Association of a Triallelic Serotonin Transporter Gene Promoter Region (5-HTTLPR) Polymorphism With Stressful Life Events and Severity of Depression
Abstract
Objective: The lower expressing allele of the serotonin transporter gene 5′ promoter region (5-HTTLPR) polymorphism is reported to be associated with susceptibility to depression and suicidality in response to stressful life events. The authors examined the relationship of a triallelic 5-HTTLPR polymorphism to stressful life events, severity of major depression, and suicidality. Method: Mood disorder subjects (N=191) and healthy volunteers (N=125), all Caucasian subjects of European origin, were genotyped for the triallelic 5-HTTLPR polymorphism (higher expressing allele: L A ; lower expressing alleles: L G , S). All subjects underwent structured clinical interviews to determine DSM-IV diagnoses, ratings of psychopathology, stressful life events, developmental history, and suicidal behavior. CSF 5-HIAA was assayed in a subgroup of subjects. Results: Lower expressing alleles independently predicted greater depression severity and predicted greater severity of major depression with moderate to severe life events compared with the higher expressing L A allele. No associations with suicidal behavior and CSF 5-HIAA were found. Conclusions: Lower expressing transporter alleles, directly and by increasing the impact of stressful life events on severity, explain 31% of the variance in major depression severity. The biological phenotype responsible for these effects remains to be elucidated.
Mood disorders are familial (1 , 2) . Twin studies have demonstrated a role for genetic and environmental factors (3 – 5) , but limited progress has been made in identifying the responsible genes. To date, linkage studies have not replicated vulnerability genes for major depression (6) , perhaps because larger samples are required to detect small effects of individual genes on complex behavioral traits (7) . Case/control association studies of candidate genes have produced mixed results (6) . Gene/environment interactions also contribute to the etiology of mood disorders (6) .
The serotonin transporter gene is of interest because major depression has been associated with fewer serotonin transporter binding sites in 1) platelets, 2) postmortem prefrontal cortex, hypothalamus, occipital cortex, and brainstem, and 3) in vivo in the brainstem and amygdala (8) . Fewer transporter sites in platelets and multiple brain regions is consistent with a lower expressing genetic variant affecting all tissues. A 44-bp insertion/deletion polymorphism has a short allele (S) with lower expression in the 5′ promotor region of the serotonin transporter gene (5-HTTLPR) compared with the long allele (L) in transformed lymphoblastoid cell lines (9) .
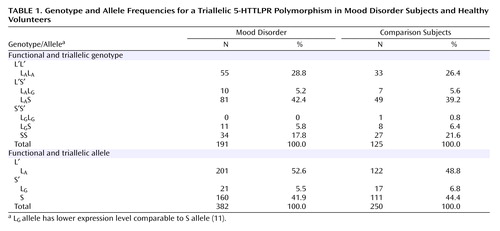
Other 5-HTTLPR variants, particularly in Japanese individuals, are rare in Caucasians (10) . A third functional allele, L G , has been described with an A>G polymorphism at position 6 of the first of two 22-bp imperfect repeats that define the 16-repeat L allele. Originally described by Nakamura et al. (10) , L G is equivalent in expression to the S allele (11) . The allelic frequency of L G is 0.09–0.14 in Caucasians and 0.24 in African-Americans, and the three alleles (S, L A , and L G ) appear to act codominantly (11) , potentially underlying some discordant published findings.
Some, but not all, studies using biallelic S/L genotyping have demonstrated a higher frequency of the low activity S allele in mood disorders and with suicidal behavior (reviewed by Anguelova et al. [12] and Arango et al. [13] ).
Childhood adversity may produce a biological and clinical diathesis for mood disorders that endures into adulthood (14) . Childhood separation from parents, sexual and physical abuse, and adult losses may precede the onset of major depression (15 , 16) . Current stressful life events have a relationship with onset of major depression, modulated at least in part via an interaction with genetic predisposition (2 – 4 , 16–18) . Life events predict depression and suicidal ideation or a suicide attempt in child, adolescent, and young adult carriers of the S allele of the 5-HTTLPR polymorphism (16 , 19 , 20) . These studies did not genotype subjects for the triallelic genotype, and no study has identified a biological intermediate phenotype. Therefore, we report here a study of the effect of the triallelic 5-HTTLPR genotype on the relationship of severity of recent stressful life events and reported childhood abuse to severity of a current major depressive episode and suicidality in adults and to CSF 5-HIAA in a subgroup of subjects.
Method
Subjects
Depressed subjects (N=191) were recruited from patients seeking evaluation and treatment of a mood disorder. Mean age was 39.8 years (SD=15.0), 136 (71%) were female, 61 (32%) had a college degree, 63 (33%) had a history of a suicide attempt, and 57 (30%) reported childhood physical or sexual abuse. All met DSM-IV criteria for a mood disorder. Exclusion criteria were current substance use disorder, head injury, or a current medical illness as determined by clinical history, physical examination, or routine laboratory test results. Diagnoses were current major depressive episode and major depressive disorder (N=115, 60%), bipolar disorder with depressed mood (N=38, 20%), or major depressive disorder in remission (N=38, 20%). Healthy volunteers (N=125) were recruited through advertisements and underwent structured clinical interviews to exclude axis I or axis II diagnoses and a family history of mood disorder, psychosis, or suicide in first-degree relatives. The volunteers also underwent a medical evaluation to exclude medical illness. The mean age of the comparison subjects was 34.6 years (SD=16.8), 63 (51%) were female, and 60% had completed college. Only Caucasians of European origin were included. Written informed consent was obtained from all subjects as approved by the institutional review board after the procedure was explained.
Clinical Phenotype Assessment
Diagnoses
Axis I and axis II diagnoses were made using the Structured Clinical Interviews for DSM-IV (i.e., SCID-I and SCID-II, respectively). Interviews were conducted by clinical psychologists with at least master’s-level degrees. Diagnoses were determined at a consensus conference utilizing data from the SCID, psychiatric clinical evaluation, and clinical charts.
Clinical traits
We rated lifetime aggression using the Brown-Goodwin Aggression Inventory (21) ; lifetime hostility with the Buss-Durkee Hostility Inventory (22) ; and impulsivity with the Barratt Impulsiveness Scale (23) . Depression severity was rated on the 17-item Hamilton Depression Rating Scale and the Beck Depression Inventory. Hopelessness was measured by the Beck Hopelessness Scale (24) .
Stressful life events
The St. Paul-Ramsey Scale (25 , 26) rated impact of significant stressful life events in the prior 6 months. The interviewer scored the impact of specific life experiences on the subject using a 7-point scale of severity: 1=no significant stressor (e.g., small bank loan, common cold, hassle with children); 2=minimal (e.g., minor violation of the law); 3=mild (e.g., broken leg); 4=moderate (e.g., new job); 5=severe (e.g., major illness in self or relative); 6=extreme (e.g., death of a close relative); and 7=multiple family deaths. The St. Paul-Ramsey Scale scores different categories of events: conjugal, other interpersonal, occupational, living situation, health, and others. The outcome variable used in this study was the highest rating score in any category. This scale is reliable in our hands with an intraclass correlation of 0.96.
Genotyping
DNA was extracted from whole blood or buccal mucosa cheek swabs (BuccalAmp DNA Extraction Kit, Epicenter, Madison, Wisc.). Both biallelic and triallelic 5-HTTLPR genotyping were performed using published methods (11 , 27) . For triallelic genotyping (11) , two fluorogenic probes specific for the L A and L G alleles were used. Polymerase chain reaction (PCR) was carried out in a 25-μl volume: 25–50 ng DNA in 1 μl, 24 μl PCR mastermix including probes (120 nmol ADP, 60 nmol ICP), PCR primers (200 nmol of each) and DMSO 4% by volume, 5 mmol/liter MgCl, 1x ABI Corebuffer, 0.2 mmol/liter dATP, 0.2 mmol/liter dGTP, 0.2 mmol/liter dCTP and 0.4 mmol/liter dUTP, 0.25 U/ml Taq Gold, and 0.01 U/ml AmpErase UNG. Each well of the 96-well optical plates (4306737, Alta.I) was sealed with optical caps (4323032, Alta.I). Amplification conditions were 2 minutes at 50 °C, 10 minutes at 95 °C, then 40 cycles at 96 °C for 15 seconds and 62.5 °C for 90 seconds. Genotypes were generated using ABIPRISM 7700 Sequence Detection system software. Genotyping standards were L A L A , L A L G , and L G L G genomic DNA whose genotypes were known by sequencing. In this study, genotypes acquired from these two procedures were combined to classify samples as one of six genotypes: SS, SL A , SL G , L A L A , L A L G , and L G L G . The low expressing S and L G alleles were designated S′ and the higher expressing L A allele was designated L′. To evaluate genotyping accuracy, one-fourth of the samples selected randomly were genotyped in duplicate. Error rate was <0.005.
Lumbar Puncture and CSF 5-HIAA
Lumbar puncture was performed on a subgroup of depressed subjects (N=40) at about 8:00 a.m. after being kept at bed rest and fasting from midnight. CSF 5-HIAA was assayed by high-performance liquid chromatography as previously described (28) .
Statistical Analysis
SPSS statistical software, edition 12.0 for Windows (2003) was used. Pearson/Spearman correlations assessed linear correlations as appropriate. The functionally equivalent L G and S alleles were grouped as the lower expressing allele (henceforth referred to as S′) and compared with the higher expressing L A allele (L′). General linear models with age and sex as covariates were performed to assess interaction between life events and genotypes (independent variables) in predicting clinical measurements such as depression (dependent variables). Results were not subjected to Bonferroni correction, since this analysis was a hypothesis-driven replication. Other analyses are exploratory. Kolmogorov-Smirnov test assessed normal distribution. Student’s t tests or analyses of variance compared scores (dependent variables) in genotypic groups (independent variables). A chi-square test assessed genotypic frequencies between groups unless Fisher’s exact test was indicated. All tests were two-tailed.
Results
Clinical and Demographic Features
Depressed and healthy subjects did not differ in level of education (t=0.30, df=314, p=0.75), but the depressed subjects were slightly older (39.8 years [SD=15.0] versus 34.6 years [SD=6.8], respectively; F=8.12, df=1, 314, p=0.005) and had a higher percentage of women (71.2% versus 50.4%; t=4.0, df=314, p<0.0001). Therefore, we controlled for sex and age and reanalyzed the findings for both sexes separately.
Reported abuse in childhood was more common in the mood disorder group than in the comparison group (33.5% [N=57] versus 5.7% [N=6], respectively; χ 2 =28.13, df=1, p<0.0001). The mood disorder group had scores of 13.5 (SD=8.6) on the Hamilton Depression Rating Scale, 22.2 (SD=13.2) on the Beck Depression Inventory, 10.2 (SD=6.2) on the Beck Hopelessness Scale, 48.1 (SD=17.0) on the Barratt Impulsiveness Scale, 16.3 (SD=3.9) on the Brown-Goodwin Aggression Inventory, and 31.4 (SD=11.6) on the Buss-Durkee Hostility Inventory. Duration of the current mood episode was 24 months (SD=116), median number of previous depressive episodes was 2.0 (SD=4.2), and the median age of onset was 24.0 years (SD=13.9). For all subjects, age and sex did not differ by genotype (age: F=0.89, df=2, 314, p=0.41; sex: χ 2 =1.34, df=1, p=0.51).
Relationship of 5-HTTLPR Genotype to Clinical Variables
Depression, suicide, and other psychopathology ratings
Genotypes were in Hardy-Weinberg equilibrium for the healthy subjects (χ 2 =1.349, df=1, p=0.24) and depressed subjects (χ 2 =0.378, df=1, p=0.54). Lower expressing L G alleles constituted 10.5% of the L alleles (N=38 of 361) and were functionally grouped with the S allele in Table 1 and designated as S′. Genotype and allele frequencies were comparable in the depression and healthy groups where genotype used the functional groupings of L′ and S′ and allelic analyses used the triallelic results (genotypes: χ 2 =1.09, df=2, p=0.57; alleles: χ 2 =1.08, df=2, p=0.58) ( Table 1 ).
Neither genotype nor allele frequencies differed within the depressed group among those who did versus did not attempt suicide (χ 2 =0.10, df=2, p=0.95). Within suicide attempters, number of suicide attempts (F=0.19, df=2, 59, p<0.57), current suicidal ideation score (F=0.45, df=2, 58, p<0.65), and medical lethality score of the most lethal attempt did not differ by genotype (F=2.23, df=2, 58, p<0.12).
In the depressed group, there were no differences by genotype in lifetime aggression (F=0.88, df=2, 161, p<0.42), impulsivity (F=0.03, df=2, 158, p<0.97), hostility (F=1.00, df=2, 156, p<0.37), and current depression (Hamilton scale [F=0.44, df=2, 181, p<0.64] or Beck Depression Inventory score [F=0.63, df=2, 174, p<0.54]), or hopelessness (F=2.06, df=2, 171, p<0.14). There was no difference in CSF 5-HIAA across genotypes (F=0.53, df=2, 38, p<0.90).
Depression and stressful life events
Severity of stressful life events in the 6 months preceding study entry in the depressed group did not differ by genotype (data not shown). A potential gene/environment interaction effect on severity of depression was tested by a general linear model with depression severity (Hamilton Depression Scale score) as the dependent variable and 5-HTTLPR genotype (S′S′, L′S′, and L′L′) and life events (St. Paul-Ramsey Scale score) as independent variables. The overall model was significant, and independent effects were found for genotype and the interaction of genotype and St. Paul-Ramsey Scale score ( Figure 1 ). The overall model explained 30.8% of the variance of depression severity, with genotype explaining 12.6% and the interaction explaining 17.3%. The S′ allele was associated with more severe depression and more lifetime major depressive episodes. For the interaction, the S′S′ genotype was associated with more severe depression (Hamilton Depression Scale score) in subjects with high versus low life events scores (19.5 [SD=8.8] versus 10.8 [SD=8.8], respectively; t=–2.47, df=17, p=0.02). Life events alone did not relate to depression severity (F=2.09, df=5, 78, p<0.08). Controlling for gender in the general linear model or analyzing the results only for female subjects produced the same findings (data not shown). The number of male subjects in the sample was too small to permit meaningful analysis.
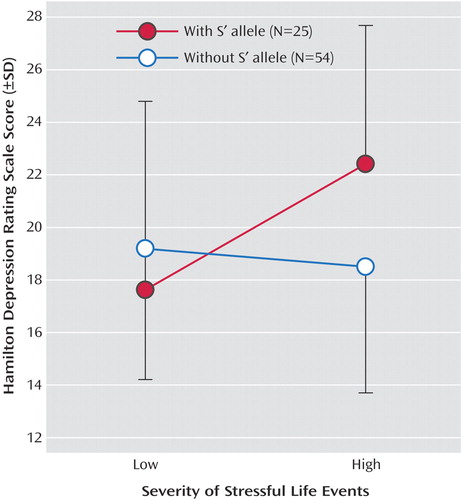
a Stressful life events score measured by St. Paul-Ramsey Scale (30, 31). High and low stressful life events were defined using a median split. The overall model was significant (F=2.22, df=13, 78, p<0.02), and independent effects were found for genotype (F=4.71, df=2, 78, p<0.02) and the interaction of genotype and St. Paul-Ramsey Scale score (F=2.27, df=6, 78, p<0.05).
Reported history of childhood abuse
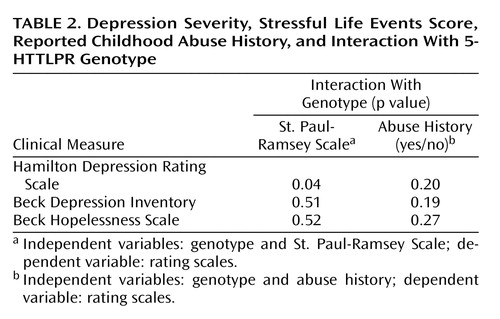
5-HTTLPR genotype was not associated with a reported history of childhood abuse (χ 2 =0.454, d=1, p<0.51). In a general linear model with depression severity (Hamilton depression scale score) as the dependent variable and 5-HTTLPR genotype and childhood abuse as independent variables, the overall model (F=0.89, df=2, 163, p<0.49), abuse history (p<0.85), and genotype-by-abuse history interaction (p<0.21) did not predict depression severity. Exploratory analyses of the effect of abuse history with genotype on number of lifetime major depressive episodes in the model, age of onset of first episode, and duration of current episode were not significant (data not shown).
The same analyses with Beck Depression Inventory or Beck Hopelessness Scale scores as dependent variables and genotype, life events, or childhood abuse as independent variables were not significant ( Table 2 ), nor were the analyses significant with the Brown-Goodwin Aggression Inventory, Buss-Durkee Hostility Inventory, or Barratt Impulsiveness Scale used as dependent variables (data not shown).
Discussion
The present study replicates and extends findings of a 5-HTTLPR/environment interaction effect on depression—reported in children (16) , female adolescents (19) , and young adults (20) —in an older study group and employing direct face-to-face severity ratings and triallelic genotyping of the 5-HTTLPR polymorphism. The triallelic approach found 10.5% of the L alleles were the lower expressing L G allele that otherwise would have been treated as the higher expressing L A allele. This is the first demonstration that genotype independently predicts severity of major depression directly and via a genotype or S′ allele-by-life events interaction effect on depression severity. This model explained 31% of the variance in severity of major depression, with genotype and the interaction term contributing comparable components. Genetic factors explain 40%–70% of the probability of major depression (4 , 5) , but genetic contribution to severity of major depression has been less studied. Major depression severity correlates with severity of recent life events (3 , 4 , 17 , 18) , but when we considered genotype, life events alone no longer had an effect. Instead, the S′ allele had both a direct effect and an indirect effect on severity of depression by modulating the impact of life events.
Like others (16 , 19) , and consistent with the alleles being codominant in terms of expression levels, we found an effect of genotype on depression severity. The severity of life events and symptoms of anxiety and depression are familial (29) , and one explanation may be that the relationship of life events to severity of depression and the experience of life events are dependent on a common genetic effect, such as the S′ allele. The relationship shown in Figure 1 is comparable to both other reports on 5-HTTLPR/environment interaction and depression (19 , 20) . The S′ allele appears associated with higher depression scores only when life events were more severe. Kaufman et al. (16) examined severe levels of maltreatment requiring separation from parents and found that therapy ameliorated the effect of the S allele. This relationship needs further study.
Measurement of depression differed among studies. We used a face-to-face diagnostic interview and clinician-rated severity of depression. Caspi et al. (19) made a diagnosis and used informant reports of depressive symptoms by mailing a brief self-report questionnaire to persons close to the proband. Eley et al. (20) gave parents a self-report questionnaire to be completed by the adolescent offspring. Kaufman et al. (16) read a self-report depression measure to the children, and most of their cases did not meet criteria for major depression. Our study and others also used different methods to assess life events over the prior 6 months. Kaufman et al. (16) defined a common stressor of sufficient maltreatment as exposure to familial violence requiring removal of the child proband from the parental home within the previous 6 months. Caspi et al. (19) employed number of events, while we scored severity and impact of stressful life events (25) . The robustness of the relationship of the 5-HTTLPR genotype-adversity interaction effect on depression is emphasized by the agreement between four studies despite methodological differences.
Of note, to minimize recall effects we assessed stressful life events in the 6 months prior to evaluation, yet the median duration of major depressive episodes was 24 months. Thus, we captured stressful life events during the depressive episode, and some stressful life events may have been a consequence of depression. Nevertheless, a genotype that increases vulnerability to the effects of life events on depression will affect the impact of a stressful life event regardless of whether it was triggered by the depression.
The biologic intermediate phenotype that mediates the 5-HTTLPR effects on depression is uncertain. Lower postmortem 5-HTT binding in the prefrontal cortex in major depression, and lower in vivo 5-HTT binding in the brainstem raphe nuclei appear unrelated to genotype (30 , 31) , although we have reported less 5-HTT gene expression in depressed suicide victims (32) . Paradoxically, serotonin reuptake inhibitors help depression. Transient 5-HTT inhibition in rodents in infancy produces a depressive behavioral phenotype (33) suggesting a developmental effect is responsible, perhaps involving heightened amygdala sensitivity to negative experiences as reported in those with the S allele (34) . Nonhuman primates having the S allele experience a persistent decline in serotonin activity after maternal separation as measured by CSF 5-HIAA (35) . This decline in serotonin function results in greater aggression, impulsiveness, and risk-taking as an adult. If such an effect of childhood adversity on serotonin function with the S allele could be demonstrated in humans, it may explain greater depression following maternal separation (16) and vulnerability to life events later in life (19 , 20) .
In agreement with Jonsson et al. (36) we failed to find an association of 5-HTTLPR with CSF 5-HIAA. However, our subgroup may have been too small to detect a weaker effect, since others (37) have reported lower CSF 5-HIAA in association with the S allele. The identification of a biologic phenotype remains a goal for future study.
Our exploratory analysis found no association of 5-HTTLPR genotype with reported history of childhood abuse, and the interaction did not predict severity of depression. Others have reported that childhood maltreatment and the S allele predict depression in childhood and young adults (16 , 19) perhaps because they collected data contemporaneously. Study group size prevented an analysis of stressful life events, genotype, and abuse history. Clearly, further study is needed to determine whether the S′ allele favors the appearance of adult major depression in those who report childhood abuse and whether this is related to an effect on serotonin function.
Eley et al. (20) examined both sexes but found a significant interaction for female subjects only. Our findings remained significant in female subjects only, but we had too few male subjects to analyze them separately. Since two previous studies have not analyzed male subjects separately, it remains to be determined whether the effect is comparable in both sexes. Stress hormone responses in nonhuman primates who were peer-reared are elevated in female but not male subjects with the S allele (38) suggesting that the gene/environment interaction might be stronger in women.
Stressful life events occurring after age 21 predict suicidal ideation or attempt at age 26 in S allele carriers (19) . Two meta-analyses of the association of suicidal behavior with 5-HTTLPR polymorphism disagree: one found a weak association with suicidal behavior (12) and the second did not (39) . We did not find an association with suicide attempts or ideation despite direct interviews quantifying severity of ideation and obtaining a lifetime suicide attempt history. Only one of three other studies (19) report on suicidal ideation and attempts. However, this study did not control for depression severity and examined attempts reported by 3% of the sample in the context of depressive episode and not lifetime. We previously reported lower postmortem ventral prefrontal cortical 5-HTT binding in suicide victims but did not find an association with 5-HTTLPR genotype (30) . We found no association with suicide (30) or suicide attempts, raising questions regarding the results of one previous report (19) . Our finding that suicides occurred only in first-degree relatives of probands with the S′ allele, while not statistically significant in our study group, is consistent with previous reports (12 , 40) .
This study replicates and extends findings in children, female adolescents, and young adults of the stress sensitivity of depression associated with the S′ allele (16 , 19 , 20) . We also found an independent effect of genotype on depression severity in adults. Larger prospective studies with more contemporaneous data on childhood abuse and assessment of recent life events prior to the onset of major depression will help clarify these gene/environment relationships in mood disorders. The biological mechanism whereby the S′ allele conveys these effects is unknown, but amygdala sensitivity to stressful events and childhood stress-induced serotonin dysfunction are candidate mechanisms.
1. Weissman MM, Gershon ES, Kidd KK, Prusoff BA, Leckman JF, Dibble E, Hamovit J, Thompson WD, Pauls DL, Guroff JJ: Psychiatric disorders in the relatives of probands with affective disorders. The Yale University−National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry 1984; 41:13–21Google Scholar
2. Sullivan PF, Neale MC, Kendler KS: Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157:1552–1562Google Scholar
3. Kendler KS, Karkowski-Shuman L: Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol Med 1997; 27:539–547Google Scholar
4. Kendler KS, Prescott CA: A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry 1999; 56:39–44Google Scholar
5. McGuffin P, Katz R, Rutherford J: Nature, nurture and depression: a twin study. Psychol Med 1991; 21:329–335Google Scholar
6. Malhi GS, Moore J, McGuffin P: The genetics of major depressive disorder. Curr Psychiatry Rep 2000; 2:165–169Google Scholar
7. Plomin R, Owen MJ, Mcguffin P: The Genetic basis of complex human behaviors. Science 1994; 264:1733–1739Google Scholar
8. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [iodine-123]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090–1098Google Scholar
9. Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, Riederer P, Lesch KP: Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect 1995; 102:247–254Google Scholar
10. Nakamura M, Ueno S, Sano A, Tanabe H: The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows 10 novel allelic variants. Mol Psychiatry 2000; 5:32–38Google Scholar
11. Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA: An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res 2005; 29:8–16Google Scholar
12. Anguelova M, Benkelfat C, Turecki G: A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter, II: suicidal behavior. Mol Psychiatry 2003; 8:646–653Google Scholar
13. Arango V, Huang YY, Underwood MD, Mann JJ: Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res 2003; 37:375–386Google Scholar
14. Heim C, Nemeroff CB: The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001; 49:1023–1039Google Scholar
15. Brown GW, Harris T, Copeland JR: Depression and loss. Br J Psychiatry 1977; 130:1–18Google Scholar
16. Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J: Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A 2004; 101:17316–17321Google Scholar
17. Moffitt TE, Caspi A, Rutter M: Strategy for investigating interactions between measured genes and measured environment. Arch Gen Psychiatry 2005; 62:473–481Google Scholar
18. Paykel ES: Life events and affective disorders. Acta Psychiatr Scand Suppl 2003; 418:61–66Google Scholar
19. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389Google Scholar
20. Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW: Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry 2004; 9:908–915Google Scholar
21. Brown GL, Goodwin FK: CSF correlates of suicide attempts and aggression. Ann N Y Acad Sci 1986; 487:175–188Google Scholar
22. Buss AH, Durkee A: An inventory for assessing different kinds of hostility. J Consult Psychol 1957; 21:343–348Google Scholar
23. Barratt ES: Impulsiveness and aggression, in Violence and Mental Disorder: Development in Risk Assessment. Edited by Monahan J, Steadman HJ. Chicago, University of Chicago Press, 1994Google Scholar
24. Beck AT, Steer RA, Kovacs M, Garrison B: Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985; 142:559–563Google Scholar
25. Paykel ES: Methodological aspects of life events research. J Psychosom Res 1983; 27:341–352Google Scholar
26. Mann JJ, Waternaux C, Haas GL, Malone KM: Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry 1999; 156:181–189Google Scholar
27. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Google Scholar
28. Scheinin M, Chang WH, Kirk KL, Linnoila M: Simultaneous determination of 3-methoxy-4-hydroxyphenylglycol, 5-hydroxyindoleacetic acid, and homovanillic acid in CSF with high-performance liquid chromatography using electrochemical detection. Anal Biochem 1983; 131:246–253Google Scholar
29. Rijsdijk FV, Sham PC, Sterne A, Purcell S, McGuffin P, Farmer A, Goldberg D, Mann A, Cherny SS, Webster M, Ball D, Eley TC, Plomin R: Life events and depression in a community sample of siblings. Psychol Med 2001; 31:401–410Google Scholar
30. Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V: A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000; 57:729–738Google Scholar
31. van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J: Central serotonin transporter availability measured with [iodine-123]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry 2004; 161:525–531Google Scholar
32. Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJX, Mann JJ: Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001; 25:892–903Google Scholar
33. Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA: Early life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004; 306:879–881Google Scholar
34. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403Google Scholar
35. Suomi SJ: Gene-environment interactions and the neurobiology of social conflict. Ann N Y Acad Sci 2003; 1008:132–139Google Scholar
36. Jonsson EG, Nothen MM, Gustavsson JP, Neidt H, Bunzel R, Propping P, Sedvall GC: Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Psychiatry Res 1998; 79:1–9Google Scholar
37. Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC: CNS serotonin function and cardiovascular responses to stress. Psychosom Med 2001; 63:300–305Google Scholar
38. Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD: Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A 2004; 101:12358–12363Google Scholar
39. Lin PY, Tsai G: Association between serotonin transporter gene promoter polymorphism and suicide: results of a meta-analysis. Biol Psychiatry 2004; 55:1023–1030Google Scholar
40. Joiner TE Jr, Johnson F, Soderstrom K: Association between serotonin transporter gene polymorphism and family history of attempted and completed suicide. Suicide Life Threat Behav 2002; 32:329–332Google Scholar