are constitutively present and, as described above for nNOS, are activated by CaM.
Functions of NO
Experimentally establishing that a substance is a neurotransmitter in the brain can be exceedingly difficult. The intestine provides an excellent model system for the study of neurotransmitters since smooth muscle function can be used to monitor neurotransmitter release from the enteric nervous system, and the enteric nervous system uses nearly all transmitters found in the brain. Indeed, the most direct evidence for NO as a neurotransmitter comes from studies of the intestine, in which many neurons express nNOS. The excitatory transmitters in the enteric nervous system, including acetylcholine, norepinephrine, and some neuropeptides, have been known for some time. In contrast, the identity of the major inhibitory transmitters has been controversial. Since inhibitory transmission persists in the presence of selective blockade of adrenergic and cholinergic receptors, inhibitory transmission was designated nonadrenergic, noncholinergic (NANC) transmission. Stimulation of intestinal neurons causes the elaboration of a vasorelaxant factor that is indistinguishable from NO (43). NOS inhibitors block NANC transmission (44–46). To further clarify the role of NO as a neurotransmitter, mice with targeted genomic deletion of the nNOS gene (nNOS knockout mice) were developed (47). In small intestinal smooth muscle preparations from these mice, we found that NANC transmission was reduced by about 50% (48). More recently, we have found that smooth muscle cells from nNOS knockout mice have abnormal resting membrane potentials, implying that basal NO production determines the excitability of intestinal smooth muscle (49). Moreover, compounds that generate NO, when added to intestinal preparations, can mimic NANC transmission. Taken together, these findings establish a role for NO as a neurotransmitter.
We also found nNOS localized to neurons that innervate the corpora cavernosae and blood vessels of the penis (50). Electrical stimulation of the cavernous nerves provokes erection, which is blocked by NOS inhibitors. These results establish NO as a neurotransmitter mediating penile erection. As in other organs, NO mediates erection through the stimulation of cyclic GMP formation. Sildenafil inhibits phosphodiesterase type 5, an enzyme that selectively degrades cyclic GMP (51). Thus, the therapeutic effect of sildenafil for patients with erectile dysfunction involves potentiation of NO neurotransmission. It is of interest that sildenafil has no demonstrable effects on mental function, aside from eliciting mild headaches, presumably by causing vasodilatation of scalp blood vessels. This may relate to the limited ability of the drug to penetrate the blood-brain barrier.
Mice lacking nNOS and therefore NO production can be studied to ascertain physiologic roles for neuronally derived NO. Gross anatomic observations have revealed only a greatly dilated stomach with hypertrophy of the pylorus (47). Recently, we found that NANC relaxation of the pyloric sphincter is abolished in nNOS knockout mice and restored by NO donors (52). Since the pylorus contains a plexus of nNOS neurons, NO appears to be the neurotransmitter mediating NANC relaxation of the pylorus. This finding has clinical relevance, as nNOS knockout mice appear to be an animal model of infantile hypertrophic pyloric stenosis. In patients with this condition, nNOS protein cannot be detected in the pylorus, and the pyloric muscle is hypertrophied as in the nNOS knockout mice (53, 54). Since the gastric dilation and pyloric dysfunction of the nNOS knockout mice resembles the gastric dysfunction observed in many diabetic patients, we reasoned that the nNOS knockout mice might also represent an animal model of diabetic gastropathy (55–57). In diabetic mice, we found that NO-dependent NANC transmission is lost in the pyloric muscle (52). At the same time, nNOS protein and mRNA are absent from the pyloric neurons, although the nerves themselves remain intact. Insulin treatment restores nNOS protein and mRNA expression in conjunction with a return of NO-mediated NANC pyloric relaxation. These results are consistent with a regulatory effect of insulin or glucose on nNOS expression, perhaps through regulatory sites in the promoter of the nNOS gene (58–60).
Insight into a physiologic role for NO in the brain comes from behavioral studies of nNOS knockout mice. These mice are extraordinarily aggressive, more so than virtually any other form of genetically mutant mice previously described (61). This behavior is dependent on testosterone, as it is abolished by castration, reproduced by testosterone administration, and absent in females (62). nNOS knockout male mice also display abnormal, excessive sexual activity. When a normal male mouse is placed together with a female that is not in estrus (the sexual receptive phase of the estrous cycle), the male will begin to mount the female but will cease when he perceives that the female is not responsive. Male nNOS knockout mice fail to heed such clues and will repeatedly mount the female (61). These findings imply that in males nNOS neurons normally restrain aggressive and sexual behaviors. In contrast, in females, maternal aggression is reduced in nNOS deficient mice (63).
NO may also play a role in learning and memory. Long-term potentiation is a model of synaptic plasticity in which powerful stimulation of a synaptic input to a neuronal system potentiates subsequent synaptic transmission in the system for long periods of time. In intact animals, long-term potentiation can persist for weeks. Chemicals that release NO facilitate long-term potentiation. There has been controversy about possible decreased long-term potentiation in nNOS-deficient mice, perhaps because of compensation by related genes like eNOS. Elegant studies by Kandel and associates show a clear decrease of long-term potentiation in mice with deletion of both eNOS and nNOS (64). Whether eNOS is located in neurons or only blood vessels in the brain remains controversial. Perhaps, NO produced in blood vessels can influence neural transmission. This possibility fits with evidence for behavioral changes in eNOS knockout mice. These mice display a pronounced decrease in aggressive behavior, opposite to the nNOS knockout mice (65). This result may seem surprising, but localizations of eNOS associated with brain vasculature differ markedly from those for nNOS, thus NO formed by the two different enzymes may influence distinct and largely unrelated brain structures.
NO dysfunction in stroke
As medical students, many of us were taught that stroke reflects permanent damage in infarcted tissue where cerebral arteries have been occluded. However, evidence accumulated over the last decade has indicated that a major fraction of the neural damage in stroke is due to oxygen-derived free radicals that are produced after reperfusion of the ischemic area or from hypoxic mitochondria in surviving neurons. We also know that hypoxia in the brain triggers a massive release of excitatory transmitters, especially glutamate. Glutamate levels may reach as high as 50 times normal levels, literally “exciting to death” partially hypoxic cells (66). Evidence for a role of glutamate in stroke includes the ability of glutamate antagonists, especially NMDA receptor antagonists, to reduce stroke damage about 50%–60% (66). Glutamate toxicity associated with stroke can be mimicked in cerebral cortical cultures where NMDA receptor activation can kill up to 90% of neurons, while NMDA receptor antagonists prevent this damage (67). Glutamate neurotoxicity is diminished in cultures from nNOS knockout mice or after treatment with NOS inhibitors (68). Stroke damage is also markedly reduced after treatment with NOS inhibitors (69–73) and in nNOS knockout mice (74).
If NO mediates neurotoxicity in stroke, how exactly does NO kill cells? Although NO is a free radical, it is not a particularly toxic one. When NO combines with superoxide (e.g., from hypoxic mitochondria), it forms peroxynitrite, which degenerates into the extremely toxic hydroxyl radical (OH&*;. Peroxynitrite and OH&*; can damage all major biomolecules, including lipids (through peroxidation of cell membranes leading to calcium entry), proteins (both directly and through calcium-dependent proteases), and DNA. All of these processes may play some role in neurotoxicity, but some evidence suggests that DNA damage is the major mechanism. OH&*; causes DNA strand breaks that activate the enzyme poly (ADP-ribose) polymerase (PARP). PARP is a nuclear enzyme that facilitates the DNA repair process. PARP’s substrate is NAD, which, when activated, transfers 50 to 200 ADP-ribose groups in branched chains to several nuclear proteins, including PARP itself. When NAD is overactivated by massive amounts of DNA damage, NAD levels are depleted, and ATP is also depleted in efforts to resynthesize NAD (75, 76). Accordingly, DNA damage leading to PARP overactivation can kill cells by energy loss and cellular starvation. Evidence that such a mechanism mediates killing of cells by NO comes from studies showing that PARP inhibitors block neuronal death in cultures elicited by NO donors or NMDA receptor activation (77). Even more strikingly, brain cultures from PARP knockout mice are completely protected from such neurotoxicity (78). PARP-mediated neuronal cell death may have clinical relevance. In PARP knockout mice, there is a 80% reduction in stroke damage after reversible occlusion of the middle cerebral artery (78, 79), and postischemic administration of a PARP inhibitor can ameliorate stroke damage (80).
Carbon Monoxide
Neurotransmitters are grouped by chemical classes, such as the biogenic amines, amino acids, and neuropeptides. Thus, we wondered whether gaseous transmitters besides NO might exist. Carbon monoxide (CO) is formed physiologically by heme oxygenase (HO). HO cleaves the porphyrin ring of heme to form biliverdin, which is rapidly reduced by biliverdin reductase to bilirubin (Figure 3). In the process, ferrous iron is released, and a one-carbon fragment is released as CO. Might CO be the second member of a new group of neurotransmitters, the gases?
HO was first identified as an inducible enzyme activated by many cellular stresses. This inducible form of HO was found to be identical to a heat shock protein and is now designated HO1. HO1 is most concentrated in the spleen, the repository of aged red blood cells. In the spleen, HO degrades heme from red blood cell hemoglobin which otherwise would be quite toxic. In other cells, HO destroys heme from mitochondrial and other enzymes. In the course of purifying HO1, Maines and associates (85, 86) discovered a second HO protein, which they designated HO2. HO2 is not inducible and is most concentrated in the brain and testes. We reasoned that if CO were to be a neurotransmitter, its biosynthetic enzyme should be discretely localized to certain neurons. In situ hybridization studies revealed that HO2 mRNA is highly localized to specific neurons in the brain, with localizations similar to those of soluble guanylyl cyclase (87). Like NO, CO can activate this enzyme, triggering cyclic GMP formation (Figure 3). In cultures of olfactory neurons, which have very high concentrations of HO2 and lack nNOS, cyclic GMP levels are depleted by HO inhibitors (88, 89).
As in the case of NO, direct evidence for CO as a neurotransmitter emerged from studies of the myenteric plexus of the intestine. Immunohistochemical staining revealed significant colocalization of HO2 and nNOS in myenteric neuronal cells, suggesting that CO and NO might be cotransmitters (48, 90). As discussed above, NANC transmission is reduced by half in intestinal preparations from nNOS knockout mice. In similar experiments, we used mice with targeted genomic deletion of HO2 (HO2 knockout mice) and found that NANC transmission was also reduced 50% in intestine in those mice (48). Recently, we have extended these findings by demonstrating depolarization of the resting membrane potential in jejunal smooth muscle cells from HO2 knockout mice (49). Intestinal preparations from these mice also have decreased NANC inhibitory junctional potentials, measured electrophysiologically. In mice deficient in both nNOS and HO2, we observed additive effects on the resting membrane potential and the inhibitory junctional potentials (49). Application of exogenous CO mimics NANC neurotransmission. Thus, CO, like NO, appears to be an inhibitory NANC neurotransmitter.
HO2 is also concentrated in nerves that innervate the vas deferens, a smooth muscle whose contractions underlie ejaculation. Reflex activity of the bulbospongiosus muscle that underlies ejaculation is profoundly reduced in preparations from HO2 knockout mice, and the mice display a marked reduction in ejaculatory function (91). Bulbospongiosus nerves do not contain nNOS. Conversely, the NO nerves mediating penile erection do not contain HO2. Although NO is critical for penile erections, CO appears to be the neurotransmitter of nerves mediating ejaculation. Thus, in the myenteric plexus, NO and CO may be cotransmitters, but in other peripheral autonomic nerves, they have distinct localizations and functions.
As described above, CO appears to physiologically regulate cyclic GMP in olfactory neurons. However, its functional link to cyclic GMP in other parts of the brain is not clear despite the apparent colocalization of HO2 and soluble guanylate cyclase. Although some relatively nonspecific inhibitors of HO diminish long-term potentiation, no major deficit in long-term potentiation is evident in HO2 knockout mice (92–94). Thus, a transmitter role for CO in the brain remains to be rigorously established. Even signaling through cyclic GMP (cGMP) in the brain is not established, as whole brain levels of cGMP are not reduced in HO2 knockout mice (R. Zakhary and S.H. Snyder, unpublished 1999 data). Although the knockout animals are useful research tools, drugs that selectively perturb transmitter actions are comparably important. Whereas potent, selective inhibitors of NOS exist, the existing HO inhibitors, mostly protoporphyrin structures, are only modestly selective for HO.
In contrast to the current paucity of evidence regarding a role for CO in normal brain function, other products of HO enzymatic activity do display physiologic roles. Ames and co-workers identified an antioxidant function for bilirubin many years ago (95, 96). Recently, we showed a neuroprotectant role for bilirubin (82). This work stemmed from our efforts to identify a means for activating HO2 analogous to the CaM activation of nNOS. Phorbol esters, which stimulate protein kinase C, protect cortical neurons from death after treatment with hydrogen peroxide. The neuroprotection of phorbol esters is greatly reduced by inhibitors of HO and is abolished in neuronal cultures from HO2 knockout mice. Bilirubin, in very low concentrations, mimics the neuroprotective effects of phorbol esters. In findings consistent with a neuroprotective role for bilirubin, stroke damage was substantially worsened in HO2 knockout mice (83). Sometimes, mice with a specific genetic deficiency are unhealthy and therefore susceptible to injury or illness in general. HO1 knockout mice are generally debilitated, with weight loss, anemia, and signs of chronic inflammation developing after 20 weeks of age. However, HO1 knockout mice do not display accentuated stroke damage. In contrast, HO2 knockout mice appear normal and healthy, though they suffer enlarged strokes in ischemic models.
The neuroprotective effects of bilirubin are surprising considering the well-known neurotoxicity of bilirubin in jaundiced babies with very high blood levels of bilirubin. Kernicterus requires high micromolar levels of bilirubin (97), a thousand times the concentrations of bilirubin that are neuroprotective in vitro. There is interesting evidence that the moderately “elevated” plasma levels of bilirubin in most normal babies may be protective, as babies with moderately elevated bilirubin levels are less susceptible to oxygen-radical-mediated injury (98).
Iron, the third product of HO, is highly toxic through the Fenton reaction, which produces OH&*; (99). Accordingly, there ought to exist some mechanism to stimulate the efflux from the cell of iron formed by HO. HO activity appears to be linked to cellular iron efflux. Thus, transfection of HO1 into mammalian cells stimulates iron efflux, and iron efflux is greatly diminished in fibroblasts from HO1 knockout mice (84). These findings fit with evidence of low serum iron and accumulation of iron in the tissues of HO1 knockout mice (100, 101). Whether HO2 similarly regulates iron efflux is not yet established, although HO2 knockout mice develop iron accumulation in their lungs in response to hyperoxia (102).
Other gases might also function as neurotransmitters. Hydrogen sulfide (H2S) exerts specific effects on the electrical properties of serotonin neurons in the dorsal raphe nucleus (103). Abe and Kimura (104) demonstrated that cystathionine beta-synthase produces H2S in the brain. In addition, exogenous H2S enhances NMDA neurotransmission and facilitates the induction of long-term potentiation in the hippocampus at concentrations comparable to those that occur physiologically (104). Cystathionine beta-synthase is also expressed in smooth muscle and where physiologically relevant concentrations of H2S enhance NO-mediated muscle relaxation (105).
d-Amino Acids: Focus on d-Serine
Organic molecules, and therefore biological molecules, are based on the chemistry of the carbon atom. Carbon atoms can have up to four bonded groups attached to them in three-dimensional space, forming a tetrahedron. Because of this structure, carbon-containing molecules can have the same four constituents, yet differ in their structure by the location of the four groups in space. This property of carbon-based molecules is known as chirality. As an example, one’s hands are mirror images of each other, but they cannot be superimposed on each other. Similarly, carbon atoms with four different groups attached occur in two forms that are nonsuperimposable mirror images of each other, known as enantiomers. The elegant precision of biological reactions requires the recognition sites in proteins to be exceedingly specific. Just as a right-handed glove will not fit a left hand, enzymes and receptors can discriminate between the enantiomers of ligands and substrates.
As biology students, we were taught that organisms exclusively employ the d-enantiomers for sugars, while amino acids for proteins are always in the l-form. d-Amino acids had been observed in bacteria and invertebrates (106), but a role for d-amino acids in higher species was deemed unlikely. However, observations in the 1990s have pointed to the existence of substantial quantities of some d-amino acids in higher species, including humans (107–111). In the brain, levels of d-serine are up to a third those of l-serine, and, in a variety of tissues including the brain and certain glands, d-aspartate levels are 20%–30% those of l-aspartate (107).
The existence of significant amounts of d-serine and d-aspartate in the brain suggested that some d-amino acids serve specific neuroactive roles. Levels of d-serine have marked variations in different regions of the brain, with highest concentrations in the forebrain, where NMDA-type glutamate receptors are enriched (107). This finding meshed with certain known features of the NMDA receptor. For instance, Ascher and others had observed that the loss of NMDA receptor activation after rapid perfusion of neural preparations can be reversed by glycine (112). Subsequent studies confirmed the existence of a glycine recognition site on the NMDA receptor and established that this receptor requires coactivation by glycine as well as glutamate (113). These conclusions were somewhat puzzling, as glycine concentrations in the CNS are lowest in forebrain, areas that are enriched in NMDA receptors, and highest in the spinal cord and hindbrain, where glycine is known to function as an inhibitory neurotransmitter. We suspected that d-serine might act as an endogenous ligand for the glycine site of the NMDA receptor, because numerous studies had established that d-serine was at least as potent as glycine.
Based on these hints, we developed antisera to d-serine and mapped its localization in the CNS. d-serine is indeed highly concentrated in areas of the brain enriched in NMDA receptors, where glycine levels are lowest (114). In contrast, in some parts of the brain, such as the adult cerebellum and the accessory olfactory bulb, glycine, rather than d-serine, may be associated with NMDA receptors (115). Most striking was our observation that d-serine occurs in glia, not neurons. d-Serine is exclusively localized to protoplasmic astrocytes that are enriched in gray matter together with NMDA receptors (Figure 4). One reason that neurotransmitters have been presumed to derive exclusively from neurons is that only neurons were thought to release transmitters after appropriate excitation. In cultures of protoplasmic type II astrocytes, activation of non-NMDA glutamate receptors stimulates release of d-serine (114). In the brain, these astrocytes ensheathe the synapse so that any d-serine released from astrocytes would have close proximity to NMDA receptors. Thus, we have proposed that synaptic release of glutamate from a presynaptic neuron triggers the release of d-serine from adjacent astrocytes to coactivate the NMDA receptors on nearby postsynaptic neurons (Figure 4).
The idea that d-serine possesses a physiologic role in the brain helped clarify a biochemical oddity first noticed in the 1930s. At that time, Hans Krebs (118) discovered an enzyme that selectively deaminates d-amino acids and designated it “d-amino acid oxidase” (DAAOX). Because d-amino acids were unknown in mammalian tissues, it was assumed that the enzyme was an evolutionary vestige from bacteria, that it was a mistake of nature, or that it served to oxidize glycine that is not chiral and therefore does not have enantiomeric forms. Our histochemical investigations showed marked regional variations in DAAOX localization, with concentrations exactly reciprocal to those of d-serine (114). These data suggest that DAAOX degrades d-serine physiologically.
How might one definitively determine whether d-serine is an endogenous modulator of NMDA receptor function? We utilized DAAOX as a tool, first establishing that d-serine is the only substance in brain preparations degraded by DAAOX (119). Then we showed that DAAOX treatment markedly reduces NMDA neurotransmission in cerebellar preparations by monitoring both NOS activity and cyclic GMP levels as well as by conducting electrophysiologic studies in slice and culture preparations of the cerebellum and the hippocampus (119, 120)
How might the brain synthesize d-serine? After developing a specific assay to monitor d-serine levels, we isolated an enzyme from mammalian brain that converts l-serine to d-serine, designated it serine racemase (116), and then cloned its complementary DNA (117). Serine racemase is localized to the d-serine-containing protoplasmic astrocytes in areas of the brain enriched in NMDA receptors (117). It is a novel protein, although it possesses some modest sequence similarity to other enzymes that use serine as a substrate. These enzymes, like serine racemase, require pyridoxal phosphate (vitamin B6) as a cofactor (116, 117).
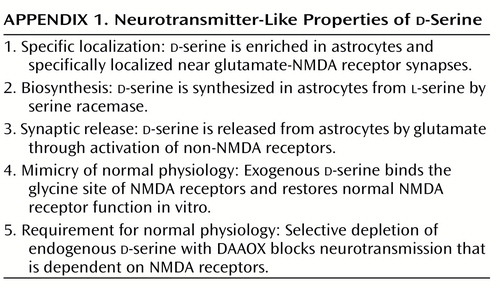
d-Serine challenges various dogma about neurotransmitters, perhaps to a greater extent even than NO and CO. The idea of a function for d-amino acids in mammals, especially as a neurotransmitter, goes against long-standing biochemical and physiological teachings. Does d-serine satisfy criteria for a neurotransmitter, shown in Appendix 1? It is localized to the sites of its receptors and possesses a dedicated biosynthetic enzyme. d-Serine is released on appropriate stimulation and mimics the actions of the physiologic transmitter. Thus, in many ways, d-serine fulfills more criteria than many neuropeptides that are well accepted as transmitters. However, the concept of a neurotransmitter arising from glia may discomfort some neuroscientists. Glia do not have synaptic vesicles, so d-serine release cannot occur through classic exocytosis. Instead, its release may result from reversing the directionality of an amino acid transporter that might otherwise remove substances from the synapse. Thus, as for NO and CO, aberrant release processes would confound those who insist that neurotransmitters adhere to traditional criteria.
The cloning of serine racemase provides a potentially powerful approach to learning about d-serine in the brain and to exploring therapeutic ramifications. Future studies will allow monitoring of NMDA neurotransmission, long-term potentiation, and overall behavior in serine racemase gene knockout mice. Inhibitors of serine racemase would be expected to diminish NMDA neurotransmission, and so, like NMDA receptor antagonists, serine racemase inhibitors might be beneficial in treating stroke and other conditions associated with excess excitation. Glutamate neurotoxicity may be relevant for the therapy of neurodegenerative diseases, whether or not excitotoxicity is directly involved in pathophysiology. We don’t know the precise etiology of conditions such as Parkinson’s disease and Alzheimer’s disease. However, regardless of the initiating event, neurons do degenerate in patients with these disorders. Because glutamate release is augmented in the presence of hypoxic neural damage, it is likely that excessive levels of glutamate are released as the disease progresses and that glutamate may work together with other insults to permit cell death. Concievably, diminishing glutamate receptor activation may block the progression of neurodegenerative conditions such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). Although reliable animal models of Alzheimer’s disease and Huntington’s disease have been difficult to establish, there are model systems for ALS and Parkinson’s disease. Glutamate receptor antagonists are therapeutic in these models, and thus serine racemase inhibitors merit examination as well.
d-Serine and NMDA transmission may be relevant to schizophrenia. The psychotic state after administration of NMDA antagonists such as phencyclidine (PCP) closely resembles certain features of schizophrenia, more than most drug psychoses. Whereas psychoses elicited by drugs such as amphetamines and cocaine manifest positive schizophrenic symptoms, psychoses associated with PCP elicit negative as well as positive symptoms (121). Besides causing the negative syndrome of emotional withdrawal, PCP elicits cognitive dysfunction characteristic of schizophrenia. Evidence for a relationship between glutamatergic and dopamine models of schizophrenia comes from studies showing altered dopamine release after PCP administration (122, 123).
According to the NMDA receptor model of schizophrenia, one would expect glutamate agonists to be therapeutic. Since glutamate itself is potentially toxic and too rapidly metabolized, researchers have administered glycine, d-serine, or cycloserine (which mimics d-serine at NMDA receptors) to assess their potential efficacy in patients with schizophrenia. Beneficial effects have been reported (124, 125).
d-Serine may not be the only d-amino acid neurotransmitter candidate. d-Aspartate was first discovered in invertebrates (126, 127). Several groups identified d-aspartate in mammalian tissues with notable concentrations in endocrine glands and the brain (128–131). D’Aniello and associates (132, 133) observed that d-aspartate is released from the testes and may stimulate testosterone synthesis, and some studies have found changes in d-aspartate levels in the brains of patients with Alzheimer’s disease (134, 135). With antisera to d-aspartate, we localized the amino acid to selected neuronal populations in the brain as well as to epinephrine-containing cells in the adrenal medulla, to the vasopressin-releasing hypothalamic neurons innervating the posterior pituitary gland, and to pinealocytes in the pineal gland (136). Although d-aspartate can activate NMDA receptors, the localizations of d-aspartate do not match those of NMDA receptors, and d-aspartate levels are far lower than glutamate levels. Thus, at present, too little is known about d-aspartate to draw any firm conclusions about its function in endocrine glands or the brain.
Conclusions
This essay has focused on NO, CO and d-serine as recently identified candidate neurotransmitters. A historical review reveals that every neurotransmitter candidate after acetylcholine has altered one or more preconceptions about the defining characteristics of a neurotransmitter. With so much controversy, one may be tempted to discard the term. Alternatively, bearing in mind these historical lessons, we might try adopting a reasonably liberal conceptualization of a neurotransmitter, such as the following: A transmitter is a molecule, released by neurons or glia, that physiologically influences the eletrochemical state of adjacent cells. Outside the CNS, those adjacent target cells need not be neurons and, in most instances, would be smooth muscle or glandular cells. In considering the CNS, researchers usually think of neurotransmitters as influencing adjacent neurons, but one need not exclude influences on glia or blood vessels. Advocating changes in classic definitions of neurotransmitters may seem heretical. However, previous assumptions about neurotransmission have been challenged repeatedly over the past 50 years. In all of these instances, we have benefited through new insights with important therapeutic implications for neuropsychiatry.
Received Dec. 7, 1999; revision received June 16, 2000; accepted June 30, 2000. From the Departments of Neuroscience, Pharmacology and Molecular Sciences, and Psychiatry and Behavioral Sciences, and Department of Medicine, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore. Address reprint requests to Dr. Snyder, Department of Neuroscience, Johns Hopkins University School of Medicine, 725 North Wolfe Street, Baltimore, MD 21205-2185; [email protected] (e-mail).Supported by grants from NIMH (MH-18501) and the National Institute on Drug Abuse (DA-00266), Research Scientist Award DA-00074 to Dr. Snyder, and a Howard Hughes Fellowship for Physicians to Dr. Ferris.The authors thank David Linden and Bertil Hille for helpful discussions.
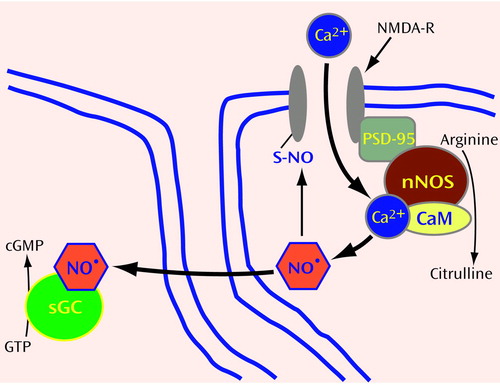
Figure 1. Production and Activity of the Nitric Oxide Radical (NO•) in Neuronsa
aNO• is generated from arginine after glutamate activation of NMDA receptors and calcium influx. In the brain, NO• production is linked to glutamate receptor activation (see text). The binding of glutamate to NMDA-type glutamate receptors (NMDA-R) results in the opening of an ion channel that allows calcium (Ca2+) to enter the neuron. Neuronal NO synthase (nNOS) is physically linked to NMDA-R through postsynaptic density protein-95 (PSD-95). After nNOS is activated by Ca2+-calmodulin (CaM), arginine is converted to NO• and citrulline. NO•, in turn, regulates NMDA-R function through direct modification of the sulfur (S) in cysteine contained within a subunit of NMDA-R. Covalent modification of S in cysteine by NO&*; is known as S-nitrosylation. Like phosphorylation, S-nitrosylation may represent a general mechanism of posttranslational modification of proteins. In addition, NO• functions as a neurotransmitter by diffusing through the membranes of postsynaptic cells, where it binds the heme in soluble guanylate cyclase (sGC), activating this enzyme to convert GTP into the second messenger cGMP.
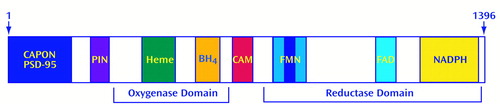
Figure 2. Structure and Activity of Neuronal Nitric Oxide Synthase (nNOS)a
anNOS contains oxygenase and reductase domains and has binding sites for multiple regulatory molecules. nNOS has 1,396 amino acids. The N-terminal region contains binding sites for interacting proteins such as PSD-95, carboxyl-terminal PDZ ligand of NOS, and protein inhibitor of NOS, as described in the text. The oxygenase domain binds heme and tetrohydrobiopterin (BH4). A calmodulin-binding site (CAM) links the oxygenase domain to the reductase domain in the C-terminal half of nNOS. The oxygenase domain displays homology to cytochrome P450 reductase and contains binding sites for flavin adenine mononucleotide (FMN), flavin adenine dinucleotide (FAD), and NADPH. nNOS contains a 42-amino-acid insert (represented by the stripe in the center of the FMN domain) that confers sensitivity to calmodulin (21). This insert is absent in inducible NOS (iNOS), which is not dependent on calmodulin.
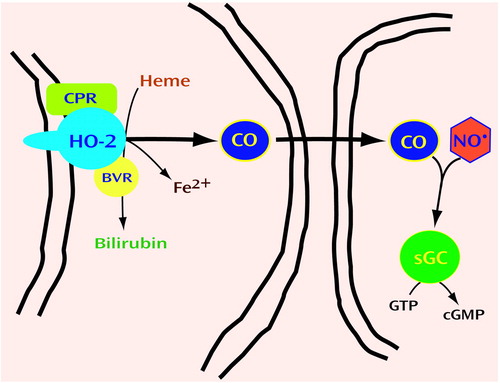
Figure 3. Production and Activity of Carbon Monoxide (CO) in Neuronsa
aHeme oxygenase-2 (HO2), together with cytochrome P450 reductase (CPR) and biliverdin reductase (BVR), converts heme into bilirubin, ferrous iron (Fe2+), and carbon monoxide (CO). HO2 is enriched in neurons, and its activity results in the production of CO that functions similarly to NO. Thus, CO diffuses through membranes to target cells, where it can bind the heme of soluble guanylate cyclase (sGC) to regulate production of cGMP from GTP. CO may modulate the effect of NO by competing for binding to the heme in sGC (81). The colocalization of nNOS and HO2 in myenteric neurons suggests that NO and CO function as coneurotransmitters (48, 49). Bilirubin production may be neuroprotective (82, 83). Fe2+ released from heme is extruded from cells to prevent cell death (84).
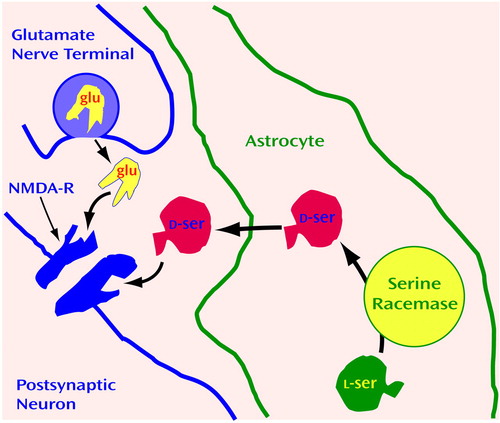
Figure 4. Activity of d-Serine in Glutamatergic Neurotransmissiona
ad-Serine is snythesized in astrocytes and released near NMDA receptors (NMDA-Rs), where it regulates glutamatergic neurotransmission. NMDA-R requires ligand binding at two sites to open an ion channel. Thus, glutamate (glu) binds to NMDA-R only if the NMDA-R glycine site is occupied by the agonist. d-Serine is synthesized from l-serine in astrocytes by serine racemase (116, 117), is released by glutamate, and binds to the glycine site of the NMDA-R.
 |
1. Hillarp NA, Fuxe K, Dahlstrom A: Demonstration and mapping of central neurons containing dopamine, noradrenaline, and 5-hydroxytryptamine and their reactions to psychopharmaca. Pharmacol Rev 1966; 18:727–741Medline, Google Scholar
2. Axelrod J: Noradrenaline: fate and control of its biosynthesis. Science 1971; 173:598–606Crossref, Medline, Google Scholar
3. Hornykiewicz O: Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev 1966; 18:925–964Medline, Google Scholar
4. Amara SG, Kuhar MJ: Neurotransmitter transporters: recent progress. Annu Rev Neurosci 1993; 16:73–93Crossref, Medline, Google Scholar
5. Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M: Histaminergic transmission in the mammalian brain. Physiol Rev 1991; 71:1–51Crossref, Medline, Google Scholar
6. Snyder SH: Adenosine as a neuromodulator. Annu Rev Neurosci 1985; 8:103–124Crossref, Medline, Google Scholar
7. Hokfelt T: Neuropeptides in perspective: the last ten years. Neuron 1991; 7:867–879Crossref, Medline, Google Scholar
8. Chang MM, Leeman SE: Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 1970; 245:4784–4790Google Scholar
9. Snyder SH: Opiate receptor in normal and drug altered brain function. Nature 1975; 257:185–189Crossref, Google Scholar
10. Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR: Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 1975; 258:577–580Crossref, Medline, Google Scholar
11. Hokfelt T, Johansson O, Goldstein M: Chemical anatomy of the brain. Science 1984; 225:1326–1334Google Scholar
12. Krieger DT: Brain peptides: what, where, and why? Science 1983; 222:975–985Google Scholar
13. Furchgott RF, Zawadzki JV: The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288:373–376Crossref, Medline, Google Scholar
14. Palmer RM, Ferrige AG, Moncada S: Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327:524–526Crossref, Medline, Google Scholar
15. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G: Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 1987; 84:9265–9269Google Scholar
16. Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA: Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther 1981; 218:739–749Medline, Google Scholar
17. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS: Macrophage oxidation of l-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 1988; 27:8706–8711Google Scholar
18. Hibbs JB Jr, Vavrin Z, Taintor RR: l-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol 1987; 138:550–565Medline, Google Scholar
19. Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF: Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med 1989; 169:1011–1020Google Scholar
20. Garthwaite J, Charles SL, Chess-Williams R: Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988; 336:385–388Crossref, Medline, Google Scholar
21. Daff S, Sagami I, Shimizu T: The 42-amino acid insert in the FMN domain of neuronal nitric-oxide synthase exerts control over Ca(2+)/calmodulin-dependent electron transfer. J Biol Chem 1999; 274:30589–30595Google Scholar
22. Bredt DS, Snyder SH: Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A 1989; 86:9030–9033Google Scholar
23. Garthwaite J, Garthwaite G, Palmer RM, Moncada S: NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol 1989; 172:413–416Crossref, Medline, Google Scholar
24. Bredt DS, Snyder SH: Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 1990; 87:682–685Crossref, Medline, Google Scholar
25. Bredt DS, Hwang PM, Snyder SH: Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990; 347:768–770Crossref, Medline, Google Scholar
26. Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH: Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 1991; 7:615–624Crossref, Medline, Google Scholar
27. Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH: Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 1991; 351:714–718Crossref, Medline, Google Scholar
28. Bredt DS, Ferris CD, Snyder SH: Nitric oxide synthase regulatory sites: phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem 1992; 267:10976–10981Google Scholar
29. Brune B, Lapetina EG: Phosphorylation of nitric oxide synthase by protein kinase A. Biochem Biophys Res Commun 1991; 181:921–926Crossref, Medline, Google Scholar
30. Nakane M, Mitchell J, Forstermann U, Murad F: Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun 1991; 180:1396–1402Google Scholar
31. Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB: The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol 1999; 12:845–848Google Scholar
32. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM: Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999; 399:601–605Crossref, Medline, Google Scholar
33. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC: Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999; 399:597–601Crossref, Medline, Google Scholar
34. Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS: Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996; 84:757–767Crossref, Medline, Google Scholar
35. Jaffrey SR, Snyder SH: PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 1996; 274:774–777Crossref, Medline, Google Scholar
36. Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH: CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 1998; 20:115–124Crossref, Medline, Google Scholar
37. Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D’Angelo DD, Lynch KR, Peach MJ: Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem 1992; 267:15274–15276Google Scholar
38. Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD: Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem 1992; 267:14519–14522Google Scholar
39. Lamas S, Marsden PA, Li GK, Tempst P, Michel T: Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A 1992; 89:6348–6352Google Scholar
40. Lyons CR, Orloff GJ, Cunningham JM: Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem 1992; 267:6370–6374Google Scholar
41. Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C: Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992; 256:225–228Crossref, Medline, Google Scholar
42. Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH: Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A 1992; 89:6711–6715Google Scholar
43. Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG: Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 1990; 345:346–347Crossref, Medline, Google Scholar
44. Stark ME, Szurszewski JH: Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology 1992; 103:1928–1949Google Scholar
45. Stark ME, Bauer AJ, Szurszewski JH: Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol (Lond) 1991; 444:743–761Crossref, Google Scholar
46. Boeckxstaens GE, Pelckmans PA, Bult H, De Man JG, Herman AG, Van Maercke YM: Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol 1990; 190:239–246Crossref, Medline, Google Scholar
47. Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC: Targeted disruption of the neuronal nitric oxide synthase gene. Cell 1993; 75:1273–1286Google Scholar
48. Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH: Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci U S A 1997; 94:14848–14853Google Scholar
49. Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH: Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci U S A 2000; 97:1851–1855Google Scholar
50. Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH: Nitric oxide: a physiologic mediator of penile erection. Science 1992; 257:401–403Crossref, Medline, Google Scholar
51. Corbin JD, Francis SH: Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem 1999; 274:13729–13732Google Scholar
52. Watkins C, Blackshaw S, Sawa A, Snyder S, Ferris C: Diabetic gastropathy in mice reflects loss of neuronal nitric oxide synthase that is restored by insulin. J Clin Invest 2000; 106:373–384Crossref, Medline, Google Scholar
53. Chung E, Curtis D, Chen G, Marsden PA, Twells R, Xu W, Gardiner M: Genetic evidence for the neuronal nitric oxide synthase gene (NOS1) as a susceptibility locus for infantile pyloric stenosis. Am J Hum Genet 1996; 58:363–370Medline, Google Scholar
54. Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ, De Laet MH: Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. N Engl J Med 1992; 327:511–515; correction 1992; 327:1252Google Scholar
55. Koch KL: Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci 1999; 44:1061–1075Google Scholar
56. Kassander P: Asymptomatic gastric retention in diabetes (gastroparesis diabeticorum). Ann Intern Med 1958; 48:797–812Crossref, Medline, Google Scholar
57. Mearin F, Camilleri M, Malagelada JR: Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology 1986; 90:1919–1925Google Scholar
58. Wang Y, Newton DC, Robb GB, Kau CL, Miller TL, Cheung AH, Hall AV, VanDamme S, Wilcox JN, Marsden PA: RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. Proc Natl Acad Sci U S A 1999; 96:12150–12155Google Scholar
59. Wang Y, Marsden PA: Nitric oxide synthases: gene structure and regulation. Adv Pharmacol 1995; 34:71–90Crossref, Medline, Google Scholar
60. Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA: Structural organization of the human neuronal nitric oxide synthase gene (NOS1). J Biol Chem 1994; 269:33082–33090Google Scholar
61. Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH: Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 1995; 378:383–386Crossref, Medline, Google Scholar
62. Kriegsfeld LJ, Dawson TM, Dawson VL, Nelson RJ, Snyder SH: Aggressive behavior in male mice lacking the gene for neuronal nitric oxide synthase requires testosterone. Brain Res 1997; 769:66–70Crossref, Medline, Google Scholar
63. Gammie SC, Nelson RJ: Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci 1999; 19:8027–8035Google Scholar
64. Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, Kandel ER: Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell 1996; 87:1015–1023Google Scholar
65. Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH: Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci 1999; 19:RC30Google Scholar
66. Choi DW: Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988; 1:623–634Crossref, Medline, Google Scholar
67. Meldrum B, Garthwaite J: Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 1990; 11:379–387Crossref, Medline, Google Scholar
68. Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM: Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 1996; 16:2479–2487Google Scholar
69. Nowicki JP, Duval D, Poignet H, Scatton B: Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur J Pharmacol 1991; 204:339–340Crossref, Medline, Google Scholar
70. Nagafuji T, Matsui T, Koide T, Asano T: Blockade of nitric oxide formation by N omega-nitro-l-arginine mitigates ischemic brain edema and subsequent cerebral infarction in rats. Neurosci Lett 1992; 147:159–162Crossref, Medline, Google Scholar
71. Buisson A, Plotkine M, Boulu RG: The neuroprotective effect of a nitric oxide inhibitor in a rat model of focal cerebral ischaemia. Br J Pharmacol 1992; 106:766–767Crossref, Medline, Google Scholar
72. Trifiletti RR: Neuroprotective effects of NG-nitro-l-arginine in focal stroke in the 7-day old rat. Eur J Pharmacol 1992; 218:197–198Crossref, Medline, Google Scholar
73. Nishikawa T, Kirsch JR, Koehler RC, Bredt DS, Snyder SH, Traystman RJ: Effect of nitric oxide synthase inhibition on cerebral blood flow and injury volume during focal ischemia in cats. Stroke 1993; 24:1717–1724Google Scholar
74. Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA: Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994; 265:1883–1885Google Scholar
75. Pieper AA, Verma A, Zhang J, Snyder SH: Poly(ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci 1999; 20:171–181Crossref, Medline, Google Scholar
76. Berger NA: Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res 1985; 101:4–15Crossref, Medline, Google Scholar
77. Zhang J, Dawson VL, Dawson TM, Snyder SH: Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 1994; 263:687–689Crossref, Medline, Google Scholar
78. Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL: Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 1997; 3:1089–1095Google Scholar
79. Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA: Ischemic brain injury is mediated by the activation of poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab 1997; 17:1143–1151Google Scholar
80. Takahashi K, Pieper AA, Croul SE, Zhang J, Snyder SH, Greenberg JH: Post-treatment with an inhibitor of poly(ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res 1999; 829:46–54Crossref, Medline, Google Scholar
81. Ingi T, Cheng J, Ronnett GV: Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron 1996; 16:835–842Crossref, Medline, Google Scholar
82. Dore S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH: Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A 1999; 96:2445–2450Google Scholar
83. Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH: Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med 1999; 5:656–663Crossref, Medline, Google Scholar
84. Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH: Heme oxygenase-1 prevents cell death by regulating cellular iron. Nature Cell Biology 1999; 1:152–157Crossref, Medline, Google Scholar
85. Trakshel GM, Kutty RK, Maines MD: Purification and characterization of the major constitutive form of testicular heme oxygenase: the noninducible isoform. J Biol Chem 1986; 261:11131–11137Google Scholar
86. Maines MD, Trakshel GM, Kutty RK: Characterization of two constitutive forms of rat liver microsomal heme oxygenase: only one molecular species of the enzyme is inducible. J Biol Chem 1986; 261:411–419Medline, Google Scholar
87. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH: Carbon monoxide: a putative neural messenger. Science 1993; 259:381–384Crossref, Medline, Google Scholar
88. Ingi T, Ronnett GV: Direct demonstration of a physiological role for carbon monoxide in olfactory receptor neurons. J Neurosci 1995; 15:8214–8222Google Scholar
89. Ingi T, Chiang G, Ronnett GV: The regulation of heme turnover and carbon monoxide biosynthesis in cultured primary rat olfactory receptor neurons. J Neurosci 1996; 16:5621–5628Google Scholar
90. Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH: Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U S A 1996; 93:795–798Crossref, Medline, Google Scholar
91. Burnett AL, Johns DG, Kriegsfeld LJ, Klein SL, Calvin DC, Demas GE, Schramm LP, Tonegawa S, Nelson RJ, Snyder SH, Poss KD: Ejaculatory abnormalities in mice with targeted disruption of the gene for heme oxygenase-2. Nat Med 1998; 4:84–87Crossref, Medline, Google Scholar
92. Shinomura T, Nakao S, Mori K: Reduction of depolarization-induced glutamate release by heme oxygenase inhibitor: possible role of carbon monoxide in synaptic transmission. Neurosci Lett 1994; 166:131–134Crossref, Medline, Google Scholar
93. Meffert MK, Haley JE, Schuman EM, Schulman H, Madison DV: Inhibition of hippocampal heme oxygenase, nitric oxide synthase, and long-term potentiation by metalloporphyrins. Neuron 1994; 13:1225–1233Google Scholar
94. Poss KD, Thomas MJ, Ebralidze AK, O’Dell TJ, Tonegawa S: Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron 1995; 15:867–873Crossref, Medline, Google Scholar
95. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN: Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235:1043–1046Google Scholar
96. Stocker R, Glazer AN, Ames BN: Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A 1987; 84:5918–5922Google Scholar
97. Gourley GR: Bilirubin metabolism and kernicterus. Adv Pediatr 1997; 44:173–229Medline, Google Scholar
98. Belanger S, Lavoie JC, Chessex P: Influence of bilirubin on the antioxidant capacity of plasma in newborn infants. Biol Neonate 1997; 71:233–238Crossref, Medline, Google Scholar
99. Meneghini R: Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med 1997; 23:783–792Crossref, Medline, Google Scholar
100. Poss KD, Tonegawa S: Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A 1997; 94:10925–10930Google Scholar
101. Poss KD, Tonegawa S: Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A 1997; 94:10919–10924Google Scholar
102. Dennery PA, Spitz DR, Yang G, Tatarov A, Lee CS, Shegog ML, Poss KD: Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J Clin Invest 1998; 101:1001–1011Google Scholar
103. Kombian SB, Reiffenstein RJ, Colmers WF: The actions of hydrogen sulfide on dorsal raphe serotonergic neurons in vitro. J Neurophysiol 1993; 70:81–96Crossref, Medline, Google Scholar
104. Abe K, Kimura H: The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996; 16:1066–1071Google Scholar
105. Hosoki R, Matsuki N, Kimura H: The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997; 237:527–531Crossref, Medline, Google Scholar
106. Corrigan JJ: d-amino acids in animals. Science 1969; 164:142–149Crossref, Medline, Google Scholar
107. Hashimoto A, Oka T: Free d-aspartate and d-serine in the mammalian brain and periphery. Prog Neurobiol 1997; 52:325–353Crossref, Medline, Google Scholar
108. Hashimoto A, Nishikawa T, Oka T, Takahashi K: Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem 1993; 60:783–786Crossref, Medline, Google Scholar
109. Hashimoto A, Kumashiro S, Nishikawa T, Oka T, Takahashi K, Mito T, Takashima S, Doi N, Mizutani Y, Yamazaki T, Kaneko T, Ootomo E: Embryonic development and postnatal changes in free d-aspartate and d-serine in the human prefrontal cortex. J Neurochem 1993; 61:348–351Crossref, Medline, Google Scholar
110. Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K: Free d-serine, d-aspartate and d-alanine in central nervous system and serum in mutant mice lacking d-amino acid oxidase. Neurosci Lett 1993; 152:33–36Crossref, Medline, Google Scholar
111. Chouinard ML, Gaitan D, Wood PL: Presence of the N-methyl-d-aspartate-associated glycine receptor agonist, d-serine, in human temporal cortex: comparison of normal, Parkinson, and Alzheimer tissues. J Neurochem 1993; 61:1561–1564Google Scholar
112. Johnson JW, Ascher P: Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987; 325:529–531Crossref, Medline, Google Scholar
113. Kleckner NW, Dingledine R: Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988; 241:835–837Crossref, Medline, Google Scholar
114. Schell MJ, Molliver ME, Snyder SH: d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A 1995; 92:3948–3952Google Scholar
115. Schell MJ, Brady RO Jr, Molliver ME, Snyder SH: d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 1997; 17:1604–1615Google Scholar
116. Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO Jr, Ferris CD, Snyder SH: Purification of serine racemase: biosynthesis of the neuromodulator d-serine. Proc Natl Acad Sci U S A 1999; 96:721–725Crossref, Medline, Google Scholar
117. Wolosker H, Blackshaw S, Snyder SH: Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci U S A 1999; 96:13409–13414Google Scholar
118. Krebs HA: CXCVII. Metabolism of amino acids, III: deamination of amino acids. Biochem J 1935; 29:1620–1644Google Scholar
119. Mothet JP, Parent AT, Wolosker H, Brady RO Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH: d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci U S A 2000; 97:4926–4931Google Scholar
120. Parent A, Mothet JP, Snyder SH, Rogawski MA: d-serine is an endogenous ligand for the glycine site of the NMDA receptor: electrophysiological characterization in cultured hippocampal neurons (abstract). Soc for Neurosci Abs 1999; 25:1714Google Scholar
121. Javitt DC, Zukin SR: Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301–1308Google Scholar
122. Jentsch JD, Roth RH: The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999; 20:201–225Crossref, Medline, Google Scholar
123. Olney JW, Newcomer JW, Farber NB: NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 1999; 33:523–533Crossref, Medline, Google Scholar
124. Tsai G, Yang P, Chung LC, Lange N, Coyle JT: d-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 1998; 44:1081–1089Google Scholar
125. Krystal JH, D’Souza DC: d-serine and the therapeutic challenge posed by the N-methyl-d-aspartate antagonist model of schizophrenia (editorial). Biol Psychiatry 1998; 44:1075–1076Google Scholar
126. D’Aniello A, Guiditta A: Presence of d-aspartate in squid axoplasm and in other regions of the cephalopod nervous system. J Neurochem 1978; 31:1107–1108Google Scholar
127. D’Aniello A, Guiditta A: Identification of d-aspartic acid in the brain of octopus vulgaris lam. J Neurochem 1977; 29:1053–1057Google Scholar
128. Lee JA, Homma H, Sakai K, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Yoshikawa M, Imai K: Immunohistochemical localization of d-aspartate in the rat pineal gland. Biochem Biophys Res Commun 1997; 231:505–508Crossref, Medline, Google Scholar
129. Sakai K, Homma H, Lee JA, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Imai K: d-aspartic acid localization during postnatal development of rat adrenal gland. Biochem Biophys Res Commun 1997; 235:433–436Crossref, Medline, Google Scholar
130. Dunlop DS, Neidle A, McHale D, Dunlop DM, Lajtha A: The presence of free d-aspartic acid in rodents and man. Biochem Biophys Res Commun 1986; 141:27–32Crossref, Medline, Google Scholar
131. Neidle A, Dunlop DS: Developmental changes in free d-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci 1990; 46:1517–1522Google Scholar
132. D’Aniello A, Di Cosmo A, Di Cristo C, Annunziato L, Petrucelli L, Fisher G: Involvement of d-aspartic acid in the synthesis of testosterone in rat testes. Life Sci 1996; 59:97–104Crossref, Medline, Google Scholar
133. D’Aniello A, Di Fiore MM, D’Aniello G, Colin FE, Lewis G, Setchell BP: Secretion of d-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett 1998; 436:23–27Crossref, Medline, Google Scholar
134. Fisher G, Lorenzo N, Abe H, Fujita E, Frey WH, Emory C, Di Fiore MM, D’Aniello A: Free d- and l-amino acids in ventricular cerebrospinal fluid from Alzheimer and normal subjects. Amino Acids 1998; 15:263–269Crossref, Medline, Google Scholar
135. D’Aniello A, Lee JM, Petrucelli L, Di Fiore MM: Regional decreases of free d-aspartate levels in Alzheimer’s disease. Neurosci Lett 1998; 250:131–134Crossref, Medline, Google Scholar
136. Schell MJ, Cooper OB, Snyder SH: d-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A 1997; 94:2013–2018Google Scholar



