Correlation Between Extraversion and Regional Cerebral Blood Flow in Response to Olfactory Stimuli
Abstract
Objective: Extraversion, a trait associated with individual differences in approach motivation and the experience of positive emotional states, is negatively correlated with certain psychiatric disorders, including depression and social phobia. The authors examined the correlation between extraversion and regional cerebral blood flow (rCBF) while participants were exposed to olfactory stimuli in order to further characterize individual differences in hedonic processing associated with this trait. Method: Twelve healthy participants were exposed to pleasant and unpleasant odors while rCBF was measured using [ 15 O] water PET. The NEO Five-Factor Inventory was used to assess extraversion. Associations between extraversion scores and rCBF in each olfactory stimulus condition were assessed by correlational analysis. Results: During the pleasant smell condition, extraversion was correlated with rCBF in the amygdala and occipital cortex. During the unpleasant smell condition, extraversion was correlated with rCBF in the occipital cortex and inferior temporal gyrus. Conclusions: These results provide important evidence for the biological basis of extraversion and indicate that there are systematic individual differences in patterns of brain activation in response to affective stimuli.
It is becoming increasingly clear that many of the maladaptive characteristics associated with certain psychiatric disorders reflect extreme manifestations of basic personality processes (1) . While there may be multiple explanations for the link between personality and psychiatric disorders, one possibility is that certain personality traits predispose people to psychiatric illness. Therefore, a better understanding of the biological processes associated with personality traits can improve our knowledge of the etiology and neurophysiology of psychiatric disorders.
Extraversion is an individual differences trait that is assessed in virtually every major multidimensional personality inventory (2) . It is negatively correlated with several psychiatric disorders, including major depression and social phobia (3) . Although commonly thought of as a trait associated with seeking out and enjoying social interactions, extraversion has been linked to a more fundamental motivational system. Tellegen (4) noted that extraversion shows strong and consistent associations with measures of positive affectivity and proposed that the trait be renamed “positive emotionality.” Tellegen (4) further theorized that extraversion reflects individual differences in the behavioral activation system, a motivational system thought to control behaviors in response to signals of reward (5) .
Individual differences in extraversion are thought to be related to the dopaminergic system arising from the midbrain to the ventral striatum and limbic regions, including the amygdala (6) . However, few brain imaging studies have examined the association between extraversion and regional cerebral blood flow (rCBF) in response to hedonic stimuli in order to investigate the neurophysiology of individual differences in emotion processing. In this study, we used positron emission tomography (PET) while study subjects were exposed to pleasant and unpleasant olfactory stimuli to examine the association between extraversion and rCBF. Previous studies have focused largely on visual stimuli (7 , 8) , some of which involve social scenes or human faces. Responses to these stimuli may represent extraverts’ sensitivity to social stimuli, but not necessarily to rewarding stimuli. Furthermore, because the olfactory cortex has direct connections to limbic structures including the amygdala, and because less cognitive processing is required for the emotional meaning of olfactory stimuli to be determined (9) , it is important to examine how extraversion scores predict rCBF in response to this stimulus modality.
Method
Participants
Twelve subjects (five of them female) 19 to 48 years of age (mean=29.8 years, SD=8.4) without psychiatric, neurological, or general medical illness participated in the study. Participants were healthy volunteers recruited as part of a larger project on schizophrenia and emotion (10) ; most of the schizophrenia patients in the larger study were smokers, and a similar smoking profile was sought in selection of the healthy comparison group. Consequently, nine of the 12 subjects in the present study were smokers. However, all participants scored at least 35 out of 40 (mean=37, SD=1.1) on the University of Pennsylvania Smell Identification Test (11) . No differences were observed between smokers and nonsmokers in intensity ratings for pleasant and unpleasant smells.
Olfactory Stimuli
During PET data acquisition, participants were first exposed to a pleasant odor solution (100 μl of vanillin) and then an unpleasant one (100 μl of 4-methylvaleric acid), each presented on a cotton ball placed 2 mm from the nostrils. Odors were presented 10 seconds prior to arrival of the [ 15 O] water bolus to the brain. After completion of the PET scan, participants rated the odors on valence (–7=extremely unpleasant to +7=extremely pleasant) and intensity (0=odorless to 7=most intense).
Personality Assessment
The NEO Five-Factor Inventory (12) was used to obtain self-report ratings of extraversion. This instrument consists of 60 items to which participants respond on a 5-point Likert scale (1=strongly disagree to 5=strongly agree).
Image Acquisition and Analysis
rCBF was measured by PET with a GE 4096 Plus whole-body scanner (GE Medical Systems, Milwaukee, Wisc.) after intravenous injection of a 50-mCi [ 15 O] water bolus. Fifteen axial slices with an intrinsic in-plane resolution of 6.5 mm full width at half maximum and a 26-cm field of view were acquired. Time from injection to bolus arrival in the brain was measured individually during a sham condition (13) . For each injection, arterial blood was sampled from time of injection for 100 sec in 5-second frames (13) . Frames from the 40 seconds immediately after bolus arrival were summed and reconstructed into 2-mm pixels (128×128 matrix) using a Butterworth filter (order=6, cutoff frequency=0.35 Nyquist units). Using this summed image and the measured arterial input function, rCBF was calculated on a pixel-by-pixel basis by the autoradiographic method and normalized by dividing by global CBF. To reduce anatomical variability, a two-dimensional 18-mm Hanning filter was applied (see reference 10 for details).
Anatomical images were obtained for each subject with a standard T 1 -weighted three-dimensional spoiled gradient-recalled echo pulse sequence on a 1.5-T GE Signa scanner (GE Medical Systems, Milwaukee, Wisc.) (TE=5 msec, TR=24 msec, flip angle=40°, number of excitations=2, field of view=26 cm, matrix=256×192, slice thickness=1.5 mm). The anterior commissure-posterior commissure line was used to realign brain images of all subjects to standardized Talairach coordinate space. Automated image registration (14) with visual verification was used to coregister PET image data to their individual magnetic resonance scans.
Statistical Analysis
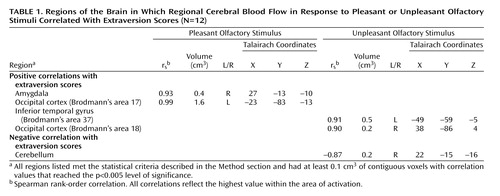
The rCBF value for each voxel in the pleasant and unpleasant smell conditions was subtracted from the value in the corresponding voxel during the resting, baseline condition. Locally developed software was used to calculate Spearman rank-order correlations between extraversion and rCBF for each voxel in each olfactory condition (subtracted from the resting condition) (15) . We used a two-tiered approach to the analysis. We first determined which areas showed significant correlations that were unlikely to be due to chance by performing a Bonferroni correction based on the number of resolution elements (resels). We found evidence for approximately 282 resels. This resulted in a p value of 0.0002, corresponding to a Spearman rank-order correlation [r s ] of 0.87, which produced 11 and eight significant correlations in the pleasant and unpleasant conditions, respectively. We then reanalyzed the data after adjusting the significance level to p<0.005 (corresponding to r s >0.75), focusing only on the regions that met the corrected significance threshold.
Results
Higher extraversion scores were associated with greater activation in the amygdala and occipital cortex ( Table 1 ). This finding is consistent with the observation that extraverts showed a modest, nonsignificant tendency to experience the pleasant smell as more pleasant (r s =0.42, p=0.18). During the unpleasant condition, extraversion was positively correlated with rCBF in the occipital and temporal cortices. There was also a negative correlation (indicating greater activity for introverts) with a region in the cerebellum. The self-report ratings confirm that extraverts interpreted the unpleasant smell differently. There was a modest, nonsignificant tendency for extraverts to report the unpleasant smell as less intense (r s =–0.46, p=0.14) and less unpleasant (r s =0.44, p=0.15).
In order to establish the specificity of the extraversion-amygdala association, we conducted further analyses with the neuroticism scale of the NEO Five-Factor Inventory. Neuroticism has strong correlations with measures of negative affectivity and is thought to reflect a biobehavioral system that responds to cues of conditioned punishment (5) . There was no evidence of a significant association between neuroticism and rCBF in the left or right amygdala to the pleasant or unpleasant smell (r s =0.10 at the same Talairach coordinates as the peak extraversion-amygdala correlation).
Discussion
Although extraversion has previously been reported (8) to be associated with increased amygdala activation in response to happy faces, it was unclear whether this result represented a greater sensitivity to pleasant stimuli in general or a specific affinity for happy faces. The present study, combined with earlier work (8) , suggests that extraverts show greater activation in the amygdala in response to a range of pleasant stimuli. Although extraversion is commonly thought of as a trait associated with seeking out and enjoying social interactions, the correlation between extraversion and rCBF in the amygdala provides important evidence that it more fundamentally represents a trait related to a biobehavioral approach system that controls appetitive motivation (2) . The self-report ratings confirmed important differences in how extraverts and introverts respond to hedonic stimuli. Furthermore, because extraversion is negatively correlated with depressive disorders (3) , depression may be associated with decreased activation in regions subserving positive evaluation of stimuli, including the amygdala. Relatively few studies have used brain imaging to investigate the association between depression and responses to positive, as compared to negative, stimuli. Therefore, researchers should investigate brain activation in response to positive, as well as negative, stimuli in subjects with depression.
Interestingly, extraversion was associated with greater activity in the inferior temporal gyrus during the unpleasant olfactory stimulus as well as with increased activity in the occipital cortex during both the pleasant and unpleasant olfactory stimuli. Considering the fact that the inferior temporal gyrus is part of the ventral visual processing stream (16) , this finding raises the interesting possibility that higher extraversion scores may be associated with enhanced visual representations of hedonic smells, providing evidence of individual differences in cross-modal sensory processing. Future studies should examine brain activation while participants are passively experiencing affective stimuli and when they are making overt responses to the stimuli. Such a design would improve our understanding of individual differences in passively experiencing versus rating affective stimuli.
1. Watson D, Gamez W, Simms LJ: Basic dimensions of temperament and their relation to anxiety and depression: a symptom-based perspective. J Res Pers 2005; 39:46–66Google Scholar
2. Watson D, Clark LA: Extraversion and its positive emotional core, in Handbook of Personality Psychology. Edited by Hogan R, Johnson JA, Briggs SR. San Diego, Academic Press, 1997, pp 767–793Google Scholar
3. Clark LA, Watson D, Mineka S: Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol 1994; 103:103–116Google Scholar
4. Tellegen A: Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report, in Anxiety and the Anxiety Disorders. Edited by Tuma AH, Maser JD. Hillsdale, NJ, Erlbaum, 1985, pp 681–706Google Scholar
5. Watson D, Wiese D, Vaidya JG, Tellegen A: The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. J Pers Soc Psychol 1999; 76:820–838Google Scholar
6. DePue RA, Collins PF: Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 1999; 22:491–517Google Scholar
7. Reuter M, Stark R, Henning J, Walter B, Kirsch P, Schienle A, Vaitl D: Personality and emotion: test of Gray’s personality theory by means of an fMRI study. Behav Neurosci 2004; 118:462–469Google Scholar
8. Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE: Amygdala responses to happy faces as a function of extraversion. Science 2002; 296:2191Google Scholar
9. Ehrlichman H, Bastone L: The use of odour in the study of emotion, in Fragrance: The Psychology and Biology of Perfume. Edited by Van Toller SD, Dodd GH. New York, Elsevier, 1992, pp 143–159Google Scholar
10. Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA 2001; 286:662–663Google Scholar
11. Doty R, Shaman P, Dann M: Development of the University of Pennsylvania Smell Test: standardized microencapsulated test for olfactory function. Physiol Behav 1984; 32:489–502Google Scholar
12. Costa PT Jr, McCrae RR: Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI): Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1992Google Scholar
13. Hurtig RR, Hichwa RD, O’Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC: Effects of timing and duration of cognitive activation in [ 15 O] water PET studies. J Cereb Blood Flow Metab 1994; 14:423–430 Google Scholar
14. Woods R, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620–633Google Scholar
15. Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC: Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport 1999; 10:2493–2496Google Scholar
16. Ungerleider LG, Mishkin M: Two cortical visual systems, in Analysis of Visual Behavior. Edited by Ingle DJ, Goodale MA, Mansfield RJW. Cambridge, Mass, MIT Press, 1982Google Scholar