High Vesicular Monoamine Transporter Binding in Asymptomatic Bipolar I Disorder: Sex Differences and Cognitive Correlates
Abstract
OBJECTIVE: It has been hypothesized that anomalies in monoaminergic function underlie some of the manifestations of bipolar disorder. In this study the authors examined the possibility that trait-related abnormalities in the concentration of monoaminergic synaptic terminals may be present in patients with asymptomatic bipolar disorder type I. METHOD: The concentration of a stable presynaptic marker, the vesicular monoamine transporter protein (VMAT2), was quantified with (+)[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. Sixteen asymptomatic patients with bipolar I disorder who had a prior history of mania with psychosis (nine men and seven women) and individually matched healthy subjects were studied. Correlational analyses were conducted to examine the relationship between regional VMAT2 binding, cognitive function, and clinical variables. RESULTS: VMAT2 binding in the thalamus and ventral brainstem of the bipolar patients was higher than that in the comparison subjects. VMAT2 concentrations in these regions correlated with performance on measures of frontal, executive function. In addition, sex differences in VMAT2 binding were detected in the thalamus of the bipolar patients; the male patients had higher binding than the women. No sex differences in binding were observed in the healthy comparison group. CONCLUSIONS: These initial results suggest that higher than normal VMAT2 expression and, by extension, concentration of monoaminergic synaptic terminals, may represent a trait-related abnormality in patients with bipolar I disorder and that male and female patients show different patterns. Also, VMAT2 concentrations may be associated with some of the cognitive deficits encountered in euthymic bipolar disorder.
Bipolar disorder is a neuropsychiatric disorder characterized by cyclic variations in mood, from depressed to manic states, and a typical onset in the late teens and 20s (1, 2). Its prevalence in the general population is approximately 1%, with equal proportions of affected men and women (3). Bipolar disorder has a high degree of inheritability. Bipolar I disorder (characterized by full manic and major depressive episodes) shows approximately 80% concordance in monozygotic twins (4). While it usually follows a relapsing, chronic course, with variable quiescent intervals between episodes, both social and cognitive functioning appear to be affected even during asymptomatic phases (5). Recently, deficits in verbal memory and executive functioning have been confirmed during asymptomatic, “euthymic” states (6).
Neuroimaging of energy consumption with metabolic or blood flow markers during the depressed phases of bipolar and unipolar major depression has shown abnormalities in the function of the prefrontal cortex and anterior cingulate regions (7, 8), which resolve after successful treatment (9). Higher than normal whole brain metabolism has been observed in bipolar disorder patients as they cycled from depression into hypomania, suggesting global state-dependent abnormalities in neuronal regulatory mechanisms (10). Less conclusive information has been obtained regarding possible stable, “trait” markers of this illness.
Structural neuroimaging studies have pointed to the possibility of either neurodevelopmental or neurodegenerative regional brain changes in patients with bipolar disorder. Low volume and metabolism of the left subgenual prefrontal cortex have been noted in patients with familial unipolar and bipolar depression (11). Large ventricles and both high and low temporal lobe volumes have been reported as well, albeit not replicated by all groups (12). More recently, higher than normal volumes of the amygdala have been observed, together with high left ventricular volumes (13). Data obtained with magnetic resonance spectroscopy point toward low phosphocreatine concentrations in the frontal lobes and high choline- and inositol-to-phosphocreatine ratios in the frontal cortex and basal ganglia. These anomalies have been observed during both symptomatic and asymptomatic states (14–16) and may reflect anomalies in cell membrane integrity.
While studies with functional and neuroanatomical imaging techniques have shown anomalies in the function or structure of brain regions possibly implicated in the pathophysiology of bipolar disorder, data addressing specific neurochemical systems are lacking. Abnormalities in monoaminergic (e.g., serotonin, norepinephrine, dopamine) function have been hypothesized to underlie some of the clinical manifestations of bipolar disorder for a quarter of a century. However, the supporting data have often been confounded by the use of peripheral or indirect measures, a paucity of postmortem studies, and the effects of medication treatment and clinical state on the markers examined (17). In the present study we focused on the possibility that abnormalities in more stable markers of monoaminergic synaptic concentrations may exist in bipolar disorder, perhaps associated with a vulnerability to this illness or reflecting trait characteristics. We examined the integrity of monoaminergic terminals with (+)[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography (PET). This radiotracer labels the central vesicular monoamine transporter (VMAT2) and allows its quantification in living human subjects (18, 19). The VMAT2 binding site is exclusively located in the membranes of presynaptic vesicles of monoaminergic neurons (20) and mediates the transport of monoaminergic neurotransmitters from the cytoplasm to their storage vesicles. Unlike levels of the synaptic membrane dopamine transporters, VMAT2 concentrations do not appear to be modulated by short- or long-term administration of a variety of drugs affecting monoaminergic function or metabolism (21–24). Furthermore, striatal VMAT2 binding is reduced after nigral 6-OH-dopamine lesions in rats, and it linearly reflects the concentration of monoaminergic synaptic projections in this region (primarily dopaminergic in the striatum) (25). Striatal binding also decreases with advancing age (approximately 0.5%–0.8% per year) and is low in neurodegenerative disorders affecting dopaminergic innervation (26, 27). These properties (e.g., insensitivity to medication effects, binding reflective of monoaminergic synaptic density) are desirable for the examination of possible trait-related abnormalities in bipolar disorder.
In the present study, [11C]DTBZ binding to VAMT2 sites was measured in three brain regions rich in monoaminergic terminals, but with differing concentrations of each monoamine, to test whether generalized or more neurochemically specific anomalies are present in bipolar disorder. These regions were the caudate nuclei, reflecting primarily dopaminergic projections, the thalamus, rich in serotonin and noradrenaline but with minimal dopaminergic content in humans, and the brainstem, containing monoaminergic cell bodies and local terminal projections. In addition, we were interested in examining whether abnormalities in VMAT2 binding would be associated with clinical features of the illness, such as age at onset or course, or with trait cognitive dysfunctions we have found (unpublished data) or those recently described by others (6). In this regard, monoaminergic input at the level of the caudate nuclei and the thalamus has been implicated in the regulation of frontal cortical networks, affecting memory consolidation, attention, and executive functions (28–31).
Method
Subjects
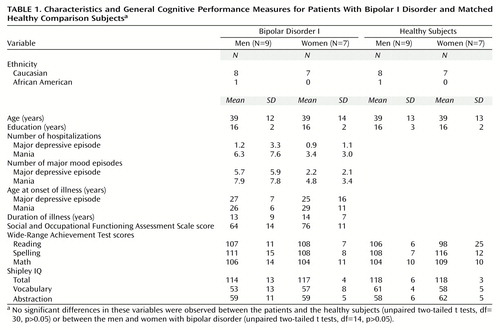
Sixteen asymptomatic (euthymic) patients diagnosed with bipolar disorder type I (DSM-IV criteria), nine men and seven women, were recruited from various treatment clinics of the Department of Psychiatry at the University of Michigan (Table 1). The diagnoses were confirmed by a structured interview (Structured Clinical Interview for DSM-IV [SCID]) (32). The asymptomatic state was defined by a Hamilton Depression Rating Scale (33) score of less than 7 and a Young Mania Rating Scale (34) score of less than 6. All patients had been asymptomatic for at least 6 months, except for two, who had recovered from hypomanic episodes 1 and 3 months before the study. Additional clinical information (onset of illness, prior symptoms, numbers of episodes and hospitalizations, prior treatments) was obtained from both clinical charts and direct patient interviews. Social functioning was rated with the DSM-IV Social and Occupational Functioning Assessment Scale (35) (Table 1). All patients had had at least one prior episode of mania with psychosis. Two of the patients did not meet the full criteria for past major depressive episodes. All patients were being maintained with one or more mood-stabilizing drugs (valproic acid, N=12; lithium, N=7; carbamazepine, N=2; lamotrigine, N=1), but three patients were also receiving low doses of antipsychotics (5 mg/day of olanzapine, N=1; 4 mg/day of thiothixene, N=2). None of the subjects had taken antidepressant medications for at least 6 months before the study. The patients took their last dose of medication 12 hours before functional brain imaging to standardize their medication status before the neuropsychological testing, which was conducted after the PET study. Blood samples for measurement of levels of mood stabilizers were obtained immediately before imaging.
Sixteen healthy comparison subjects were recruited through advertisement. They were matched individually to the patients by age, sex, educational level, and ethnicity (Table 1). Undiagnosed psychiatric illnesses were ruled out by use of a structured interview (SCID).
All subjects, patients and the comparison group, were free of acute medical and neurological illnesses, ascertained by review of medical history and review of systems. They received complete physical examinations, including ancillary laboratory tests (liver, kidney, thyroid, electrolytes, fasting glucose) and a comprehensive urine toxicology screen. All subjects were right-handed, did not smoke, and had no prior history of alcohol or other substance abuse or dependence according to DSM-IV criteria. In addition, none of the subjects had been exposed to reserpine, a compound known to reduce VMAT2 concentrations. All of the women studied had a history of regular menstrual cycles and were scanned during the follicular phase of their cycles (days 2 to 12 after the onset of menses). A comprehensive neuropsychological testing battery was also completed shortly after PET imaging, typically within 1–2 hours. After complete description of the study to the subjects, written informed consent was obtained. All the procedures and experimental protocols were approved by the University of Michigan Institutional Review Boards.
Scanning Procedures
PET imaging was performed on a Siemens/CTI ECAT Exact-47 scanner (Knoxville, Tenn.) in three-dimensional mode (septa retracted). A 12-minute transmission scan was obtained immediately before emission scanning for attenuation correction. (+)-α-[11C]DTBZ was administered intravenously at a mean dose of 18 mCi (SD=1) (666 MBq, SD=37) per subject. One-half of the dose was administered as a bolus over 1 minute, and the remainder was given as a continuous infusion over the subsequent 59 minutes. The 50:50 fractionation between the bolus and infusion portions was based on calculations of the rate of clearance from plasma from prior bolus studies so that tracer equilibrium between plasma and brain was achieved between 30 and 60 minutes after tracer administration (36). No-carrier-added (+)-α-[11C]DTBZ (250 to 1000 Ci/mmol at time of injection) was prepared as previously described (37). Scanning was performed during the 0–4 minutes after DTBZ injection in a single frame, followed by three 10-minute scans from 30 to 60 minutes.
Data were reconstructed by using a Hanning filter with a cutoff of 0.5 cycles/projection ray with scatter and attenuation corrections, and a reconstructed resolution of approximately 9 mm full width at half maximum was achieved.
Blood samples were drawn by using a radial artery catheter placed in the nondominant wrist. For the first minute, samples were obtained approximately every 6 seconds; for the second minute, approximately every 10 seconds. Additional samples were taken at 2.5, 3.0, 3.5, and 4.0 minutes and then at 30, 40, 50, and 60 minutes. Plasma was separated from red cells by centrifugation and counted in an NaI well counter. The plasma radioactivity time course was corrected by the presence of radiolabeled metabolites by using a rapid chromatographic technique, as previously described (26). Radioactive fiducial markers placed on the subject’s scalp were used to coregister the images and to correct for patient motion that may have occurred during the study (36).
Two sets of parametric images were then calculated on a pixel-by-pixel basis by using the dynamic scan data. The first set represented the total distribution volume of the radiotracer at equilibrium (Figure 1, second row). This measure is obtained by dividing the concentration of (+)-α-[11C]DTBZ in brain tissue (measured with PET) by the metabolite-corrected arterial plasma concentration averaged over the portion of the study after equilibrium conditions were achieved (30–60 minutes after tracer administration) (36). It represents the sum of specific VMAT2 binding and nonsaturable (free ligand plus nonspecific binding) [11C]DTBZ levels and is proportional to the distribution of VMAT2 binding sites (26). The second type of parametric image, the rate of transport of the radiopharmaceutical (K1), was then calculated by an autoradiographic method using the corresponding distribution volume estimate for each pixel (38) (Figure 1>, first row).
Structural magnetic resonance (MR) images were obtained for all subjects by using axial T1-weighted spoiled gradient/recall acquisition in the steady state (SPGR), T2, and proton density sequences (TR=14 msec, TE=5.5 msec, 1.5-mm thickness; TR=4000 msec, TE=20 msec, 3-mm thickness; and TR=4000 msec, TE=100 msec, 3-mm thickness, respectively). The MR images were used to rule out structural abnormalities and to aid in the placement of regions of interest. The orientation of the images was then standardized by realigning the T1 and K1 images with the line between the anterior commissure and the posterior commissure (AC-PC line), and applying the same transformation matrix to the distribution volume images (39) (Figure 1). Four-by-four-pixel regions of interest (1.875 × 1.875 × 3.375 mm per pixel, x, y, z axis) were placed on the K1 images. The spatial location of the regions of interest was standardized by using a predefined region-of-interest template (based on stereotactic coordinates, to be described) and following the same AC-PC line orientation and the regional definitions of the Talairach and Tournoux atlas (40). To avoid a full nonlinear stereotactic transformation of the images, which may be unreliable for subcortical structures, the regions of interest were adjusted to the individual anatomy when necessary by a trained operator blind to subject and diagnosis and were confirmed by one of us (J.-K.Z.), also blinded. The regions of interest were then transferred to the distribution volume images. The regions of interest were placed in three regions in which specific binding has been previously described (41) (one region of interest on each side): caudate, thalamus, and brainstem. The caudate and brainstem were further subdivided into ventral and dorsal areas, since they subserve different functional networks. Each region and subregion was sampled in three consecutive slices. The middle region of interest for each structure was centered by the predefined template in the following stereotactic locations (x, y, z axes, in millimeters): dorsal caudate, 10, 15, 8 and –10, 15, 8; ventral caudate, 10, 10, –7 and –10, 10, –7; thalamus, 10, –10, 8 and –10, –10, 8; dorsal brainstem, 5, –30, –12 and –5, –30, –12; ventral brainstem, 10, –15, –12 and –10, –15, –12. The occipital cortex was sampled to provide an estimate of nonsaturable activity, as previously described (26) (four regions of interest on each side). The occipital cortex values were then used to normalize the K1 values (regional K1 values were divided by occipital cortex K1 values) to reduce interexperimental variability due to global scaling factors (e.g., variability in the plasma metabolite correction procedures, calibration factors for scanner or well counters). They were also used to obtain an estimate of normalized specific DTBZ binding, commonly referred to as binding potential (42), which is based on the distribution volume at equilibrium: (regional distribution volume – occipital cortex distribution volume) / occipital cortex distribution volume, or distribution volume ratio – 1. The binding potential is equivalent to the Bmax/Kd for this receptor site. Brain time-activity curves from a representative subject for the aforementioned regions and for white matter are shown in Figure 2.
The values for all regions of interest for each anatomical area were then averaged across slices to yield one single value. Furthermore, no significant differences in either K1 or distribution volume were noted between the right and left sides of each region (two-tailed paired t tests, p>0.05), so they were again averaged to reduce the number of comparisons.
Neuropsychological Measures
Recent data from our laboratory and other authors (6) have confirmed that euthymic patients with bipolar disorder show worse performance on tests reflecting verbal learning and executive and psychomotor functions. Therefore, we were interested in examining relationships between possible anomalies in regional VMAT2 concentrations and the scores obtained in the neuropsychological tests in our laboratory. The tests selected on the basis of those data were as follows: 1) for memory, the Wechsler Memory Scale paired associates test, immediate recall (43, pp. 451–454), 2) for executive, frontal functions, the Wisconsin Card Sorting Test (43, pp. 621–625) and Stroop Color and Word Test (43, pp. 373–375), and 3) for psychomotor function, the Manual Imitation Test (44) and Bead-Tap Test (45, 46). The testing battery was administered 1–2 hours after completion of the imaging protocol.
Statistics
Group differences in regional radioligand transport rates (normalized K1) and binding (distribution volume ratio – 1, or binding potential) were tested with independent two-tailed unpaired t tests for the selected regions of interest. Possible relationships between binding potentials and neuropsychological test results were explored with Pearson correlations. The threshold level of statistical significance was set at p<0.05. However, only the tests previously found to show differences between groups and regions found to be different between patients and comparison subjects were used for the correlational analyses. Other statistical comparisons are indicated in the text.
Results
Subjects
There were no significant differences in age, educational level, or measures of scholastic aptitude or IQ between the patients with bipolar I disorder and the healthy comparison group (Table 1). Similarly, no significant differences in demographic characteristics, general measures of cognitive function, or clinical characteristics between the men and women with bipolar I disorder were noted (Table 1). The women showed a tendency toward higher social functioning (score on the Social and Occupational Functioning Assessment Scale) and a slightly earlier onset of depressive episodes and later onset of mania; however, these differences did not reach statistical significance (Table 1).
Tracer Transport and Binding
The proportion of authentic (unmetabolized) [11C]DTBZ in the plasma sampled over the scanning period did not significantly differ between the patients and the comparison group at any time, according to a repeated measures analysis of variance (ANOVA) of the group-by-time interaction (F=0.86, df=8, 232, p=0.55).
The K1 values did not significantly differ between diagnostic groups for any the brain regions examined: caudate (t=0.15, df=30, p=0.98), thalamus (t=0.49, df=30, p=0.63), ventral midbrain (t=0.32, df=30, p=0.75), or dorsal midbrain (t=0.75, df=30, p=0.46).
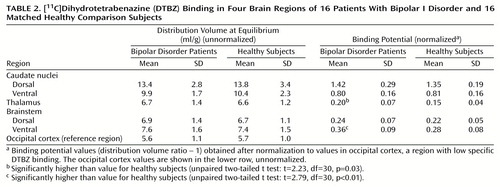
Differences in binding potential between diagnostic groups, with higher binding in the bipolar disorder group, were detected in the thalamus (mean difference between groups, 31%) and ventral midbrain (mean difference between groups, 28%) (Table 2).
Relation of Thalamic and Ventral Brainstem Binding to Clinical Variables
Possible relationships between regional binding potentials and clinical variables were examined for the thalamus and brainstem. We found no significant correlations between binding potentials in these regions and any of the clinical variables examined, which included the Hamilton Depression Rating Scale score and Young Mania Rating Scale score immediately before imaging, numbers of hospitalizations for depression and mania, numbers of episodes of depression and mania, years of illness, Social and Occupational Functioning Assessment Scale ratings, years of exposure to mood stabilizers, antidepressants, and antipsychotic drugs, and blood levels of mood stabilizers before imaging.
Relation of Thalamic and Ventral Brainstem Binding to Neuropsychological Measures
Thalamic binding potential correlated positively with Wisconsin Card Sorting Test perseverative responses (r=0.43, df=29, p=0.01) and perseverative errors (r=0.39, df=29, p=0.03). There was a slightly lower correlation, which did not reach statistical significance, between thalamic binding and the score on the Wechsler Memory Scale paired associates subtest of immediate recall (r=–0.34, df=28, p=0.06). Ventral brainstem binding correlated negatively with the test of immediate recall (r=–0.38, df=28, p=0.04) and with the score on the Stroop Color and Word Test (r=–0.38, df=29, p=0.03). There was a lower, nonsignificant correlation between binding in this region and the Wisconsin Card Sorting Test categories score (r=–0.32, df=29, p=0.07).
Sex Differences
A secondary exploratory analysis was conducted to determine whether sex effects would have influenced the preceding group differences in regional binding potentials. A two-way (diagnosis, sex) factorial ANOVA was used for the thalamus and ventral brainstem to determine whether sex effects and sex-by-diagnosis interactions were present in the data.
ANOVAs showed significant diagnosis (F=4.99, df=1, 28, p=0.03) and sex (F=5.28, df=1, 28, p=0.02) effects in the thalamus but no diagnosis-by-sex interaction (F=1.27, df=1, 28, p=0.27). In the ventral midbrain, a significant diagnosis effect was noted (F=7.00, df=1, 28, p=0.01), as expected, but not a sex effect (F=1.13, df=1, 28, p=0.29) or sex-by-diagnosis interaction (F=1.39, df=1, 28, p=0.25).
In the thalamus, the region where a sex effect was detected, the healthy men and women and the women with bipolar I disorder showed similar mean values and distributions for binding potentials. The men with bipolar I disorder had higher binding potentials (mean difference of 42%) than the women in the same diagnostic group.
Discussion
In this study we examined selected regional concentrations of a stable presynaptic monoaminergic protein, VMAT2, in euthymic patients with bipolar I disorder and in matched healthy volunteers, using [11C]DTBZ and PET. The purpose of this study was to determine whether trait-related abnormalities in the concentrations of VMAT2 sites, a marker of monoaminergic synaptic density, could be detected. While dysfunctions of monoaminergic networks have been postulated in the mood disorders (17), to our knowledge the integrity of these neurochemical systems has not been directly examined in patients with bipolar disorder.
The main finding of this study was the observation that the concentrations of VMAT2 binding sites were higher in the thalamus and ventral brainstem of the patients with bipolar I disorder than in the healthy subjects, with mean differences between groups of 31% and 28%, respectively.
In addition, the VMAT2 concentrations in the thalamus and ventral brainstem correlated negatively with performance on tests of frontal, executive function and with a test of verbal learning. Lower than normal performance on these and similar tests have been reported in patients with bipolar disorder, even after prolonged euthymic states (6).
A secondary finding was the sex difference in the concentration of VMAT2 binding sites in the bipolar disorder patients. ANOVA showed an effect of sex on the concentration of VMAT2 binding sites in the thalamus of the bipolar disorder patients but not the healthy comparison group. The women with bipolar I disorder showed thalamic VMAT2 concentrations in the same range as that of the comparison men and women, while the male patients with bipolar disorder demonstrated greater binding in this region than the female patients (mean difference, 42%).
We found no consistent associations between thalamic or ventral brainstem VMAT2 concentration and clinical variables, blood levels of mood stabilizers, or prior exposure to psychotropic medications.
The greater thalamic and ventral brainstem binding values were not coupled with similarly high levels of regional tracer transport, a measure proportional to blood flow. This indicates that the observed differences in binding values were not likely to be secondary to nonspecific differences in variables such as regional brain volume or anomalies in initial tracer uptake. The presence of anomalies in regional brain volumes in bipolar disorder still remains a controversial issue, with both high and low volumes having been reported (12, 13). However, the fact that the abnormalities in volume so far observed are relatively small in comparison to the effects noted in the present paper indicates that they are not likely to entirely account for the findings reported. The sizes of the regions of interest applied to the image data (approximately one full width at half maximum of their resolution) and the fact that they were centered over relatively large anatomical regions also made the measures obtained less susceptible to nonspecific volumetric differences between groups (47).
[11C]DTBZ is a selective marker for VMAT2 (18, 19). This protein, located in the membrane of the synaptic vesicles (20), mediates the transport of the monoamines from the cytoplasm into presynaptic storage vesicles. It reflects the concentration of synaptic vesicles in monoaminergic cells, which in turn is thought to provide an estimate of the density of synaptic contacts, since reductions in their concentration are typically associated with degenerative changes in monoaminergic neurons (48–50).
We measured VMAT2 binding sites as a possible trait marker in bipolar I disorder because of their apparent insensitivity to medication effects and because their binding reflects synaptic terminal concentrations (25). The high-affinity synaptic membrane transporters, while also located presynaptically, are regulated by neuronal function and by a variety of drugs affecting the release or metabolism of the monoamines. This does not appear to be the case for the VMAT2 sites, at least for commonly used compounds, such as antipsychotics and various antidepressants (21–24), albeit their possible regulation by mood stabilizers has not been specifically examined. However, the disadvantage of using the VMAT2 site as a marker of monoaminergic synaptic concentration is its nonselectivity for the various monoamines (51), which is conferred to the specific neurons by the synaptic membrane transporters (the serotonin transporter, dopamine transporter, and noradrenergic transporter). However, some degree of neurochemical specificity may be inferred from the regional distribution of VMAT2 binding sites.
The brainstem area contains the cell bodies and local projections of serotonergic (raphe nuclei) and dopaminergic (retrorubral, substantia nigra, and ventral tegmental area) neurons. In an attempt to relate possible findings to more selective neuronal populations, the brainstem was subdivided into ventral and dorsal regions, as previously described by other authors (52). The former is rich in dopaminergic cells (regions A8, A9, and A10); however, it receives dense input from serotonergic cells located in the raphe nuclei. The dorsal area contains the serotonergic cells of the nucleus raphe dorsalis (53). It should be noted, however, that because of the relatively poor resolution of PET cameras, the values obtained from regions in close proximity are likely to be contaminated by spillover of activity from one to the other. This applies to the subdivision of the brainstem into dorsal and ventral portions or the subdivision of the caudate into dorsal and ventral portions (the latter region including the nucleus accumbens). Therefore, the separation between ventral and dorsal brainstem and between ventral and dorsal caudate should be taken with caution, and mostly as an approximation to binding values in those specific brain regions.
Prior studies with the nonselective serotonin transporter and dopamine transporter ligand [123I]β-CIT and single photon emission computed tomography have shown that brainstem [123I]β-CIT labeling in humans and nonhuman primates is displaceable with serotonin transporter blockers but not dopamine transporter blockers (54, 55). This suggests that the majority of monoaminergic presynaptic terminals in this brain area are serotonergic, a possibility that agrees with literature on animal research describing dense serotonergic projections from the dorsal raphe to midbrain dopaminergic cells (56). This is supported by the finding in our study that the higher levels in the ventral brainstem were not coupled with parallel abnormalities in dopaminergic-terminal-rich areas (caudate nucleus/nucleus accumbens). On the other hand, differences of similar magnitude were noted in the thalamus, a region with a high density of serotonergic and noradrenergic terminals but negligible dopaminergic innervation in humans (57). Another, perhaps less likely possibility is that the higher levels noted in the thalamus are based on binding to noradrenergic terminals. In this regard, abnormally high indexes of noradrenergic turnover in the thalamus and cortical regions have been observed in a postmortem study of bipolar disorder patients (58). However, resolving the specific neurochemical network or networks involved in the higher levels observed would require the development of specific radioligands labeling other transporters or receptors involved in serotonergic or noradrenergic responses.
As already noted, [123I]β-CIT binding in the brainstem has been used as an indicator of serotonin transporter concentrations in this brain region in various studies. Serotonin transporter concentrations have been found to be low in patients with unipolar major depression (59) and in male abstinent alcohol-dependent volunteers (52). In the study by Mallison et al. (59), [123I]β-CIT binding was approximately 20% lower in the brainstem of patients with major depression than in healthy subjects. In the alcohol-dependent volunteers (52), [123I]β-CIT binding was a mean of 30% lower in dorsal but not ventral brainstem. In this study group, only subjects with an early onset of alcohol dependence showed low binding, perhaps suggesting the presence of vulnerability factors related to monoaminergic function in alcohol dependence.
We observed negative correlations of performance on measures of verbal learning and frontal, executive functions with thalamus and ventral brainstem [11C]DTBZ binding. Although these correlations do not imply a causal relationship, the data obtained suggest that higher VMAT2 binding in these regions may bear a relationship with the poorer performance of patients with bipolar I disorder on specific cognitive measures. Lower scores on measures of verbal learning, executive functions, and motor coordination in the absence of more global cognitive deficits have been observed by our group and by other authors (6). Monoaminergic function at the level of the thalamus and brainstem is thought to regulate the activity of the basal ganglia and thalamocortical networks (28, 30, 60–62). These in turn influence cortical processes such as selective attention, learning of associative tasks, and adaptive responses, as well as the processing of emotion-related stimuli (29, 63–67). In the case of serotonin, acute tryptophan depletion has been associated with improvements in Stroop test performance (68), suggesting that serotonin may regulate some cognitive functions by an inhibitory mechanism.
This study opens several lines of questioning regarding the pathophysiology of bipolar I disorder. Higher than normal levels of VMAT2 binding in the thalamus and ventral brainstem during euthymic states, correlating with poorer performance on tests of verbal learning and executive functions, may represent trait abnormalities in the concentration of monoaminergic synaptic terminals or synaptic vesicles in bipolar disorder. In addition, we found sex differences in VMAT2 binding in the thalamus of the patients with bipolar disorder but not in the healthy subjects. To determine the clinical implications of this finding, such as its possible relationship with reproductive status or sex differences in episode frequency or pattern (69, 70), would require the examination of a larger patient group. Further studies are warranted to increase the power of the data presented, to ascribe the aforementioned anomalies to specific monoaminergic networks, and to examine possible sex differences in the distribution of these markers. Also, such work is needed to confirm that the differences in the thalamus and ventral brainstem indeed represent a trait abnormality and not the result of either medication exposure or illness progression, although these possibilities do not appear to be supported by the data obtained. For that purpose, it would be of interest to examine both newly diagnosed patients and subjects who are at high risk for developing this illness (e.g., those with a prominent family history of bipolar disorder). This should be followed by a prospective examination of illness progression and the possible changes in monoaminergic innervation associated with it or with the long-term use of mood-stabilizing drugs.
 |
 |
Received Nov. 22, 1999; revision received May 8, 2000; accepted May 15, 2000. From the Mental Health Research Institute and Division of Neuropsychology, Department of Psychiatry, and the Division of Nuclear Medicine, Department of Internal Medicine, University of Michigan. Address reprint requests to Dr. Zubieta, Mental Health Research Institute, Neuroscience Building, 1103 East Huron St., Ann Arbor, MI 48104-1687; [email protected] (e-mail).Supported by the General Clinical Research Center at the University of Michigan (grant RR-00042 from the National Center for Research Resources), by the Mental Illness Research Association’s Arthur Forrest Tull II Research Fund, and by the National Alliance for Research on Schizophrenia and Depression.The authors thank PET nuclear medicine technologists Jill M. Rothley, Edward J. McKenna, Andrew R. Weeden, and Todd M. Hauser.
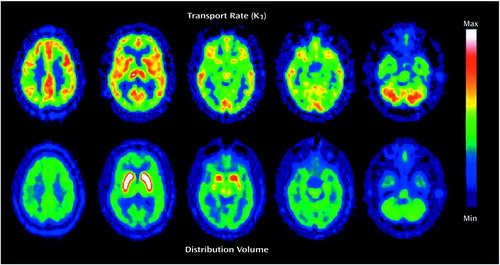
Figure 1. Parametric PET Images of (+)[11C]Dihydrotetrabenazine Transport and Total Distribution Volume at Equilibrium From a Representative Subject With Bipolar I Disordera
aThe images, from a pixel-by-pixel estimation (see Method), are shown in five transaxial slices from the supraventricular (left-most column) to the posterior fossa (right-most column) levels. The maximum and minimum values are noted in the pseudocolor scale on the right.
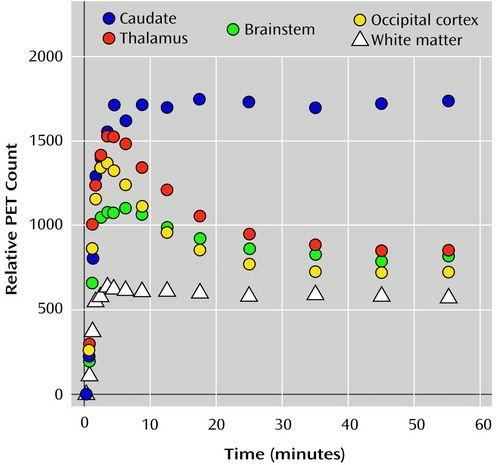
Figure 2. Binding of (+)[11C]Dihydrotetrabenazine Over 60 Minutes in Four Brain Regions and White Matter of a Representative Subject With Bipolar I Disordera
aAreas with detectable specific binding (caudate nucleus, thalamus, brainstem) are shown in comparison with regions in which very low or no specific binding could be detected (occipital cortex, white matter).
1.. Coryell W, Andreasen NC, Endicott J, Keller M: The significance of past mania or hypomania in the course and outcome of major depression. Am J Psychiatry 1987; 144:309–315Link, Google Scholar
2.. Angst J: Course of affective disorders, in Handbook of Biological Psychiatry. Edited by Van Praag HM, Lader MH, Rafaelsen OJ, Sachar EJ. New York, Marcel Dekker, 1981, pp 225–242Google Scholar
3.. Boyd JH, Weissman MM: Epidemiology of affective disorders: a reexamination and future directions. Arch Gen Psychiatry 1981; 38:1039–1046Google Scholar
4.. Gershon ES: Genetics, in Manic Depressive Illness. Edited by Goodwin FK, Jamison KR. New York, Oxford University Press, 1990, pp 373–401Google Scholar
5.. Strakowski SM, Keck PE, McElroy SL, West SA, Sax KW, Hawkins JM, Kmetz GF, Upadhyaya VH, Tugrul KC, Bourne ML: Twelve month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry 1998; 55:49–55Crossref, Medline, Google Scholar
6.. van Gorp WG, Altshuler L, Theberge DC, Wilkins J, Dixon W: Cognitive impairment in euthymic bipolar patients with and without alcohol dependence. Arch Gen Psychiatry 1998; 55:41–46Crossref, Medline, Google Scholar
7.. Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, Lohr J, Wu J, Lottenberg S, Jerabek PA, Trenary M, Tafalla R, Reynolds C, Bunney WE Jr: Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Arch Gen Psychiatry 1992; 49:935–942Crossref, Medline, Google Scholar
8.. Mayberg HS, Lewis PJ, Regenold W, Wagner HN Jr: Paralimbic hypoperfusion in unipolar depression. J Nucl Med 1994; 35:929–934Medline, Google Scholar
9.. Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C: Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 1997; 41:15–22Crossref, Medline, Google Scholar
10.. Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Crossref, Medline, Google Scholar
11.. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Crossref, Medline, Google Scholar
12.. Norris SD, Krishnan KR, Ahearn E: Structural changes in the brain of patients with bipolar affective disorder by MRI: a review of the literature. Progr Neuropsychopharmacol Biol Psychiatry 1997; 21:1323–1337Google Scholar
13.. Strakowsky SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER: Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 1999; 56:254–260Crossref, Medline, Google Scholar
14.. Sharma R, Venkatasubramanian PN, Barany M, Davis JM: Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res 1992; 8:43–49Crossref, Medline, Google Scholar
15.. Kato T, Hamakawa H, Shioiri T, Murashita J, Takahashi Y, Takahashi S, Inubushi T: Choline-containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. J Psychiatry Neurosci 1996; 21:248–254Medline, Google Scholar
16.. Kato T, Shioiri T, Murashita J, Hamakawa H, Takahashi Y, Inubushi T, Takahashi S: Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol Med 1995; 25:557–566Crossref, Medline, Google Scholar
17.. Goodwin FK, Jamison KR: Manic-Depressive Illness. New York, Oxford University Press, 1990Google Scholar
18.. Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC, Kilbourn MR, Kuhl DE: Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab 1996; 16:1288–1299Google Scholar
19.. Lee LC, Vander Borght T, Sherman PS, Frey KA, Kilbourn MR: In vitro and in vivo studies of benzisoquinoline ligands for the brain synaptic vesicle monoamine transporter. J Med Chem 1996; 39:191–196Crossref, Medline, Google Scholar
20.. Henry JP, Scherman D: Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem Pharmacol 1989; 38:2395–2404Google Scholar
21.. Krejci E, Gasnier B, Botton D, Isambert MF, Sagne C, Gagnon J, Massoulie J, Henry JP: Expression and regulation of the bovine vesicular monoamine transporter gene. FEBS Lett 1993; 335:27–32Crossref, Medline, Google Scholar
22.. Naudon L, Leroux-Nicollet I, Costentin J: Short-term treatments with haloperidol or bromocriptine do not alter the density of the monoamine vesicular transporter in the substantia nigra. Neurosci Lett 1994; 173:1–4Crossref, Medline, Google Scholar
23.. Vander Borght TM, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA: The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 1995; 294:577–583Crossref, Medline, Google Scholar
24.. Wilson JM, Kish SJ: The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci 1996; 16:3507–3510Google Scholar
25.. Vander Borght TM, Sima AA, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA: [3H]Methoxytetrabenazine: a high specific activity ligand for estimating monoaminergic neuronal integrity. Neuroscience 1995; 68:955–962Crossref, Medline, Google Scholar
26.. Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE: Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann Neurol 1996; 40:873–884Crossref, Medline, Google Scholar
27.. Gilman S, Frey KA, Koeppe RA, Junck L, Little R, Vander Borght TM, Lohman M, Martorello S, Lee LC, Jewett DM, Kilbourn MR: Decreased striatal monoaminergic terminals in olivopontocerebellar atrophy and multiple system atrophy demonstrated with positron emission tomography. Ann Neurol 1996; 40:885–892Crossref, Medline, Google Scholar
28.. Bubser M, Feenstra MG, Erdtsieck-Ernste EB, Botterblom MH, Van Uum HF, Pool CW: Modulatory role of catecholamines in the transsynaptic expression of c-fos in the rat medial prefrontal cortex induced by disinhibition of the mediodorsal thalamus: a study employing microdialysis and immunohistochemistry. Brain Res 1997; 749:214–225Crossref, Medline, Google Scholar
29.. Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S, Hitzemann R, Smith G, Logan J: Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155:344–349Link, Google Scholar
30.. Wise SP, Murray EA, Gerfen CR: The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol 1996; 10:317–356Crossref, Medline, Google Scholar
31.. Coull JT: Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 1998; 55:343–361Crossref, Medline, Google Scholar
32.. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
33.. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
34.. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
35.. Goldman HH, Skodol AE, Lave TR: Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992; 149:1148–1156Google Scholar
36.. Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE: Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab 1997; 17:919–931Crossref, Medline, Google Scholar
37.. Jewett DM, Kilbourn MR, Lee LC: A simple synthesis of [11C]dihydrotetrabenazine (DTBZ). Nucl Med Biol 1997; 24:197–199Crossref, Medline, Google Scholar
38.. Herscovitch P, Markham J, Raichle ME: Brain blood flow measured with intravenous H2(15O), I: theory and error analysis. J Nucl Med 1983; 24:782–789Medline, Google Scholar
39.. Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE: Automatic detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med 1993; 34:322–329Medline, Google Scholar
40.. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
41.. Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR: Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab 1999; 19:1376–1384Google Scholar
42.. Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ: A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984; 15:217–227Crossref, Medline, Google Scholar
43.. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
44.. Reynolds C, Bigler ED: Test of Memory and Learning Examiner’s Manual. Austin, Tex, PRO-ED, 1994Google Scholar
45.. Taylor AE, Saint-Cyr JA, Lang AE: Frontal lobe dysfunction in Parkinson’s disease. Brain 1986; 109:845–883Crossref, Medline, Google Scholar
46.. Talland G, Schwab RS: Performance with multiple sets in Parkinson’s disease. Neuropsychologia 1964; 2:45–53Crossref, Google Scholar
47.. Links JM, Zubieta JK, Meltzer CC, Stumpf MJ, Frost JJ: Influence of spatially heterogeneous background activity on “hot object” quantitation in brain emission computed tomography. J Comput Assist Tomogr 1996; 20:680–687Crossref, Medline, Google Scholar
48.. Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ: Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry 1999; 156:238–245Abstract, Google Scholar
49.. Naudon L, Leroux-Nicollet I, Costentin J: Consequences of an intrastriatal injection of kainic acid on the dopaminergic neuronal and vesicular uptake systems. Brain Res 1992; 593:32–38Crossref, Medline, Google Scholar
50.. Varoqui H, Erickson JD: Vesicular neurotransmitter transporters: potential sites for the regulation of synaptic function. Mol Neurobiol 1997; 15:165–192Crossref, Medline, Google Scholar
51.. Hoffman BJ, Hansson SR, Mezey E, Palkovits M: Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol 1998; 19:187–231Crossref, Medline, Google Scholar
52.. Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M: Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998; 155:1544–1549Google Scholar
53.. Azmitia E, Whitaker-Azmitia P: Awakening the sleeping giant: anatomy and plasticity of the brain serotonergic system. J Clin Psychiatry 1991; 52(suppl 12):4–16Google Scholar
54.. Laruelle M, Baldwin R, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS, Sybirska EH, Zimmermann RC, Wisniewski G, Neumeyer JL, Milius RA, Wang S, Smith EO, Roth RH, Charney DS, Hoffer PB, Innis RB: SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 1993; 13:295–309Crossref, Medline, Google Scholar
55.. Pirker W, Asenbaum S, Kasper S, Walter H, Angelberger P, Koch G, Pozzera A, Deecke L, Podreka I, Brucke T: β-CIT SPECT demonstrates blockade of 5-HT uptake site by citalopram in the human brain in vivo. J Neural Transm Gen Sect 1995; 100:247–256Crossref, Medline, Google Scholar
56.. Imai H, Steindler DA, Kitai ST: The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol 1986; 243:363–380Crossref, Medline, Google Scholar
57.. Oke AF, Carver LA, Gouvion CM, Adams RN: Three-dimensional mapping of norepinephrine and serotonin in human thalamus. Brain Res 1997; 763:69–78Crossref, Medline, Google Scholar
58.. Young LT, Warsh JJ, Kish SJ, Shannak K, Hornykeiwicz O: Reduced brain 5-HT and elevated NE turnover and metabolites in bipolar affective disorder. Biol Psychiatry 1994; 35:121–127Crossref, Medline, Google Scholar
59.. Mallison RT, Price LH, Berman R, vanDyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 22:1090–1098Google Scholar
60.. Coull JT, Buchel C, Friston KJ, Frith CD: Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage 1999; 10:705–715Crossref, Medline, Google Scholar
61.. McCormick DA: Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol 1992; 9:212–223Crossref, Medline, Google Scholar
62.. Bennett-Clarke CE, Lane RD, Rhoades RW: Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain Res 1995; 702:255–260Crossref, Medline, Google Scholar
63.. Kalivas PW, Churchill L, Romanides A: Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann NY Acad Sci 1999; 877:64–70Crossref, Medline, Google Scholar
64.. Rauch SL, Whalen PJ, Curran T, McInerney S, Heckers S, Savage CR: Thalamic deactivation during early implicit sequence learning: a functional MRI study. Neuroreport 1998; 9:865–870Crossref, Medline, Google Scholar
65.. Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SCR, Checkley SA: Functional MRI study of the cognitive generation of affect. Am J Psychiatry 1999; 156:209–215Abstract, Google Scholar
66.. Servan-Schreiber D, Carter CS, Bruno RM, Cohen JD: Dopamine and the mechanisms of cognition, part II: D-amphetamine effects in human subjects performing a selective attention task. Biol Psychiatry 1998; 43:723–729Crossref, Medline, Google Scholar
67.. Servan-Schreiber D, Bruno RM, Carter CS, Cohen JD: Dopamine and the mechanisms of cognition, part I: a neural network model predicting dopamine effects on selective attention. Biol Psychiatry 1998; 43:713–722Crossref, Medline, Google Scholar
68.. Schmitt JAJ, Jorissen BL, Sobczak S, van Boxtel MPJ, Hogervorst E, Deutz NEP, Riedel WJ: Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacol 2000; 14:21–29Crossref, Medline, Google Scholar
69.. Rehavi M, Goldin M, Roz N, Weizman A: Regulation of rat brain vesicular monoamine transporter by chronic treatment with ovarian hormones. Mol Brain Res 1998; 57:31–37Crossref, Medline, Google Scholar
70.. Angst J: The course of affective disorders, II: typology of bipolar manic-depressive illness. Arch Psychiatr Nervenkr 1978; 226:65–73Crossref, Medline, Google Scholar



