Identification of Functional Polymorphisms in the Promoter Region of the Human PICK1 Gene and Their Association With Methamphetamine Psychosis
Abstract
Objective: Protein interacting with C-kinase-1 (PICK1) plays a role in the targeting and clustering of dopamine transporter, which is the primary target site for the abused drug methamphetamine. Based on the interaction of PICK1 with dopamine transporter, it is of particular interest to investigate the association between the PICK1 gene and methamphetamine abusers. Method: The authors studied the association between PICK1 gene polymorphisms and methamphetamine abusers in a Japanese group. Two hundred and eight methamphetamine abusers and 218 healthy comparison subjects were enrolled in the study. Furthermore, the authors also examined the effects of single nucleotide polymorphisms (SNPs) in the promoter and 5′-untranslated region on transcription levels of PICK1. Results: The authors identified four highly frequent SNPs, rs737622 (–332 C/G) and rs3026682 (–205 G/A) in the promoter region and rs713729 (T/A) in intron3 and rs2076369 (T/G) in intron4. Of these SNPs, rs713729 was significantly associated with methamphetamine abusers in general, and rs713729 and rs2076369 were significantly associated with those with spontaneous relapse of psychosis. Furthermore, haplotype analysis revealed that specific haplotypes of these SNPs were associated with methamphetamine abusers. A gene reporter assay revealed that the two SNPs in the promoter region significantly altered transcriptional activity. Conclusions: Our findings suggest that the PICK1 gene may be implicated in the susceptibility to spontaneous relapse of methamphetamine psychosis and that, as an intracellular adapter protein, PICK1 may play a role in the pathophysiology of methamphetamine psychosis.
Methamphetamine is one of the most widely used illicit drugs, and its abuse continues to be a growing problem worldwide. Accumulating evidence has suggested that genetic factors play a role in vulnerability to methamphetamine abuse and the psychiatric symptoms related to methamphetamine abuse (1 – 5) . The principal target for the action of methamphetamine is the dopamine transporter, which removes dopamine from the extracellular space at the synapse and thereby controls dopamine signals (6 , 7) . Both the activity and the surface availability of the dopamine transporter are believed to be tightly regulated by different cellular mechanisms, the best characterized being modulation by protein kinase C activation (8 , 9) . Recent positron emission tomography (PET) studies of methamphetamine abusers have demonstrated that the density of dopamine transporter is significantly low in the caudate/putamen of methamphetamine abusers (10 , 11) , suggesting that the long-term use of methamphetamine leads to damage of dopaminergic neurons in the human brain. Of interest, the variable number of tandem repeats polymorphism of the human dopamine transporter gene has been shown to be a risk factor for a prognosis of prolonged-type methamphetamine psychosis (12) .
A protein interacting with C kinase (PICK1), one of the PSD95/disk-large/ZO-1 (PDZ) domain-containing synaptic proteins, was originally identified by a yeast two-hybrid system on the basis of its interaction with protein kinase C alpha (13 , 14) . PICK1 plays a role in the targeting and, when serving as a scaffold, in the localization of synaptic membrane proteins such as the dopamine transporter (15) . PICK1 interacts with dopamine transporter through the PDZ domain of PICK1 and the last three residues of the carboxyl terminal of dopamine transporter (16) . Thus, it is likely that the interaction of PICK1 with dopamine transporter results in a clustering of dopamine transporter on the cell surface and a subsequent enhancement of dopamine transporter uptake activity due to an increase in plasma membrane dopamine transporter density in mammalian cells and dopamine neurons in culture.
The PICK1 gene has been mapped to chromosome 22q13.1, a region thought to contain a gene for schizophrenia (17) . It is well known that methamphetamine psychosis is similar to the psychosis associated with schizophrenia (18) . In a case-control study, Hong et al. (19) reported that the PICK1 gene was associated with schizophrenia in the Taiwanese population. Furthermore, in a case-control association study with well-characterized Japanese subjects, Fujii et al. (20) reported an association of the PICK1 gene with schizophrenia, which is more prominent in people with the disorganized type of schizophrenia. Taken together, these findings point to the possibility of an association between the PICK1 gene and methamphetamine psychosis.
The present study was undertaken to examine the association between PICK1 gene polymorphisms and methamphetamine abuse. Using a gene reporter assay, we also investigated the effects of the single nucleotide polymorphisms (SNPs) in the promoter and 5′-untranslated regions on the levels of PICK1 transcription.
Materials and Methods
Subjects
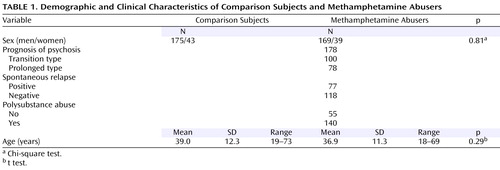
The subjects were 208 patients (169 men and 39 women, ages: mean=36.9 years, SD=11.3, age range=18–69) with methamphetamine dependence and a psychotic disorder meeting the ICD-10-DCR criteria (F15.2 and F15.5) who were outpatients or inpatients of psychiatric hospitals affiliated with the Japanese Genetics Initiative for Drug Abuse and 218 age-, gender-, and geographical origin-matched normal comparison subjects (175 men and 43 women, age: mean=39.0 years, SD=12.3, age range=19–73) with no past history and no family history of drug dependence or psychotic disorders ( Table 1 ). The age of the normal subjects did not differ from that of the methamphetamine abusers ( Table 1 ). The research was performed after approval was obtained from the ethics committees of each institute of the Japanese Genetics Initiative for Drug Abuse, and all subjects provided written informed consent for the use of their DNA samples as part of this study.
Background of Methamphetamine Abusers
Diagnoses were made by two trained psychiatrists based on interviews and available information, including hospital records. Subjects were excluded if they had a clinical diagnosis of schizophrenia, another psychotic disorder, or an organic mental syndrome. All subjects were Japanese and were born and living in restricted areas of Japan, including northern Kyushu, Setouchi, Chukyo, Tokai, and Kanto. The patients were divided into subgroups by characteristic clinical features ( Table 1 ).
Prognosis of Psychosis
The prognosis of methamphetamine psychosis varied among patients, some of whom showed continued psychotic symptoms, even after methamphetamine discontinuance, as previously reported (21 , 22) . Accordingly, the patients were categorized by prognosis into two groups, a transient type and a prolonged type, based on the duration of the psychotic state after methamphetamine discontinuance. The transient type is defined as those whose symptoms improved within 1 month, and the prolonged type is those whose psychosis continued for more than 1 month after methamphetamine discontinuance and the start of treatment with neuroleptics . In this study, there were 100 transient type and 78 prolonged type patients with methamphetamine psychosis ( Table 1 ). One of the issues in categorizing was the difficulty in distinguishing patients who coincidently developed schizophrenia. Therefore, we excluded cases in which the predominant symptoms were of the negative and/or disorganized type in order to maintain the homogeneity of the subgroup.
Spontaneous Relapse
It has been well documented that once methamphetamine psychosis has developed, patients in a state of remission are susceptible to spontaneous relapse without reconsumption of methamphetamine (21 , 22) . It has thus been postulated that a sensitization phenomenon induced by the repeated consumption of methamphetamine develops in the brain of patients with methamphetamine psychosis, which provides a neural basis for an enhanced susceptibility to relapse. Therefore, the patients in this study were divided into two groups according to the presence or absence of spontaneous relapse. In this study, 77 patients underwent a spontaneous relapse, and 118 did not ( Table 1 ).
Polysubstance Abuse
The patients were divided according to polysubstance abuse status; 55 patients had abused only the drug methamphetamine in their lifetime, and 140 patients had abused both methamphetamine and other drugs in the present or past. After methamphetamine abuse, organic solvents and marijuana were the most frequently used substances. Cocaine and heroin were rarely abused in this group of subjects.
Identification of SNPs
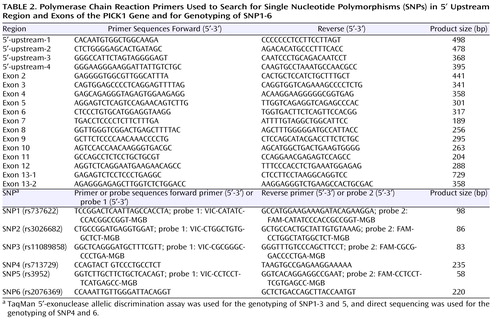
The association between the SNPs of the PICK1 gene and schizophrenia has been reported by two groups. Hong et al. (19) reported a case-control study of the PICK1 gene polymorphism (rs3952) and schizophrenia patients in a Chinese sample. In a Japanese sample, Fujii et al. (20) demonstrated an association between two SNPs (rs713729 and rs2076369) of the PICK1 gene and schizophrenia. However, it remained unclear whether highly common SNPs exist in the 5′-upstream region and the exons of the PICK1 gene in the Japanese population. Therefore, we searched for SNPs in the 5′-upstream region and in all 13 exons with the flanking intronic region of the PICK1 gene using a direct sequencing method. We designed a total of 34 primers for polymerase chain reactions ( Table 2 ) based on information about the PICK1 gene obtained from a public database (the PICK1 gene sequence was assigned as a portion of AL031587, May 18, 2005, i.e., as protein kinase C alpha binding protein; http://www.ncbi.nlm.nih.gov/). Amplification was carried out with an initial denaturation at 95°C for 1 minute, followed by 40 cycles at 95°C for 1 minute, 60°C for 1 minute, and 72°C for 40 seconds, with a final extension at 72°C for 5 minutes. The sequencing reaction was performed on an ABI 310 genetic analyzer (PE Biosystems, Foster City, Calif.) following the manufacturer’s protocol.
For the screening of the 5′-upstream region, pairs of polymerase chain reaction primers were designed to amplify 368–498-bp fragments in approximately 1000 bp of the 5′-upstream region ( Table 2 ). To determine the transcription start position, we used a large-insert cDNA library made from human fetal brain (Clontech Laboratories, Inc., Mountain View, Calif.). Based on SMART technology (Clontech), the cDNA library contains high-fidelity full-length transcripts. We performed polymerase chain reactions with 5′-sequencing primer supplied by the manufacturer and the 5′-3R primer we designed in our laboratory ( Table 2 ). By using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.), the polymerase chain reaction product was cloned into TA plasmids according to the manufacturer’s instructions. Then the inserted 5′-upstream region was direct-sequenced with sequencing primers provided with the TA cloning kit.
For all polymerase chain reaction products, we first analyzed the sequences of the 32 comparison subjects, and we identified three SNPs in the 5′-upstream region and 11 SNPs in the exons and their flanking intronic regions ( Figure 1 ). Of these 14 SNPs, minor allele frequencies of two SNPs in the 5′-upstream region and two SNPs in introns 3 and 4 were more than 10%. By referring to the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/), we confirmed that two of these SNPs in the 5′-upstream region were rs737622 (SNP1) and rs3026682 (SNP2) ( Figure 1 ). Although none of the SNPs was described as highly frequent in all exons observed, we found that rs713729 (SNP4) in intron 3 and rs2076369 (SNP6) in intron 4 were highly frequent; these results are in good agreement with those of a previous study (20) ( Figure 1 ).
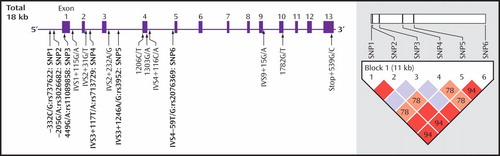
a The rectangles and horizontal lines represent exons and introns, respectively. Of these single nucleotide polymorphisms (SNPs), six (SNPs 1–6, indicated in boldface) were highly frequent. The haplotype block structure with linkage disequilibrium parameters D′ is shown in the right hand panel. The D′ values were calculated from comparison groups.
Genotyping of Identified SNPs
To investigate the putative association between PICK1 gene polymorphisms and methamphetamine abuse, we selected the following SNPs for genotyping: rs737622 (C/G: SNP1), rs3026682 (G/A: SNP2), rs110898858 (G/A: SNP3), rs713729 (T/A: SNP4), and rs2076369 (T/G: SNP6). To compare the present results with those of previous reports (19 , 20) , we also selected rs3952 (A/G: SNP5) for genotyping. For four of these SNPs, i.e., SNP1, 2, 3, and 4, genotyping was performed by TaqMan 5′-exonuclease allelic discrimination assay in accordance with the manufacturer’s protocol. The primers and probes used for these SNPs are shown in Table 2 .
For SNP4 (rs713729) and SNP6 (rs2076369), genotyping was performed by direct sequencing, and the primers used for polymerase chain reactions are shown in Table 2 .
Dual-Luciferase Gene Reporter Assays
Reporter plasmids containing the rs737622 (-332C/G: SNP1), rs3026682 (-205G/A: SNP2), and rs11089858 (449G/A: SNP3) polymorphic sites were constructed, and 1039-bp fragments (from –373 to +666, Figure 2 ) were amplified from the genomic DNAs with the identified genotypes as templates. The polymerase chain reaction primers were as follows: forward, 5′-CGACGCGTCCGGACTCAATTAGCCACCT-3′ (including a Mlu I site) and reverse, 5′-CGCTCGAGTCGGAACCAAGAACGAGAAC-3′ (including an Xho I site). The polymerase chain reaction products of four haplotypes (C-332/G-205/G+449: Pr1, C-332/G-205/A+449: Pr2, G-332/A-205/A+449: Pr3, and G-332/A-205/A+449: Pr4) were cloned into the pGL-3 Basic Plasmid (Promega Corporation, Madison, Wis.). The inserted sequences were confirmed with direct sequencing by using an ABI 310 genetic analyzer (PE Biosystems, Foster City, Calif.) according to the manufacturer’s protocol.

a The numbers indicate the nucleotide positions cited from the NCBI database AL031587. A bold black arrow indicates the transcription start position we identified, which was 513 bp before the start position (114471) reported in the database. Blue characters indicate exons of PICK1, and the translation start codon, ATG, is orange. The positions of the three SNPs we identified are indicated in red.
Two cell lines, human neuroblastoma SK-N-SH and human glioblastoma U-87, were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Luciferase reporter plasmids containing the four haplotypes were transiently transfected into these cells by using the TransFast lipofection reagent (Promega Corporation, Madison, Wis.). The renilla luciferase expression plasmid phRL-TK was cotransfected as an internal standard. After 48 hours, the cells were harvested, and the luciferase reporter activity was measured by using a TD-20/20 luminometer and a Dual-Luciferase Assay Kit (Promega Corporation, Madison, Wis.). All experiments were repeated at least three times.
Statistical Analysis
Allele and genotype frequencies were calculated, and the differences between groups were evaluated with Fisher’s exact test. Case-control haplotype analysis was performed by the maximum-likelihood method by using SNPAlyse (DYNACOM, Yokohama, Japan, http://www.dynacom.co.jp/); p values of haplotypes were obtained by 1000-fold permutation to correct for bias due to multiple tests. For the luciferase assay, one-way analysis of variance (ANOVA) followed by post hoc Bonferroni tests were performed for comparison of relative luciferase activity among four types of inserted vectors. The analysis was performed with SPSS software (SPSS version 12.0J, Tokyo). All statistically significant p values were set at <0.05.
Results
Identification of SNPs and Association Studies
In searching the transcription start position, we found that exon 1 turned out to stretch beyond the position reported in the public database ( Figure 2 ). Namely, we found that the transcription start position was at 113958, which is 513 bp before the start position (114471) reported in AL031587 (http://www.ncbi.nlm.nih.gov/).
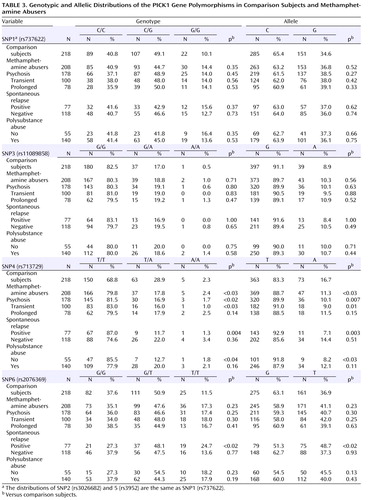
We searched for the SNPs in the PICK1 gene, including the promoter region approximately 500 bp ahead of the transcription start position, the entire 5′-untranslated sequence from the translation start position in exon 2, and all 13 exons and their neighboring sequences. In this study, we found 14 SNPs in the PICK1 gene ( Figure 1 ). Of these SNPs, rs737662 (-332C/G: SNP1), rs3026682 (-205G/A: SNP2), rs11089858 (449 G/A: SNP3), rs713729 (IVS3+117T/A: SNP4), and rs2076369 (IVS4-59T/G: SNP6) were found to be highly frequent (the minor allele >10%) ( Figure 1 ). Subsequent genotyping was performed for these five SNPs (SNP1, 2, 3, 4, and 6) and rs3952 (IVS3+1246A/G: SNP5). Both the genotype and the allele distributions of SNP1, SNP2, and SNP5 were completely the same ( Table 3 ). The allele frequencies and genotype distributions of SNP1, 3, 4, and 6 in methamphetamine abusers and comparison subjects are shown in Table 3 . The genotype distributions were within the Hardy-Weinberg equilibrium.
We found significantly different frequencies between comparison subjects and methamphetamine abusers in SNP4 ( Table 3 ). The frequency (88.7%) of carrying the T allele among the methamphetamine abusers was significantly higher (odds ratio=1.58, 95% confidence interval [CI]=1.06–2.34, p<0.03) than that of the comparison subjects (83.3%), and we also detected a different distribution of genotype (p<0.03). Positive associations were detected in the subgroup of those who experienced psychosis (alleles, p=0.007, odds ratio=1.79, 95% CI=1.17–2.74, genotype, p<0.02), transient-type psychosis (alleles, p=0.01, odds ratio=2.03, 95% CI=1.17–3.51, genotype, p<0.03), and psychosis with spontaneous relapse (alleles, p=0.003, odds ratio=2.61, 95% CI=1.35–5.07, genotype, p=0.004) and in abusers without polysubstance abuse (alleles, p<0.03, odds ratio=2.26, 95% CI=1.09–4.67, genotype, p<0.04) ( Table 3 ). For SNP6, the frequency (48.7%) of the T allele among methamphetamine abusers who experienced psychosis with spontaneous relapse was significantly higher (odds ratio=1.62, 95% CI=1.19–2.35, p<0.02) than that of the comparison subjects (36.9%), and we also detected a different distribution of genotype (p<0.02) ( Table 3 ). In contrast, no differences for SNP1, 2, 3, and 5 were detected between methamphetamine abusers and comparison subjects ( Table 3 ).
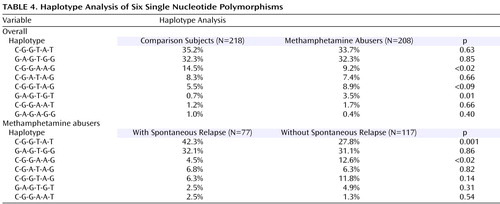
As shown in Figure 1 , a strong linkage disequilibrium was observed in five of these six SNPs. Two haplotypes, C(SNP1)-G(SNP2)-G(SNP3)-A(SNP4)-A(SNP5)-G(SNP6) and G(SNP1)-A(SNP2)-G(SNP3)-T(SNP4)-G(SNP5)-T(SNP6), were significantly different between comparison subjects and methamphetamine abusers ( Table 4 ). The frequency (9.2%) of the CGGAAG haplotype in the methamphetamine abusers was significantly lower (odds ratio=0.60, 95% CI=0.45–0.79, p<0.02) than that of the comparison subjects (14.5%), and the frequency (3.5%) of the GAGTGT haplotype in the methamphetamine abusers was significantly higher (odds ratio=5.2, 95% CI=2.27–11.6, p=0.01) than that (0.7%) of the comparison subjects ( Table 4 ). Of interest, a haplotype analysis between methamphetamine abusers with and without spontaneous relapse of psychosis showed the significant difference in the most major haplotype (CGGTAT) as well as the CGGAAG type. The frequency (42.3%) of CGGTAT type in the methamphetamine abusers with spontaneous relapse was significantly higher (odds ratio=2.2, 95% CI=1.80–2.61, p=0.001) than that in those without spontaneous relapse (27.8%) ( Table 4 ). As to the frequency of the CGGAGG type, the frequency (4.5%) in methamphetamine abusers with spontaneous relapse was significantly lower (odds ratio=0.33, 95% CI=0.23–0.47, p<0.02) than that in those without spontaneous relapse ( Table 4 ).
Transcriptional Effects of SNPs in the Promoter Region
The transcriptional effects of four promoter haplotypes on SK-N-SH cells and U-87 cells were also examined. As shown in Figure 3 , the results for these two cell lines differed. For SK-N-SH cells, a substitution variant, Pr3 (G-332/A-205/A+449), showed significantly increased relative luciferase activity (1.54 for Pr3/Pr1, p<0.001, 2.03 for Pr3/Pr2, p<0.001, 1.74 for Pr3/Pr4, p<0.001). In contrast, for U-87 cells, every substitution showed significantly lower relative luciferase activity than that of the major type, Pr1 (C-332/G-205/G+449) (0.51 for Pr2/Pr1, p<0.001, 0.51 for Pr3/Pr1, p<0.001, 0.59 for Pr4/Pr1, p<0.001).
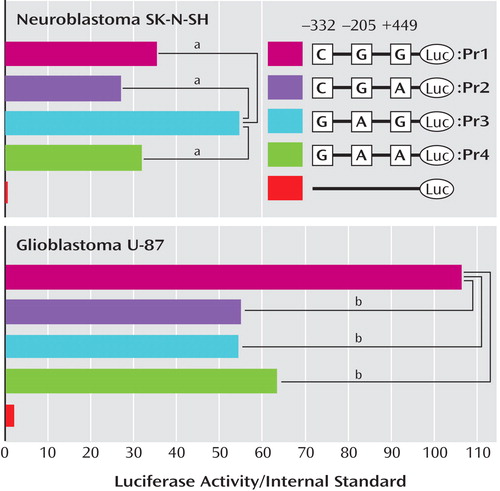
a The phRL-TK vector used was a negative control. The pGL3 Basic vector, which does not contain any promoter sequences, was used as a negative control. Each value is shown as the mean for three independent experiments.
b p<0.001.
Discussion
The major findings of the present study were the discovery of an association between PICK1 gene polymorphisms and methamphetamine abusers and the identification of functional SNPs (SNP1 and SNP2) in the promoter region of the PICK1 gene. It was of great interest to find that SNP4 and SNP6 were significantly associated with methamphetamine abusers who experienced spontaneous relapse of psychosis. In addition, the haplotype analysis demonstrated that specific haplotypes, C(SNP1)G(SNP2)G(SNP3) A(SNP4)A(SNP5)G(SNP6) and GAGTGT, were significantly associated with methamphetamine abusers in general. Furthermore, we also found that the frequencies of major haplotypes CGGTAT and CGGAAG were significantly different between methamphetamine abusers with and without spontaneous relapse of psychosis. Spontaneous relapse of psychosis among methamphetamine abusers is known as “flashbacks,” which are known to follow nonspecific stress, even after the consumption of methamphetamine has ceased and drug treatment has begun, and it appears that a psychotic state might be induced by excess dopaminergic activity (21 , 22) . Given the role of dopamine systems in the pathogenesis of methamphetamine psychosis, it is possible that a functional alteration of dopamine transporter may be caused by genetic variations in PICK1 and can lead to dysfunction of the dopamine system. Taken together, these results suggest that the CGGTAT and CGGAAG haplotypes in the PICK1 gene are likely to be associated with the psychosis of methamphetamine abusers who experience spontaneous relapse. The different distributions of those two haplotypes between methamphetamine abusers with and without spontaneous relapse of psychosis also suggest the difference in genetic backgrounds between the two groups. In the present study, the group of subgroups was small. Because of the small size of subcategories, type I error cannot be ruled out. Therefore, further studies with a large group with subcategories would reveal the associations between the PICK1 gene and methamphetamine-induced psychosis.
In the 5′-upstream region of the PICK1 gene, we identified three SNPs (SNP1: –332 C/G, rs737622, SNP2: -205 G/A, rs3026682, and SNP3: 449G/A, rs11089858). A luciferase assay revealed the functional effects of these SNPs on transcriptional activities. Although the threshold scores were low, the TFSEARCH program (http://mbs.cbrc.jp/research/db/TFSEARCH.html) predicted that the major transcription factors, including GATA1 (for SNP1, score 78.3) and AML-1a (for SNP2, score 83.7), bind to either position of SNPs in the PICK1 promoter position. Of course, it is likely that unidentified transcription factors may also be involved in the transcriptional process because we found that the levels of PICK1 expression could be altered by nucleotide substitutions of these SNPs in the promoter region. After consideration of the role of PICK1 in the proper targeting and surface clustering of dopamine transporter (16) , it is possible that altered PICK1 expression might lead to altered dopamine transporter function in synaptic dopamine signal transmission, which would in turn influence the pathogenesis of methamphetamine abuse and related psychotic symptoms.
In this study, we found that transcriptional effects of SNPs in the promoter region of the PICK1 gene differed in SK-N-SH and U-87 cells. The nucleotide substitutions (C→G at –332 and G→A at –205) showed significantly increased luciferase activity in SK-N-SH cells (neuronal cells), whereas the substitutions (C→G at –332 and G→A at –205) showed significantly decreased luciferase activity in U-87 cells (glial cells). Although the mechanisms underlying the discrepancy in these two cell lines are currently unknown, these findings suggest that PICK1 expression could be affected in different ways by these SNPs in neuronal and glial cells. Fujii et al. (20) reported that a haplotype, T(rs713729)-A(rs3952)-T(rs2076369), revealed a statistically significant association with disorganized schizophrenia in methamphetamine abusers in relation to comparison subjects (p<0.02). The TAT haplotype, discussed by Fujii and coworkers, was found to correspond to C(rs737622: SNP1)-G(rs3026682: SNP2)-G(rs11089858: SNP3)-T(rs713729: SNP4)-A(rs3952: SNP5)-T(rs2076329: SNP6) in our study, and it was the most frequent haplotype in both comparison subjects and methamphetamine abusers. As discussed, the frequency (42.3%) of the CGGTAT haplotype in methamphetamine abusers with spontaneous relapse was significantly higher (p=0.001) than that of those without spontaneous relapse (27.8%). These findings also suggest that methamphetamine abusers who experience a spontaneous relapse of methamphetamine psychosis might share a similar genetic susceptibility to schizophrenia.
It has been demonstrated that PICK1 interacts with other proteins, including AMPA receptors (14 , 23) and metabotropic glutamate receptor 7 (mGluR7) (24 , 25) , which have been implicated in the pathophysiology of drug abuse as well as in schizophrenia (26 – 29) . Thus, it seems that interactions of PICK1 with AMPA receptors and metabotropic glutamate receptors are likely to be involved in the pathogenesis of methamphetamine psychosis. Furthermore, Fujii et al. (20) identified PICK1 as a protein interactor with the D-serine synthesizing enzyme serine racemase in glial cells (30) . After consideration of the role of D-serine in the pathophysiology of schizophrenia (31 – 35) , it is likely that the interaction of PICK1 with serine racemase in glial cells may play a role in the pathophysiology of methamphetamine psychosis, although further studies will still be necessary.
In conclusion, the present findings revealed that PICK1 gene polymorphisms are associated with methamphetamine abusers, suggesting that the PICK1 gene plays a major role in a genetic susceptibility to methamphetamine psychosis.
1. Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ: Familial transmission of substance use disorders. Arch Gen Psychiatry 1998; 55:973–979Google Scholar
2. Kosten TR, Markou A, Koob GF: Depression and stimulant dependence: neurobiology and pharmacotherapy. J Nerv Ment Dis 1998; 186:737–745Google Scholar
3. Kendler KS, Karkowski LM, Neale MC, Prescott CA: Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry 2000; 57:261–269Google Scholar
4. Uhl GR, Liu QR, Naiman D: Substance abuse vulnerability loci: converging genome scanning data. Trends Genet 2002; 18:420–425Google Scholar
5. Goldman D, Oroszi G, Ducci F: The genetics of addictions: uncovering the genes. Nat Rev Genet 2005; 6:521–532Google Scholar
6. Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG: Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci 1998; 18:4861–4869Google Scholar
7. Frey K, Kilbourn M, Robinson T: Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol 1997; 334:273–279Google Scholar
8. Blakely RD, Bauman AL: Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol 2000; 10:28–36Google Scholar
9. Robinson MB: Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem 2002; 80:1–11Google Scholar
10. Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 2001; 158:377–382Google Scholar
11. Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N: Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 2001; 158:1206–1214Google Scholar
12. Ujike H, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y, Sora I, Iyo M, Katsu T, Nomura A, Nakata K, Ozaki N: Nine- or fewer repeat alleles in VNTR polymorphism of the dopamine transporter gene is a strong risk factor for prolonged methamphetamine psychosis. Pharmacogenomics J 2003; 3:242–247Google Scholar
13. Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN: Pick1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol 1995; 128:263–271Google Scholar
14. Xia J, Zhang X, Staudinger J, Huganir RL: Clustering of AMPA receptors by the synaptic PDZ domain-containing protein pick1. Neuron 1999; 22:179–187Google Scholar
15. Deken SL, Beckman ML, Quick MW: Picking on transporters. Trends Neurosci 2001; 24:623–625Google Scholar
16. Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, Caron MG: Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 2001; 30:121–134Google Scholar
17. Stober G, Meyer J, Nanda I, Wienker TF, Saar K, Knapp M, Jatzke S, Schmid M, Lesch KP, Beckmann H: Linkage and family-based association study of schizophrenia and the synapsin III locus that maps to chromosome 22q13. Am J Med Genet 2000; 96:392–397Google Scholar
18. Snyder SH: Catecholamines in the brain as mediators of amphetamine psychosis. Arch Gen Psychiatry 1972; 27:169–179Google Scholar
19. Hong CJ, Liao DL, Shih HL, Tsai SJ: Association study of PICK1 rs3952 polymorphism and schizophrenia. Neuroreport 2004; 15:1965–1967Google Scholar
20. Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J, Yamada T, Ozeki Y, Kawahara R, Okawa M, Huganir RL, Ujike H, Snyder SH, Sawa A: Serine racemase binds to PIC1: potential relevance to schizophrenia. Mol Psychiatry 2006; 11:150–157Google Scholar
21. Sato M, Chen CC, Akiyama K, Otsuki S: Acute exacerbation of paranoid psychotic state after long-term abstinence in patients with previous methamphetamine psychosis. Biol Psychiatry 1983; 18:429–440Google Scholar
22. Sato M, Numachi Y, Hamamura T: Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr Bull 1992; 18:115–122Google Scholar
23. Hanley JG, Henley JM: PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J 2005; 24:3266–3278Google Scholar
24. Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S: PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci 2000; 20:7252–7257Google Scholar
25. Perroy J, El Far O, Bertaso F, Pin JP, Betz H, Bockaert J, Fagni L: PICK1 is required for the control of synaptic transmission by the metabotropic glutamate receptor 7. EMBO J 2002; 21:2990–2999Google Scholar
26. Bellone C, Luscher C: Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGLUR-dependent long-term depression. Nat Neurosci 2006; 9:636–641Google Scholar
27. Kenny PJ, Markou A: The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 2004; 25:265–272Google Scholar
28. Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW: Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 2003; 421:70–75Google Scholar
29. Javitt DC: Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry 2004; 9:984–997Google Scholar
30. Wolosker H, Blackshaw S, Snyder SH: Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA 1999; 96:13409–13414Google Scholar
31. Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M: Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 2003; 60:572–576Google Scholar
32. Hashimoto K, Shimizu E, Iyo M: Dysfunction of glia-neuron communication in pathophysiology of schizophrenia. Curr Psychiatry Rev 2005; 1:151–163Google Scholar
33. Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, Nakamura K, Shimizu E, Itokawa M, Mori N, Iyo M, Yoshikawa T: Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry 2005; 57:1493–1503Google Scholar
34. Boehning D, Snyder SH: Novel neural modulators. Annu Rev Neurosci 2003; 26:105–131Google Scholar
35. Mustafa AK, Kim PM, Snyder SH: D-serine as a putative glial neurotransmitter. Neuron Glia Biol 2004; 1:275–281Google Scholar