Fronto-Striatal Overactivation in Euthymic Bipolar Patients During an Emotional Go/NoGo Task
Abstract
Objective: Although euthymic bipolar patients show minimal manic and depressive symptoms, they continue to show impaired emotional regulation and cognitive functioning. Few studies have directly examined the interference of emotional information with cognitive processes and its underlying cerebral mechanisms in euthymic bipolar patients. The authors examined the emotional modulation of cognitive processes and its underlying neural mechanisms in euthymic bipolar patients. Method: Seventeen euthymic bipolar patients and 17 healthy comparison subjects underwent functional magnetic resonance imaging (MRI) while performing an emotional and nonemotional go/nogo task. Neural responses associated with the overall task performance, as well as with the impact of emotional information on the task performance, were assessed. Results: Bipolar disorder patients exhibited increased activity in the temporal cortex, specifically to emotional go/nogo conditions, as well as in the orbitofrontal cortex, the insula, the caudate nuclei, and the dorsal anterior and posterior cingulate cortices when inhibiting emotional stimuli compared with neutral stimuli. Conversely, no global attentional deficits were observed on either a behavioral or neural response level, indicated by similar task performances for all task conditions and similar brain activation patterns when comparing all the go/nogo conditions with the resting state. Conclusions: The present study provides evidence of an altered emotional modulation of cognitive processing in euthymic bipolar patients, indicated by an overactivation in ventral-limbic, temporal, and dorsal brain structures during emotional go/nogo conditions in patients relative to comparison subjects.
Bipolar disorder has been associated with deficits in emotional processing (1 , 2) and impaired executive functioning (3) that persist even in euthymic states, during which patients with bipolar disorder show minimal manic and depressive symptoms. It has been suggested that the emotional dysregulation accounts for cognitive disturbances by reciprocal interactions between cognitive and emotional brain networks (4) . However, direct evidence of the persisting cognitive-emotional interference in euthymic bipolar patients and its underlying neural mechanisms is scarce (5) .
In light of neurobiological models of normal emotion perception (6) and emotional dysregulation in affective disorders (4 , 7 , 8) , the supposed cognitive-emotional interference in affective disorders has been attributed to a lack of inhibitory feedback mechanisms, arising from the following two functionally altered neural routes: first, a hypoactive dorsal-cognitive network, including the dorsolateral/dorsomedial prefrontal cortices, the dorsal anterior cingulate cortex, and the posterior cingulate cortex, which has been involved in attentional executive processing (9) ; second, a hyperactive ventral-limbic network encompassing the ventrolateral prefrontal cortex, the orbitofrontal cortex, the ventral/subgenual anterior cingulate cortex, the amygdala, the insula, the hypothalamus, and the striatum that has been implicated in emotional regulation and socioemotional behaviors (6) . Because of its cytoarchitectural characteristics and reciprocal connections with both ventral and dorsal networks, the rostral anterior cingulate cortex is considered to serve as a functional link between these circuits, ensuring a normal integrative processing of mood and cognitive behaviors (7) .
In line with the previously described models, functional magnetic resonance imaging (fMRI) studies (10 , 11) have provided preliminary evidence of an increased activity in ventral-limbic brain structures during a purely cognitive-attentional task in euthymic bipolar patients with preserved behavioral performances. It has been hypothesized that patients with bipolar disorder attach an emotional valence to a task for which no processing of emotional information is required. These findings (10 , 11) indeed suggest an impact of sustained emotional dysregulation on cognitive control in remitted bipolar disorder patients. However, a direct interaction between these two processes and their relative impact on each other cannot be drawn from these previous studies, since the used tasks did not require the processing of emotional information.
To date, only one study has investigated the cognitive-emotional interference and its underlying neural mechanisms in remitted bipolar disorder patients (5) by using an emotional Stroop task. Whereas the patients’ task accuracy was preserved, remitted bipolar disorder patients showed a decreased activation in the ventral-limbic brain network as well as in the superior and middle temporal gyri in response to affective words. Yet, as no nonemotional control task was employed in that study (5) , it remains unclear whether these functional alterations represent a specific correlate of an altered emotion-cognition interaction in bipolar disorder patients or whether they represent generally altered activation patterns in response to cognitive demands, regardless of their emotionality. Indeed, a decreased activity in similar brain regions was observed in two other studies, employing a nonemotional attentional task (color-word Stroop test) (12 , 13) .
Based on the models we have described and previous findings in bipolar disorders, we tested the hypothesis of an increased cognitive-emotional interference in bipolar disorder patients that would be associated with altered neural response patterns in brain regions of the ventral-limbic network and superior/middle temporal cortices that have been previously shown to be altered in euthymic and symptomatic bipolar disorder patients (10 , 11 , 14) and that are involved in emotion regulation and emotion recognition. Furthermore, we hypothesized between-group differential activations in structures of the dorsal brain network implicated in attentional control.
To test our hypotheses, we conducted an fMRI go/nogo paradigm comprising both emotional and nonemotional task conditions. This paradigm was chosen because emotional go/nogo tasks (verbal or facial stimuli) have shown to activate parts of the ventral and dorsal neural pathway in healthy subjects (15 , 16) . Furthermore, the emotional go/nogo paradigm appears to produce differential brain activation patterns in prefrontal and cingulate cortices as well as in the thalamus and the insula in patients with mania (17) and recurrent unipolar depression (18) . Finally, response inhibition seems to be a sensible mechanism not only in symptomatic but also euthymic bipolar patients, since recent findings have revealed a disrupted response inhibition brain network, involving the middle frontal gyrus, the middle and superior temporal gyri, and the striatum (12) in those patients.
Method
Subjects
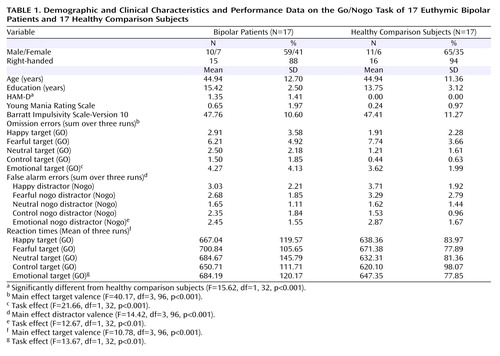
Twenty euthymic patients with bipolar disorder were recruited from the Psychiatry Department of Albert Chenevier and Henri Mondor Hospitals (Créteil, France). Euthymia was defined by a Hamilton Depression Rating Scale (HAM-D) (19) score <5 and a Young Mania Rating Scale (20) score <8 for at least 8 weeks. Nineteen healthy comparison subjects were recruited through public announcements and were closely matched to the patients’ age, gender, level of education, and handedness. Three bipolar disorder patients and two healthy comparison subjects were discarded from fMRI analysis because of excessive head movements and susceptibility artifacts. Thus, 17 bipolar disorder patients and 17 healthy comparison subjects were included in the final analyses ( Table 1 ).
Inclusion criteria for study participation comprised no history of alcohol or drug abuse/dependence, no history of mental retardation, and no current or past cardiac or neurological disease. In addition, healthy comparison subjects were free of any past or present psychiatric disorder (as assessed by the Mini-International Neuropsychiatric Interview [21] ). The study was approved by the local Bicêtre Ethics Committee. After complete study description to the subjects, written informed consent was obtained.
Clinical Assessment
Patients with bipolar disorder were diagnosed by experienced research clinicians, using the French version of the Diagnostic Interview for Genetic Studies (22) . Ten bipolar disorder patients were diagnosed with bipolar I disorder and seven with bipolar II disorder. The mean age of illness onset was 22.6 (SD=10.5) years and illness duration was 21.9 (SD=12.7) years. One patient fulfilled the criteria for a comorbid panic disorder. Two patients were medication-free, and 15 were taking psychotropic medication. Among those who were on regular medication, seven received a mood-stabilizing monotherapy (lithium, valproate) and eight a combined pharmacological treatment (e.g., mood stabilizer, atypical antipsychotics, serotonin reuptake inhibitors).
All subjects were administered the HAM-D and the Young Mania Rating Scale. Additionally, they completed the French version of the Barratt Impulsivity Scale, Version 10 (23) . Handedness was assessed using the Edinburgh Inventory (24) .
Task Procedure
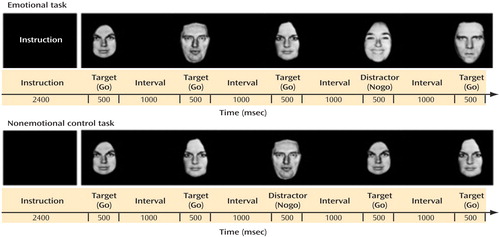
We used a go/nogo fMRI paradigm ( Figure 1 ) with emotional task conditions comprising fearful, happy, and neutral facial stimuli (pictures of facial affect [25] ) and nonemotional control task conditions containing neutral female and male faces. Subjects were presented with active task blocks, where they had to respond as quickly as possible to a target emotion (Go) and to withhold the response to a distractor emotion (Nogo), and with a block of rest in which only a fixation cross was presented. A fully factorial design, in which each emotional expression served once as target (Go) and once as distractor (Nogo), was used (e.g., Go happy-Nogo neutral; Go fearful-Nogo happy), resulting in six emotional go/nogo conditions. In addition, two control conditions, in which targets and distractors were defined on the basis of the faces’ gender, were included (Go male-Nogo female; Go female-Nogo male). The task was designed as a block paradigm, in which each of the previously described conditions represented one block (36 s). In each block, 16 targets and eight distractors were presented for 500 msec in a pseudorandomized order, followed by a 1000 msec interstimulus interval. Prior to each condition/block, subjects were given a written instruction (2400 msec) indicating to which type of face they should respond. Responses and reaction times were recorded online. A total of three functional runs were conducted, each comprising all active task conditions in a randomized order and the block of rest at the end of the run.
Imaging Procedure
Participants were trained on the task prior to the scanning session. During the imaging procedure, the stimuli were rear-projected from a computer onto a screen, which was visible by the subject via a mirror placed above the head coil. The stimulus presentation was programmed using E-Prime (Psychology Software Tools, Inc. [www.pstnet.com]), which also recorded the subjects’ responses and reactions times. Subjects gave their responses with their right thumb via a single button.
One high-resolution T1-weighted, three-dimensional magnetic resonance imaging (MRI) sequence (slice thickness=1.3 mm; field of view=24 cm; 256×256×128 matrix) as well as three functional runs with a length of 10 minutes and 36 seconds each were acquired on a 1.5 T GE Signa scanner. For each functional run, 265 volumes, each comprising 36 T2-weighted interleaved axial slices (slice thickness=3 mm) were acquired, using a single shot echo planar sequence (time to repeat=2400 msec, echo time=35 msec, flip angle=90°, 64×64 matrix). Because of the T1 stabilization process, the first five volumes of each functional run were discarded from further analyses.
Data Analysis
Imaging data were preprocessed and analyzed according to standard procedures using statistical parametric mapping (SPM2) (http://www.fil.ion.ucl.ac.uk/spm). Subjects were excluded from fMRI analysis when they exceeded minimum movement thresholds of 2.5 mm or 2° in any direction.
Functional scans were realigned to the first scan of each run as reference. Functional and anatomical data were coregistered, and fMRI data were normalized into the Talairach and Tournoux stereotaxic space (26) using MNI templates. During normalization, images were resampled every 4 mm using sinc interpolation and were smoothed with an 8×8×8 mm Gaussian kernel to decrease spatial noise.
We performed individual statistical analyses, using a General Linear Model with a delayed boxcar waveform to model blood oxygen level dependent signal changes to the different conditions. To remove variations in signal because of movement artifacts, movement parameters calculated during the realignment were included in the model as parameters of no interest.
For each subject, one image for each of the following three contrasts of interest was calculated: (1) all go/nogo conditions versus the resting state, in order to assess the activation related to global attentional effects, independent of the task’s emotional valence; (2) emotional versus control (i.e., nonemotional) go/nogo conditions for assessing the impact of emotional information on the task performance, given that the cognitive demand was equivalent for both task conditions; and (3) within emotional task conditions, those with emotional distractors (fearful, happy) versus those with neutral distractors, to estimate the effects of emotional information on response inhibition.
For each contrast of interest, these individual statistical parametric maps were smoothed using a 6 mm Gaussian kernel and entered in two types of second-level, random-effects analyses in order to generate the following: (1) group-specific voxelwise t statistics (one-sample t tests) in order to assess significant activation clusters within each experimental group and (2) between-group voxelwise t statistics (two-sample t test) in order to assess significant group differences. T statistics were normalized to Z scores to provide a statistical measure that was independent of the cohort size.
For brain regions where we had anatomical a priori hypothesis, significant clusters of activation were defined with a height threshold of p voxel-level <0.001 (uncorrected; Z>3.00) and retained when they passed an extent threshold of p cluster-level <0.05 (k=15 voxels) (18 , 27 , 28) .
Since the aim of this study was to identify brain regions where bipolar disorder patients differed significantly from healthy comparison subjects, results of the within-group analyses are reported only for those contrasts that did not yield significant group differences. Otherwise, results from the between-group analyses are reported.
The task performance data (reaction times; omission errors=no response to go trials; false alarm errors=response to nogo trials) were submitted to repeated-measures analyses of variance (ANOVAs) with the group as between-subject factors, and the emotional performance data (happy, fearful, neutral, control) were submitted as within-subject factors. Additionally, one repeated-measure ANOVA, including the pooled data for the emotional and nonemotional (control) go/nogo conditions, was performed. For group differences in demographical and clinical data, one-way ANOVAs were performed.
Results
Demographics and Task Performance
Euthymic patients with bipolar disorder and healthy comparison subjects were closely matched with regard to demographic variables. Whereas no significant between-group differences were observed for manic symptoms and impulsivity, bipolar disorder patients displayed significantly higher, although very low, HAM-D scores.
Euthymic bipolar patients and healthy comparison subjects performed similarly on the task with respect to both omission errors (F=0.31, df=1, 32, p=0.58), false alarm errors (F=0.05, df=1, 32, p=0.83), and reaction times (F=1.06, df=1, 32, p=0.31). For all performance data, a main effect of the target/distractor type was found ( Table 1 ). No significant interactions with the experimental group were found. Analyses of the pooled data of all emotional go/nogo and the control go/nogo conditions revealed significant task effects ( Table 1 ), indicating more omission and false alarm errors and longer reaction times for the emotional go/nogo relative to control go/nogo conditions in all subjects.
Imaging Results
The between-group comparisons between all go/nogo conditions and the rest revealed no significant regional brain activation differences. Within-group regional activations are displayed in Figure 2 . A table containing within-group regional activation for the contrast “all go/nogo conditions versus rest” is presented in a supplemental table accompanying the online version of this article.
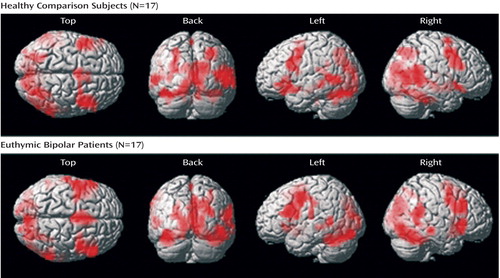
Both groups showed increased neural responses bilaterally in the inferior frontal cortex (Brodmann’s area 44), the precentral gyrus (Brodmann’s area 6), the supplemental motor area (Brodmann’s area 6), the superior temporal gyrus (Brodmann’s area 22/41), the middle temporal gyrus (Brodmann’s area 20/21), the right superior parietal gyrus (Brodmann’s area 7), the left supramarginal gyrus (Brodmann’s area 40/48), as well as primary and associative visual regions (cuneus: Brodmann’s area 17/18/19; calcarine: Brodmann’s area 17/18; middle occipital gyrus: Brodmann’s area 18/19; lingual gyrus: Brodmann’s area 17/18). Additionally, healthy comparison subjects activated the right and left superior temporal poles (Brodmann’s area 38).
Further, both groups exhibited increased neural responses in the hippocampus bilaterally, the left parahippocampal gyrus, the thalamus, the left insula, the striatum (putamen, caudate), the amygdala bilaterally, and the cerebellum bilaterally.
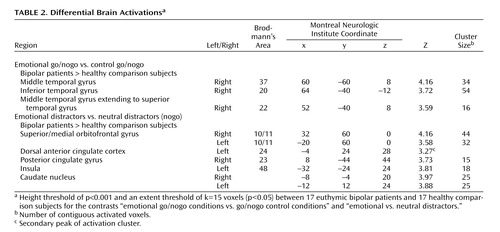
The contrast between emotional go/nogo and nonemotional go/nogo control conditions revealed increased neural responses in the right inferior temporal gyrus (Brodmann’s area 20), middle temporal gyrus (Brodmann’s area 37), and superior temporal gyrus (Brodmann’s area 22) in bipolar disorder patients relative to healthy comparison subjects ( Table 2 , Figure 3 ). No region was significantly more activated in healthy comparison subjects relative to bipolar disorder patients.
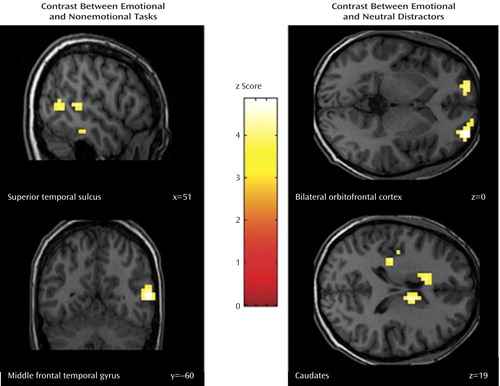
a Contrast between a) the emotional and nonemotional task conditions in the superior temporal sulcus (upper left panel) and middle temporal gyrus (lower left panel) and b) emotional and neutral distractors in the bilateral orbitofrontal cortex (upper right panel) and the caudates (lower right panel). Numbers in the lower right corner indicate the respective coordinate in the Talairach space.
Moreover, euthymic bipolar patients showed increased brain activation to emotional versus neutral distractors in the right medial and left superior orbitofrontal cortices (Brodmann’s area 10/11), the left anterior cingulate cortex (Brodmann’s area 24), the right precuneus extending to the posterior cingulate cortex (Brodmann’s area 23), the left insula (Brodmann’s area 48), and in the caudate bilaterally ( Table 2 , Figure 2 ).
Discussion
Increased neural responses were detected in euthymic bipolar patients in predominantly ventral-limbic regions, but they were also detected in dorsal brain regions during the inhibition of responses to emotional stimuli in an affective go/nogo task. Furthermore, patients with bipolar disorder recruited additional structures in the temporal cortex (inferior temporal gyrus, superior temporal sulcus) during the emotional task conditions relative to healthy comparison subjects. In contrast to these emotion-specific neural differences, euthymic patients with bipolar disorder showed no behavioral performance deficit on either the emotional or nonemotional go/nogo task, suggesting different neural mechanisms in euthymic bipolar patients to reach a similar performance level than healthy subjects.
When all go/nogo conditions were compared with the resting state, no regional brain activation differences between the groups were observed. Yet, both groups revealed increased activation in brain networks hypothesized to be involved in such activation paradigm: a fronto-striatal network known to mediate response inhibition (29) (inferior frontal cortex, supplemental motor area, striatum) and a distributed network that has been documented to play a major role in emotion and face recognition (30) as well as social cognition (31) (prefrontal cortex, insula, parahippocampal gyrus, amygdala, superior temporal gyrus, middle temporal gyrus, occipital gyrus). The lack of significant group differences, together with the preserved task performance, suggests that there are no global cognitive-attentional deficits in euthymic patients with bipolar disorder.
In contrast, we observed significant group differences in brain activation patterns resulting from those comparisons that assessed specifically the emotional modulation of the required cognitive operations during the go/nogo tasks.
When comparing the emotional with the nonemotional go/nogo conditions, where attentional demands were matched, bipolar disorder patients showed increased activation in the right middle temporal gyrus extending to the superior temporal gyrus (encompassing the superior temporal sulcus) and the right inferior temporal gyrus, relative to healthy comparison subjects. The observed increased activity in this region might suggest that patients with bipolar disorder rely on the recruitment of different neural structures, to achieve similar behavioral performances than healthy subjects when emotional information interferes with cognitive processing. On this note, a specifically enhanced activity in the right superior temporal sulcus for selective attention to facial emotion compared with attention to the face per se has been documented in a recent report (32) . Thus, our finding of an increased superior temporal sulcus and inferior temporal gyrus activity to emotional go/nogo task conditions in euthymic patients with bipolar disorder might indicate enhanced attentional processes in bipolar disorder patients when identifying the expressed emotion in another person’s face compared with its gender. Furthermore, patients with bipolar disorder may have allocated more cerebral resources for selecting relevant information and for inhibiting irrelevant information, specifically when it was emotional, indicating a prominent impact of emotional information on executive functioning.
Consistently, the comparison between emotional and neutral distractors revealed an increased neural response in a fronto-striatal circuit (bilateral orbitofrontal cortex, caudates), the dorsal anterior cingulate cortex, the insula, and the posterior cingulate cortices in patients with bipolar disorder, relative to healthy subjects. Interestingly, Elliott et al. (15) similarly reported increased orbitofrontal activation to happy distractors relative to neutral distractors in manic bipolar disorder patients and to sad versus neutral distractors in depressed patients with preserved task performances. Since the orbitofrontal cortex has consistently been shown to be activated when subjects were required to withhold a prepotent response (29 , 33) , an enhanced orbitofrontal response might thus indicate that irrelevant emotional information (i.e., distractors) captures more attention in euthymic bipolar patients and places greater demands on inhibition mechanisms than in healthy comparison subjects. Consequently, they have to recruit increased neural resources in brain regions that mediate response inhibition and impulse control to achieve a similar level of behavioral accuracy. Consistently, in the present study, bipolar disorder patients additionally overactivated the left and right caudate, a region which is known to be involved in inhibitory processes (16 , 34) .
Our finding of increased neural activity in ventral-limbic structures confirms previous results in euthymic bipolar patients (10 , 11) that used cognitive tasks. However, our results contradict those of Malhi et al. (5) , who observed diminished neural responses in the ventrolateral prefrontal cortex, the superior temporal gyrus, the middle temporal gyrus, as well as the basal ganglia in response to affective versus neutral words in euthymic bipolar patients, relative to healthy comparison subjects during an emotional Stroop task. Based on recent findings of a disturbed social perception and social cognition in remitted and symptomatic pediatric bipolar disorder patients (35) , the results of the present study may be interpreted as an enhanced neural response specifically to facial emotional stimuli, i.e., cues that provide socially relevant information.
In contrast to previous studies (13 , 36) , bipolar disorder patients in this study further exhibited an increased neural response in structures of the dorsal-cognitive network, i.e., dorsal anterior cingulate cortex, posterior cingulate cortex. Given the functional role of the dorsal anterior cingulate cortex and posterior cingulate cortex, this overactivation might indicate an increased selective attention and higher cognitive demands (37) in bipolar disorder patients relative to healthy comparison subjects, and thus is in line with the increased neural response in the orbitofrontal cortex and striatum.
Limitations
The findings of the present study have to be considered in light of some limitations. First, nearly all bipolar disorder patients were on psychotropic medication. However, since no global group differences in brain activation patterns (all go/nogo versus rest) were observed, a systematic effect of the medication on neural responses is unlikely. Yet, in line with Lawrence et al. (38) , we assessed possible confounding medication effects in greater detail by dividing patients with bipolar disorder into different medication-related subgroups (medication-free, lithium, anticonvulsive, antipsychotic, antidepressant) and comparing individual mean signal changes (extracted with MarsBaR, implemented in SPM2 [http://marsbar.sourceforge.net/]) in those brain regions that revealed significant group differences. No significant differences between the patient subgroups were identified (Kruskal-Wallis: all χ 2 <1.80, all p>0.40). Compared with healthy comparison subjects, each subgroup exhibited significantly increased neural responses in the respective brain regions (Mann-Whitney: all Z<–2.12; all p<0.05, corrected for multiple comparisons).
Second, the combination of bipolar I and bipolar II disorder patients in our cohort might have affected the significant group differences. However, additional statistical parametric mapping whole-brain analyses (two-sample t test) for the contrasts of interest (emotional versus control go/nogo; emotional versus neutral distractors) revealed no significant differences between bipolar I and bipolar II disorder patients. Likewise, no differences were observed when limiting analyses to those regions differentially activated by healthy comparison subjects and bipolar disorder patients. This is consistent with a recent literature review reporting that no significant anatomical or functional differences between these two subgroups could be inferred from the existing literature (39) .
Conclusions
Given the similarities between the findings from our cohort of remitted patients with bipolar disorder and those from emotional go/nogo studies in manic bipolar (17) and depressed patients (18) , the present findings further suggest that an overactivation of brain structures mediating emotion recognition, selective attention, and response inhibition could represent a trait-marker of bipolar disorder. This hypothesis is strongly supported by a recent study by Chang et al. (40) in euthymic adolescent patients with familial bipolar disorder, reporting an overactivation in the orbitofrontal cortex, anterior cingulate cortex, and striatum to positive valenced stimuli with preserved task performance. The hypothesized trait abnormalities seem to operate at a uniquely neural and not behavioral level, since no behavioral deficits were observed in the respective studies (17 , 18 , 38) . However, from the present data, we can only speculate about possible trait abnormalities, since we did not investigate the same patients in their euthymic, manic, and depressive states, nor did we investigate nonaffected relatives of the patients. Such studies, although difficult to conduct, would help in delineating trait markers of the pathology.
In conclusion, the present study provides evidence of a cognitive-emotional interference in euthymic bipolar patients. Since both groups examined performed similarly on the go/nogo tasks and since no global activation difference has been detected, the observed emotion-specific neural differences highlight an altered emotional modulation of cognitive processing in euthymic bipolar patients.
1. Harmer CJ, Grayson L, Goodwin GM: Enhanced recognition of disgust in bipolar illness. Biol Psychiatry 2002; 51:298–304Google Scholar
2. Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD: fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2000; 2:237–248Google Scholar
3. Bearden CE, Hoffman KM, Cannon TD: The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord 2001; 3:106–150Google Scholar
4. Strakowski SM, DelBello MP, Adler CM: The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 2005; 10:105–116Google Scholar
5. Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R: An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord 2005; 5:58–69Google Scholar
6. Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception, II: Implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Google Scholar
7. Mayberg S: Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatr Clin Neurosci 1997; 9:471–481Google Scholar
8. Blumberg HP, Charney DS, Krystal JH: Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry 2002; 7:243–254Google Scholar
9. Dolcos F, McCarthy G: Brain systems mediating cognitive interference by emotional distraction. J Neurosci 2006; 26:2072–2079Google Scholar
10. Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP: A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology 2004; 29:1734–1740Google Scholar
11. Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM: Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 2004; 6:540–549Google Scholar
12. Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC: Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry 2005; 162:1697–1705Google Scholar
13. Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, Andrew CM, Phillips ML: Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord 2006; 8:28–39Google Scholar
14. Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET: Behavioral and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med 2004; 34:795–802Google Scholar
15. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport 2000; 11:1739–1744Google Scholar
16. Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ: Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry 2005; 57:624–632Google Scholar
17. Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ: Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry 2004; 55:1163–1170Google Scholar
18. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002; 59:597–604Google Scholar
19. Hamilton M: A Rating Scale for Depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
20. Young RC, Biggs JT, Ziegler VE, Meyer DA: A Rating Scale for Mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Google Scholar
21. Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar GC: The MINI International Neuropsychiatric Interview (MINI): a short diagnostic structured interview. Eur Psychiatry 1997; 12:224–231Google Scholar
22. Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T (NIMH Genetics Initiative): Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry 1994; 51:849–859Google Scholar
23. Bayle FJ, Bourdel MC, Caci H, Gorwood P, Chignon JM, Ades J, Loo H: Factor analysis of french translation of the Barratt Impulsivity Scale (BIS-10). Can J Psychiatry 2000; 45:156–165Google Scholar
24. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Google Scholar
25. Ekman P, Friesen WV: Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
26. Talairach J, Tournoux B: Coplanar Stereotaxic Atlas of the Human Brain: Three-dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
27. Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS: Blunted activation in orbitofrontal cortex during mania: a functional MRI study. Biol Psychiatry 2005; 58:763–769Google Scholar
28. Poline JB, Worsley KJ, Evans AC, Friston KJ: Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 1997; 5:83–96Google Scholar
29. Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL: Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:374–383Google Scholar
30. Ishai A, Schmidt CF, Boesiger P: Face perception is mediated by a distributed cortical network. Brain Res Bull 2005; 67:87–93Google Scholar
31. Adolphs R: Social cognition and the human brain. Trends Cogn Sci 1999; 3:469–479Google Scholar
32. Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y: Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res Cogn Brain Res 2001; 12:225–231Google Scholar
33. Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW: Response inhibition and impulsivity: an fMRI study. Neuropsychologia 2003; 41:1959–1966Google Scholar
34. Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M: Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp 2006; May 8 (Epub ahead of print)Google Scholar
35. McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E: Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry 2005; 162:1644–1651Google Scholar
36. Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SC, Simmons A, Giles N, Lloyd AJ, Harrison CL, Seal M, Murray RM, Ferrier IN, Young AH, Curtis VA: A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord 2004; 6:550–564Google Scholar
37. Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Google Scholar
38. Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML: Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Google Scholar
39. McGrath BM, Wessels PH, Bell EC, Ulrich M, Silverstone PH: Neurobiological findings in bipolar II disorder compared with findings in bipolar I disorder. Can J Psychiatry 2004; 49:794–801Google Scholar
40. Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A: Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Arch Gen Psychiatry 2004; 61:781–792Google Scholar