Is Decreased Prefrontal Cortical Sensitivity to Monetary Reward Associated With Impaired Motivation and Self-Control in Cocaine Addiction?
Abstract
Objective: This study attempted to examine the brain’s sensitivity to monetary rewards of different magnitudes in cocaine abusers and to study its association with motivation and self-control. Method: Sixteen cocaine abusers and 13 matched healthy comparison subjects performed a forced-choice task under three monetary value conditions while brain activation was measured with functional magnetic resonance imaging. Objective measures of state motivation were assessed by reaction time and accuracy, and subjective measures were assessed by self-reports of task engagement. Measures of trait motivation and self-control were assessed with the Multidimensional Personality Questionnaire. Results: The cocaine abusers demonstrated an overall reduced regional brain responsivity to differences between the monetary value conditions. Also, in comparison subjects but not in cocaine abusers, reward-induced improvements in performance were associated with self-reports of task engagement, and money-induced activations in the lateral prefrontal cortex were associated with parallel activations in the orbitofrontal cortex. For cocaine abusers, prefrontal cortex sensitivity to money was instead associated with motivation and self-control. Conclusions: These findings suggest that in cocaine addiction 1) activation of the corticolimbic reward circuit to gradations of money is altered; 2) the lack of a correlation between objective and subjective measures of state motivation may be indicative of disrupted perception of motivational drive, which could contribute to impairments in self-control; and 3) the lateral prefrontal cortex modulates trait motivation and deficits in self-control, and a possible underlying mechanism may encompass a breakdown in prefrontal-orbitofrontal cortical communication.
Drug addiction is characterized by impaired response inhibition and salience attribution, in which the motivation to procure drugs overpowers the drive to attain most other, non-drug-related goals (1) . In clinical practice, a similar notion that motivated goal-directed behavior is limited to drug-related rewards has been integrated into the core diagnostic definition of substance dependence (DSM-IV), prompting the use of motivational interviewing as a brief therapeutic intervention (2) . However, although cocaine-addicted subjects show lower corticolimbic activations when viewing nondrug compared to drug rewards (an erotic video versus a cocaine video [3] ), there is still a paucity of work on the underlying neurobiological markers of motivation and response to reward in human drug addiction.
The brain reward circuit has classically included the mesocorticolimbic dopaminergic network, spanning the prefrontal cortex, amygdala, and mesencephalon, but also the thalamus and cerebellum, which are associated with the processing of salient stimuli (4) . Within the prefrontal cortex, the orbitofrontal cortex and anterior cingulate cortex mediate the sustained activation of goal-directed (including drug-seeking) behavior (5) . The lateral prefrontal cortex has been implicated in the cognitive aspects of reward expectancy, possibly integrating cognitive and motivational operations (6) by attending to internally generated emotional (7) and crucial feedback (8) information.
In the current study, comparing drug-addicted individuals to nonaddicted subjects, our goals were to 1) quantify the neural responsivity to nondrug reward (money), 2) examine intercorrelations between measures of state motivation, and 3) examine associations between the prefrontal cortex and trait motivation and self-control. We hypothesized that in cocaine abusers, 1) neural sensitivity to different levels of money would be reduced, 2) a disrupted perception of internally generated motivational drives would be indicated by a discrepancy between self-reported motivation and actual task performance, and 3) decreased prefrontal cortex sensitivity to reward would be related to impaired perception of inner motivation and decreased self-control.
Method
Subjects
The participants were 16 cocaine abusers and 13 comparison subjects who were matched by gender, race, English as first language, handedness, education, socioeconomic status, general intellectual functioning, and self-reported depression. Significant group differences were observed in age and cigarette smoking (data supplement Table 1, available online at http://ajp.pyschiatryonline.org). Initial screening by telephone and subsequent onsite evaluation by a neurologist and a clinical psychologist ensured that the cocaine abusers were not using marijuana, barbiturates, amphetamines, or opiates (this was ensured by prescan urine tests in all subjects), that they were free of illnesses that required hospitalization or regular monitoring, and that they had a DSM-IV diagnosis of substance dependence or abuse (see data supplement Table 1 for drug use histories). The subjects were fully informed of the nature of the research and provided written consent for their involvement in this study in accordance with the local institutional review board.
Fifteen of the 16 cocaine abusers fulfilled criteria for current cocaine dependence (N=9) or cocaine early remission (N=6). One cocaine abuser, who admitted to weekly use of cocaine, did not meet current abuse or dependence criteria but met DSM-IV criteria for past polysubstance abuse, which included crack cocaine. The nine abusers with current cocaine dependence reported using cocaine the night before the study; their urine was positive for cocaine, indicating that they had used cocaine within the previous 72 hours. We chose not to exclude subjects with recent cocaine use because specific regional changes in blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) can be measured reliably even after acute cocaine infusion (9) ; moreover, all measures return to baseline by 2 hours postinfusion (9) because of cocaine’s short half-life in the brain (20 minutes, reference 10 ). Nevertheless, drug urine status in the cocaine abusers as well as the age and smoking differences between the groups were accounted for in the analyses, as described in the Results section.
fMRI Activation Paradigm and State Motivation
After training, the subjects either responded (pressed a button) or refrained from responding during a trigger, depending on one of two preceding instructional stimuli (adapted from Thut et al., 11). There were nine pairs of press and no-press trials within each of three identical conditions. These were distinguished only by blocked levels of monetary rewards received for correct performance on this forced-choice task: high money (45 cents), low money (1 cent), and no money (0 cents). Each monetary condition/block was of 63 seconds’ duration, preceded by a 35-second fixation cross to preclude carryover effects. Every three (different) monetary blocks constituted a run for a total of six runs. To simulate real-life incentive motivation, the subjects received up to $50 for this task, seeing a numeral designating the reward contingencies before each monetary block and immediately after each trial. This was a relatively substantial amount of money because it doubled the subjects’ total earnings during the complete study day.
The task was presented by means of MRI-compatible goggles. Reaction time and accuracy data were collected across all trials. Upon task completion, the subjects were asked to rate their interest and excitement in the three monetary conditions on two visual analogue scales (range = 0 to 7, boring to interesting and dull to exciting, respectively). These ratings were averaged to represent self-reported task engagement. Monetary differentials (45 cents > 0 cents) were calculated for the reaction time, accuracy, and averaged rating: the first two were used as objective and the latter as subjective measures of state motivation. In this fMRI incentive task, we did not establish a propensity to go (the ratio of go to no-go trials was 50%); therefore, reaction time was not considered a state measure of inhibitory control.
Trait Evaluations
The Multidimensional Personality Questionnaire by Tellegen and Waller (12) was available for 10 comparison subjects and 10 cocaine abusers (data supplement Table 1 ). The Multidimensional Personality Questionnaire achievement and self-control scales were used as trait measures of incentive motivation and inhibitory control, respectively.
fMRI Data Processing and Image Analysis
MRI scanning was performed on a 4-T whole-body MRI scanner (Varian, Palo Alto, Calif./Siemens, Berlin). BOLD responses were measured with a T 2 *-weighted single-shot gradient-echo echoplanar imaging sequence (TE=20, TR=3500 msec, 4-mm slice thickness, 1-mm gap, typically 33 coronal slices, field of view=20 cm, 64×64 matrix size, 90° flip angle, 200-kHz bandwidth with ramp sampling, 91 time points, and four dummy scans). Padding was used to minimize motion, which was inside the accepted threshold of 1-mm maximum displacement (32% of the voxel size) and 1° rotation, as determined immediately after each run (13) . A T 1 -weighted three-dimensional modified equilibrium Fourier transform sequence (14) was used for structural imaging; all MRI images were inspected to rule out gross morphological brain abnormalities.
All time series were converted into the statistical parametric mapping 99 format (Wellcome Department of Cognitive Neurology, London). A six-parameter rigid body transformation (three rotations, three translations) was used for image realignment to correct for head motion. The realigned data sets were normalized to the Talairach frame with a 12-parameter affine transformation (15) by using a voxel size of 3×3×3 mm 3 . An 8-mm full-width half-maximum Gaussian kernel was used to smooth the data. A general linear model (16) and a box car design convolved with a canonical hemodynamic response function were used to calculate the activation maps. The time series were band-pass filtered with the hemodynamic response function as a low-pass filter and a 1/750-second cutoff frequency as a high-pass filter.
Statistical Analyses
Goal 1
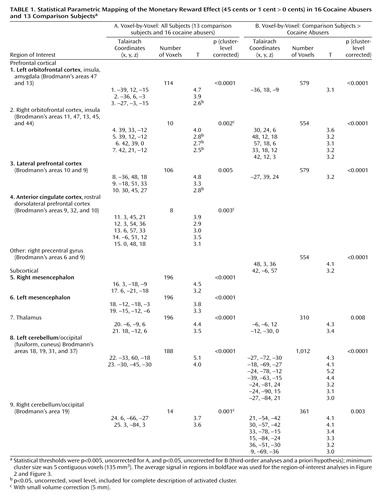
To identify brain areas activated specifically to monetary reward compared to a neutral cue, a voxel-based (whole-brain) statistical analysis with two positive contrasts (45>0 and 1>0) was applied for each run, separately for each subject (fixed-effects analyses). Maps of BOLD signals for individual subjects were then averaged by using a custom program written in Interactive Data Language (Research Systems, Boulder, Colo.) across all six runs and included in a combined statistical analysis. For this random-effects second-level analysis, a repeated-measures between-subjects analysis of variance (ANOVA) was conducted by statistical parametric mapping (a mask of the general task activations, i.e., 45, 1, or 0 > a fixation baseline was used at p<0.05, voxels = 0 for the purposes of mask inclusiveness). Statistical thresholds were 0.005, uncorrected for the main effect of monetary reward (45 cents or 1 cent > 0 cents) across all subjects (second-order analysis), and 0.05, uncorrected for the between-subjects monetary comparison (third-order analysis and our first a priori hypothesis). Thus, the threshold was reduced from 0.005 to 0.05 because of the anticipated loss of power associated with the increased rigorousness of the analyses from second to third order (comparing money conditions between groups versus studying each effect in isolation).
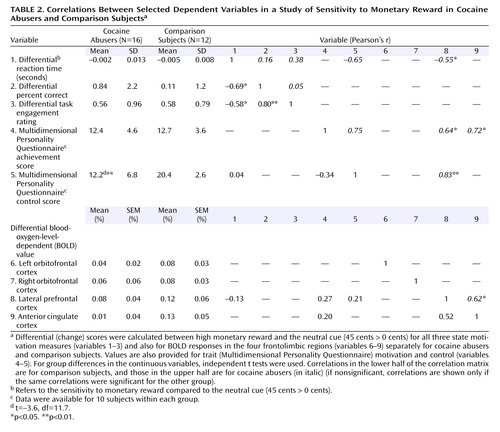
Similarly to our other fMRI studies (e.g., reference 17 ), functional regions of interest with a relatively large volume of 729 mm 3 (27 voxels) were then defined at the cluster centers of the regions that showed a significant monetary reward effect across all study subjects; within each region, the estimated BOLD fMRI signal was calculated and expressed as a percentage of change for each monetary condition from baseline and then averaged to represent all significantly activated clusters. Clarification of anatomical specificity was corroborated with a coplanar stereotaxic atlas of the human brain (18) . These regions of interest were used to complement the statistical parametric mapping analyses; a two-by-three (group-by-money) repeated-measures ANOVA was performed for each of the clusters, and the main effects of group and money or their interaction were followed by independent (diagnosis differences) or paired (reward differences) t tests. In addition, planned comparisons were performed to test our a priori first hypothesis that cocaine abusers would display reduced sensitivity to gradients of reward. The statistical significance for these region-of-interest analyses was defined at p<0.05. Note that in these analyses, one comparison subject was removed because of loss of 70% of the BOLD fMRI data. Therefore, all subsequent analyses are reported for 12 comparison subjects.
Goal 2
Correlations were conducted specifically for sensitivity to monetary reward compared to the neutral cue (45 cents > 0 cents) between the selected three state motivation measures, separately for each study group.
Goal 3
First, correlations were conducted specifically for sensitivity to monetary reward compared to the neutral cue (random effects = 45 cents > 0 cents) between the prefrontal cortex regions of interest with the selected three state-motivation measures and with the achievement and self-control Multidimensional Personality Questionnaire scales, separately for each group. Second, similar region-of-interest correlations were conducted for the lateral prefrontal cortex and the orbitofrontal cortex, in this case, between the “absolute” BOLD responses to monetary rewards (45 cents > baseline). Simple linear voxel-based (whole-brain) correlation analyses were used to validate these region-of-interest correlations. The significance threshold for the first a priori analysis was set at p<0.05, uncorrected; for the second analysis, it was p<0.005, uncorrected. A small volume correction (19) was used for the a priori region of interest (the prefrontal cortex). Minimum cluster size was 100 contiguous voxels (2700 mm 3 ) for both analyses, masked with general task activations. Here, a large volume was selected to protect against type I error in these correlation analyses.
Results
Goal 1: Monetary Reward Neural Effect
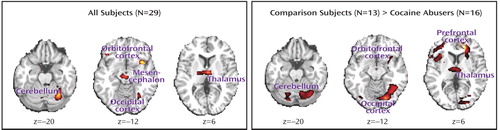
The statistical parametric mapping analyses of the monetary main effect (45 cents or 1 cent > 0 cents) in all subjects revealed activations in 25 regions comprising nine clusters that included the right and left lateral orbitofrontal cortex, the lateral and ventromedial (including the anterior cingulate cortex) prefrontal cortex, and the mesencephalon, thalamus, and cerebellum (but also the occipital lobe), all bilaterally ( Table 1 A and Figure 1 , left). However, consistent with our first a priori hypothesis, direct group analyses revealed that the activations in the comparison subjects but not the cocaine abusers were driving these results ( Table 1 B and Figure 1 , right).
a Statistical thresholds were p<0.005, uncorrected, for the leftmost box (T=2.7–5.1) and p<0.05, uncorrected, for the rightmost box (T=1.7–7) (third-order analyses and a priori hypothesis); minimum cluster size was five contiguous voxels (135 mm 3 ).
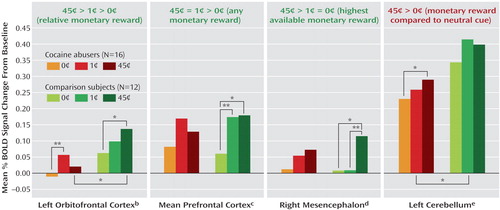
The complementary region-of-interest analyses revealed a significant monetary main effect in six of these clusters ( Table 1 , clusters in boldface, and Figure 2 ). Furthermore, a money-by-group interaction was significant in the left orbitofrontal cortex ( Figure 2 ); indeed, an overall test of coincidence of the study groups’ regression lines was statistically significant (F=3.49, df=2, 80, p<0.05), indicating different lines of best fit (from lowest to highest monetary reward) in this region of interest as a function of group. All other significant results are marked in Figure 2 and are further described in the Discussion section.
a df=2, 25 (money) or d=1, 26 (group), df=11 (comparison subjects), df=15 (cocaine abusers), df=26 (group differences); all significant t>|2.1|.
b Analysis of variance (ANOVA) was F=4.7 (money), F=3.5 (money by group).
c ANOVA was F=5.6 (money).
d ANOVA was F=8.2 (money).
e ANOVA was F=5.2 (money).
*p<0.05. **p<0.01.
We examined the effect of age, urine status, and cigarette smoking by conducting correlations or t tests with all nine clusters’ responses to absolute (45 cents, 1 cent, or 0 cents > baseline) or relative (45 cents > 0 cents, and 1 cent > 0 cents) monetary reward as the dependent variables (three covariates by nine regions of interest by five reward conditions = 35 analyses); even with a lenient Bonferroni correction (p<0.01), there were no significant correlations between age and any of these regions of interest, nor did monetary responses in these regions of interest differ as a function of urine status or cigarette smoking history in each of the study groups or in the complete sample.
Goal 2: State Motivation
All three behavioral measures of state motivation (45 cents > 0 cents) were significantly intercorrelated in the comparison subjects (variables 1–3 in Table 2 , lower half) but not in the cocaine abusers ( Table 2 , upper half). In the cocaine abusers, there was instead a correlation between the differential reaction time with the Multidimensional Personality Questionnaire self-control scale such that the faster the reaction time to the higher monetary reward, the more the self-reported trait control. Again, age, urine status, and cigarette smoking did not affect these results.
Goal 3: Brain-Behavioral Associations
The differential signal change (45 cents > 0 cents) in the lateral prefrontal cortex correlated significantly with motivation at both the state (differential reaction time) and trait (Multidimensional Personality Questionnaire achievement scale) levels and also with trait self-control, but only in the cocaine abusers (variable 8 with variables 1 and 4–5, Table 2 ). Voxel-based correlations in the cocaine abusers confirmed the involvement of the lateral prefrontal cortex in reaction time (data supplement Table 2), achievement (data supplement Table 2), and self-control (data supplement Table 2 and Figure 3, top). Selected drug use variables (data supplement Table 1) did not correlate with the differential BOLD response in the lateral prefrontal cortex or with the Multidimensional Personality Questionnaire self-control scale; furthermore, this correlation ( Figure 3 , top) remained unchanged after we added control with partial correlations for age, urine status, and smoking history (see data supplement Figure 1 ).
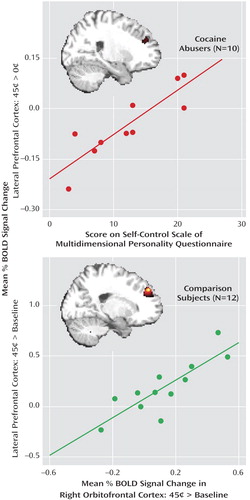
a The upper scatterplot shows the association between the blood-oxygen-level-dependent (BOLD) signal change for monetary reward compared to the neutral cue (45 cents > 0 cents) in the lateral prefrontal cortex (x=33, y=36, z=15) and the Multidimensional Personality Questionnaire self-control scale (r=0.88, p=0.001); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (data supplement Table 2C). Thresholded at p<0.05, uncorrected. The lower scatterplot shows the association between the BOLD signal change for monetary reward compared to baseline (45 cents > baseline) in the lateral prefrontal cortex (x=–21, y=48, z=36) and same responses in the orbitofrontal cortex (x=42, y=33, z=–12) (r=0.84, p=0.001); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (data supplement Table 2D). Thresholded at p<0.005, uncorrected. Minimum cluster size = 100 contiguous voxels, 2700 mm 3 , for both.
Among the comparison subjects, there was a significant correlation between the lateral prefrontal cortex and the right orbitofrontal cortex (45 cents > baseline for both) in both voxel-based (whole-brain) (data supplement Table 2D and Figure 3, bottom) and region-of-interest ( Figure 3 , linear regression) analyses. These correlations were not significant in the cocaine abusers.
Discussion
Here we report for the first time, to our knowledge, a compromised neuronal sensitivity to monetary reward in cocaine abusers. Furthermore, we report novel correlations between the prefrontal cortex sensitivity to money and motivation and self-control in cocaine abusers but not in comparison subjects, who instead demonstrated an association between reward-induced change in performance and self-reported task engagement and an intact association between the lateral prefrontal cortex and orbitofrontal cortex signals to money.
Goal 1: Reduced Complexity of Neuronal Responses to a Nondrug Reward in Addiction
Replicating and extending previous findings in healthy subjects (e.g., reference 20 ), sustained monetary reward was associated with a robust and complex neuronal activation pattern in the comparison subjects ( Figure 1 and Figure 2 ): there was a tendency for the left orbitofrontal cortex to respond in a graded fashion (45 cents > 1 cent > 0 cents), the lateral and medial prefrontal cortex responded instead to the two conditions of monetary value equally (45 cents = 1 cent > 0 cents), while the mesencephalon displayed a third pattern of sensitivity to the highest available reward only (45 cents > 1 cent = 0 cents) ( Figure 2 ). In general, these results are consistent with the role of 1) the orbitofrontal cortex in relative reward processing in the primate (21) and in healthy human subjects (20 , 22–25) , 2) the prefrontal cortex in the control of attention (8) , possibly irrespective of reward magnitude (26) , and 3) the mesencephalon in all-or-nothing reward processing in the primate (27) and in healthy human subjects (20) .
The cocaine-addicted subjects did not display this complex pattern of activation to monetary reward, demonstrating either a reduced regional BOLD signal in the between-group analyses or less sensitivity to differences between the monetary conditions in the within-group analyses ( Table 1 , Figure 1 , and Figure 2 ). Attenuated mesocorticolimbic neural activations to monetary reward have been previously reported in adolescence (28) and in Parkinson’s disease (29) . Our study extends these results to drug-addicted individuals. The importance of this finding lies in the conditioning between monetary availability and drug procurement. Therefore, it is possible that for the drug-addicted individual, only more immediate drug-related cues (e.g., pictures or a video; see reference 3 ) or the drug itself could have activated this circuit at a comparable level with that induced by a non-drug-related reward in non-drug-addicted individuals.
A relative exception was the left cerebellum, in which only the cocaine abusers displayed a significant monetary effect (45 cents > 0 cents; note, however, that the between-group analysis still showed larger reward-related activations in the comparison subjects) ( Figure 2 ). This within-subjects result is consistent with reports of compensatory mechanisms in the cerebellum in psychopathology, e.g., overreliance on the cerebellum by cocaine abusers during a working memory task (30) and by Parkinson’s patients during a rewarded task (29) .
Goal 2: Impaired Drive Perception in Addiction
Our second major finding concerns intercorrelations between all three state (task-related) behavioral measures of motivation in the comparison subjects but not the cocaine abusers ( Table 2 , variables 1–3). Thus, in the former group only, the faster and more accurate the responses for the high monetary condition compared to the neutral cue, the higher the self-reported engagement in the task. In contrast, the cocaine subjects’ reports of task engagement were disconnected from their actual task performance (speed or accuracy). This disconnect between the objective and subjective measures of state motivation in the cocaine abusers may reflect not only a discrepancy between actual behavior and explicit knowledge of rules of behavior (31) or reward and punishment outcome (32) but indeed a disruption in the ability to perceive inner motivational drives. This disruption may contribute to long-term self-control deficits, as further suggested by our results ( Table 2 , variables 1 and 5).
Goal 3: The Lateral Prefrontal Cortex in Trait Self-Control in Drug Addiction
In the cocaine abusers, we observed significant correlations between the lateral prefrontal cortex and state (differential reaction time) and trait (Multidimensional Personality Questionnaire achievement score) motivation and with trait self-control (Multidimensional Personality Questionnaire control score, Figure 3 ). In particular, the latter correlation suggests that hyposensitivity to reward in the prefrontal cortex mediates the reduced self-control reported by the cocaine abusers ( Table 2 , a significant between-group difference in variable 5). This result is consistent with the role of the prefrontal cortex in control of behavior as previously reviewed (1 , 33) and with prior research in our laboratory pointing to an association between the prefrontal cortex and inhibitory control in drug addiction (34 – 36) . Our current results for the first time highlight the role of neural sensitivity to reward in trait inhibitory control.
The Underlying Mechanism: Disruption of Frontal Neuronal Network Communications
The mechanism underlying impaired perception of motivational drives and disrupted inhibitory control in drug addiction may involve a breakdown in frontal corticolimbic neuronal network communications. Thus, while in the comparison subjects the orbitofrontal cortex tended to respond in a monotonically positive pattern to reward ( Figure 2 ) and its responses to the high monetary reward were significantly associated with parallel responses in the lateral prefrontal cortex ( Figure 3 ), both of these patterns were lacking in the drug-addicted subjects. Therefore, it is possible that in drug addiction a disrupted sensitivity to gradients in monetary value in the orbitofrontal cortex contributes to the disrupted functioning of the lateral prefrontal cortex, creating a communication breakdown that augments the cognitive, behavioral, and emotional difficulties in these individuals. Indeed, changes in frontal white matter integrity (37) and their association with impulsivity (38) were recently reported in cocaine-dependent subjects.
Limitations
Causal attributions should not be made without replication of the current correlational results (e.g., between BOLD signal and behavior or self-report) by using an experimental design (e.g., manipulation of the value of reward, motivation, and inhibitory control in the same task). Also, these results need to be replicated in larger sample sizes and with more homogeneous groups of drug-addicted individuals (e.g., all current versus all detoxified cocaine abusers). In this regard, it is important to recall the age and abstinence differences between the two study groups; age (29) and abstinence from cigarette smoking (39) or cocaine (1 , 40) could decrease neural sensitivity to reward. However, analyses revealed that in the current study these possibly confounding factors were not related to the fMRI BOLD or behavioral dependent variables or to their associations (e.g., data supplement Figure 1); nevertheless, the contribution of these factors needs to be further investigated in larger sample sizes. In addition, the experimental design did not allow for investigation of different epochs of reward processing (for example, anticipation versus consummation, see reference 28 ), and future studies could investigate whether the observed between-group differences are specific to distinct phases of reward processing (this could be accomplished with event-related designs). Finally, this study could not distinguish whether the disrupted patterns of activation to monetary reward in the cocaine abusers reflects the long-term use of drugs or whether they antedated drug use and may have constituted a vulnerability factor for addiction (this could be accomplished with longitudinal designs).
Conclusions
We attribute the deficits in the subjective perception of motivational drives in the cocaine abusers to reduced orbitofrontal cortex responsivity to gradients in a non-drug-related reward and its effect on the control of behavior by the lateral prefrontal cortex. These abnormalities may contribute to the ascribed motivational impairments and deficits in controlling drug-taking behavior in drug-addicted individuals.
Recommendations
Our results of impaired reward processing and the perception of inner drives in the cocaine abusers provide a possible neuropsychological explanation for the deterioration over time in the effectiveness of insight-oriented dynamically driven psychotherapies in drug-addicted individuals (41) . Using interventions aimed at helping drug abusers to recognize external situations that produce stress, craving, or the risk of relapse and teaching them cognitive behavior skills to counteract these situations may prove beneficial. In particular, therapeutic skill development could include cognitive strategies targeted at strengthening prefrontal cortex control of behavior, especially under salient emotional situations.
1. Goldstein RZ, Volkow ND: Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002; 159:1642–1652Google Scholar
2. Rollnick S, Heather N, Bell A: Negotiating behaviour change in medical settings: the development of brief motivational interviewing. J Ment Health 1992; 1:25–37Google Scholar
3. Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA: Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157:1789–1798Google Scholar
4. Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P: Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 2005; 25:3932–3939Google Scholar
5. Kalivas PW, Volkow ND: The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005; 162:1403–1413Google Scholar
6. Hikosaka K, Watanabe M: Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex 2000; 10:263–271Google Scholar
7. Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC: Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 2004; 16:1746–1772Google Scholar
8. Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE: Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 2004; 16:463–478Google Scholar
9. Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, Foley M, Hyman SE, Rosen B, Weisskoff R: Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab 1998; 18:724–734Google Scholar
10. Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, Wolf A: Is methylphenidate like cocaine? studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995; 52:456–463Google Scholar
11. Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL: Activation of the human brain by monetary reward. Neuroreport 1997; 8:1225–1228Google Scholar
12. Tellegen A, Waller NG: Exploring personality through test construction: development of the Multidimensional Personality Questionnaire, in Personality Measures: Development and Evaluation, Vol 1. Edited by Briggs SR, Cheek JM. Greenwich, Conn, JAI Press, 1997, pp 1–67Google Scholar
13. Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T: K-space based summary motion detection for functional magnetic resonance imaging. Neuroimage 2003; 20:1411–1418Google Scholar
14. Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K: High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med 1995; 34:308–312Google Scholar
15. Ashburner J, Neelin P, Collins DL, Evans A, Friston K: Incorporating prior knowledge into image registration. Neuroimage 1997; 6:344–352Google Scholar
16. Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS: Statistical parametric maps in functional imaging: a general approach. Hum Brain Mapp 1995; 2:189–210Google Scholar
17. Tomasi D, Ernst T, Caparelli EC, Chang L: Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage 2004; 23:1414–1421Google Scholar
18. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical 1988Google Scholar
19. Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC: A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996; 4:58–73Google Scholar
20. Elliott R, Newman JL, Longe OA, Deakin JF: Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 2003; 23:303–307Google Scholar
21. Tremblay L, Schultz W: Relative reward preference in primate orbitofrontal cortex. Nature 1999; 398:704–708Google Scholar
22. Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P: Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001; 30:619–639Google Scholar
23. Kringelbach ML, O’Doherty J, Rolls ET, Andrews C: Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 2003; 13:1064–1071Google Scholar
24. Knutson B, Westdorp A, Kaiser E, Hommer D: FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 2000; 12:20–27Google Scholar
25. O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C: Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 2001; 4:95–102Google Scholar
26. Watanabe M: The appropriateness of behavioral responses coded in post-trial activity of primate prefrontal units. Neurosci Lett 1989; 101:113–117Google Scholar
27. Tobler PN, Fiorillo CD, Schultz W: Adaptive coding of reward value by dopamine neurons. Science 2005; 307:1642–1645Google Scholar
28. Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW: Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci 2004; 24:1793–1802Google Scholar
29. Goerendt IK, Lawrence AD, Brooks DJ: Reward processing in health and Parkinson’s disease: neural organization and reorganization. Cereb Cortex 2004; 14:73–80Google Scholar
30. Hester R, Garavan H: Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 2004; 24:11017–11022Google Scholar
31. Bechara A, Tranel D, Damasio H: Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000; 123(Part 11):2189–2202Google Scholar
32. Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A: The involvement of the orbitofrontal cortex in the experience of regret. Science 2004; 304:1167–1170Google Scholar
33. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318–325Google Scholar
34. Goldstein RZ, Volkow ND, Chang L, Wang GJ, Fowler JS, Depue RA, Gur RC: The orbitofrontal cortex in methamphetamine addiction: involvement in fear. Neuroreport 2002; 13:2253–2257Google Scholar
35. Goldstein RZ, Alia-Klein N, Leskovjan AC, Fowler JS, Wang GJ, Gur RC, Hitzemann R, Volkow ND: Anger and depression in cocaine addiction: association with the orbitofrontal cortex. Psychiatry Res 2005; 138:13–22Google Scholar
36. Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S: Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport 2001; 12:2595–2599Google Scholar
37. Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP: Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 2002; 51:890–895Google Scholar
38. Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA: Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology 2005; 30:610–617Google Scholar
39. Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS: Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155:1009–1015Google Scholar
40. Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A: Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 1991; 148:621–626Google Scholar
41. McCambridge J, Strang J: Deterioration over time in effect of motivational interviewing in reducing drug consumption and related risk among young people. Addiction 2005; 100:470–478Google Scholar