Conversion Disorder
The diagnosis of psychogenic nonepileptic seizures has become easier with the assimilation of studies on the clinical categorization of seizure-like events (1) , video EEG monitoring (2) , measurement of serum prolactin (3) , the selective use of neuropsychological tests (4) , and various other diagnostic methods. Much less information is available to the clinician on what to do next. How should the diagnosis best be presented? What is optimal treatment, and how best should it be individualized? How well does treatment work? We describe a patient with nonepileptic seizures and psychogenic tremors as a starting point for a discussion about how to proceed after establishing a diagnosis of conversion disorder.
Case Presentation
“Ms. A,” a 53-year-old left-handed woman, was admitted to our epilepsy monitoring unit for evaluation of a 4-month history of tremors, head bobbing, and episodic loss of awareness. The onset of these symptoms was 1 week after she had visited an emergency department for a sudden-onset headache. In the emergency department, she developed transient numbness in her left face and arm and a left facial droop; she also became increasingly distressed by the long delay in being attended to. Ultimately, she left, quite frustrated because a physician never examined her, although she had laboratory tests and imaging studies. Magnetic resonance imaging (MRI) showed a right cerebellar lacunar infarct, suggestive of a prior stroke, but subsequent imaging proved it to be artifactual.
Six days later, Ms. A started having events in which her speech became progressively more syllabic in cadence. Her arms, head, and then her body would shake for minutes without loss of consciousness. Lorazepam provided transient relief.
Ms. A later visited a naturopath, who began neck manipulations, which triggered new episodes (i.e., she uttered, “Ooh…ooh,” while clapping both hands and feet, sometimes accompanied by visual changes, tongue deviation, and unresponsiveness). These episodes lasted up to 5 hours and occurred daily. Anxiety, music, and stress worsened her symptoms, whereas sleep improved them. Between episodes, she had a continuous head tremor.
Ms. A was then admitted to an epilepsy monitoring unit for diagnosis with video EEG recording. At admission, she was taking 40 mg/day of citalopram (later switched to escitalopram), 3 mg/day of lorazepam, 1 mg/day of benztropine, 600 mg/day of gabapentin, 0.1 mg/day of levothyroxine sodium, 75 mg/day of clopidogrel, 1 mg/day of clonazepam, 30 mg/day of nifedipine, and 180 mg/day of fexofenadine. Except for levothyroxine sodium and fexofenadine, all medications had been prescribed for neuropsychiatric symptoms.
Several typical episodes with shaking, tremor, and abnormal movements were recorded, with no clinically significant accompanying EEG changes. All event types were captured during the patient’s monitoring stay.
Initial evaluation by a psychologist uncovered a history of childhood and adolescent sexual abuse by Ms. A’s father with a later discovery that he had also molested her sisters and daughters. Ms. A’s symptoms began around the seventh anniversary of her father’s death, after she discovered his abuse of her daughters.
Her verbal IQ was 94, her performance IQ was 105, and her full-scale IQ was 99. Her verbal and visual memory functions were intact. On the MMPI-2 (5) , Ms. A showed a classic “conversion V pattern”: on scale 1 (hypochondriasis) and scale 3 (hysteria), her scores were elevated and considerably higher than on scale 2 (depression).
Conversion disorder, the nonepileptic seizures subtype, was diagnosed on the basis of video EEG recordings, history, and psychological testing. The diagnosis was explained by a neuropsychologist and a neurologist before Ms. A’s discharge from our video EEG monitoring unit. She was initially skeptical and angry about this diagnosis but ultimately accepting. She was referred to a psychotherapist experienced in working with patients with conversion disorder. A movement disorder specialist diagnosed a psychogenic tremor and voice disturbance. The episodic shaking events largely remitted, but Ms. A continued to have voice disturbances and head bobbing, which made her self-conscious, and she was no longer able to work.
When she was first seen by one of us (C.M.S.), Ms. A reported that the depression caused by her loss of function had lasted more than a year but was improving. Her psychotherapist was helping her identify her normal emotions, express her feelings more directly, and make choices that gave her a greater sense of control. The therapist also had pointed out the significance of the feelings that were elicited in the emergency department. As a teenager, Ms. A had suicidal ideation triggered by an abortion that her father reportedly performed. Afterward, she visited an emergency department for persistent bleeding, where she experienced terror, anger, and loss of control when left unattended for hours.
We affirmed the diagnosis of conversion disorder, together with major depression (recurrent in partial remission) and an anxiety disorder. Despite some histrionic and dependent traits, Ms. A was not considered to have a personality disorder. She was given a prescription for aripiprazole (5 mg b.i.d.) for residual depression, anxiety, and mood lability, in conjunction with discussion about its off-label use and its potential to induce abnormal movements. She was encouraged to return for follow-up to learn self-hypnosis to control the head tremor.
One month later, Ms. A reported improved energy, focus, and concentration and said that she felt less overwhelmed with everyday stress. She still had the head tremor and the effortful near-monosyllabic speech. In her second therapy session, she was found to be highly hypnotizable with the Hypnotic Induction Profile (6) , scoring 9.5 on a 10-point scale.
Ms. A was then taught self-hypnosis; she focused on a visual image of herself floating on water with her head stabilized in a floating ring. She successfully used this image to eliminate the head bobbing. During the therapy session, she practiced turning the bobbing on and off at will while in a trance-like state. She was instructed to practice self-hypnosis 10 times a day. More than a year later, she continued to use this technique successfully. However, the head bobbing recurred when she did not practice regular self-hypnosis. Her interpersonal psychotherapy visits decreased to monthly, her daily functioning improved, and she had no recurrence of the seizure-like episodes. She stopped taking lorazepam, clonazepam, benztropine, and gabapentin but continued to take escitalopram and aripiprazole. She has tried discontinuing aripiprazole but felt anxious and functioned less well without it.
Terminology
“Conversion disorder” is the term used in the DSM-IV classification system, originating from the description by Breuer and Freud (7) of pseudoneurological symptoms resulting from conversion of an unconscious psychological conflict to somatic representation. Other adjectives historically used to describe the same phenomena include “hysterical” and “psychogenic.” The seizure subtype of conversion disorder is often referred to as “pseudoseizures,” but we chose to use the term “nonepileptic seizures.” The term “pseudoseizure” may incorrectly imply to the patient that the symptom is not real. “Nonepileptic seizures” correctly describes the symptoms without invoking a cause, and patients tend to prefer this term. Beginning treatment with a power struggle over terminology weakens the doctor-patient relationship, and successful outcome often depends on good rapport.
Pathophysiology
At present, treatment is not based on an understanding of the underlying pathophysiology of conversion disorder. Recent functional neuroimaging studies point to a neurophysiological basis for conversion, albeit triggered by psychological processes. Functional imaging data suggest that neural circuits linking volition, movement, and perception are disrupted in conversion disorder (8) , although conclusions have been limited by the small number of subjects, varying study designs, and heterogeneous populations.
Frontal-subcortical circuits mediate many aspects of human behavior (9) . The orbitofrontal cortex serves as a control center, coordinating various regions of the thalamus, amygdala, and cortex. Both the orbitofrontal cortex and the anterior cingulate cortex mediate emotional and central executive functions and are activated when subjects suppress competing responses, suggesting an inhibitory role (10) . The anterior cingulate cortex has been implicated in the mediation of consciousness (11) . Blood flow to the anterior cingulate cortex is positively correlated with emotional awareness (12) .
Preliminary evidence suggests that during conversion reactions, primary perception is intact, but modulation of sensory and motor planning is impaired by disruption of the anterior cingulate cortex, orbitofrontal cortex, and limbic brain regions (8) . Furthermore, reduced activation of the frontal and subcortical areas involved in motor control is observed during conversion paralysis (13) , reduced activation in somatosensory cortices is seen during conversion anesthesia (14) , and reduced activation in the visual cortex is noted during conversion blindness (15) .
Marshall and colleagues (16) measured regional cerebral blood flow in a woman with left-side conversion paralysis as she attempted to move her paralyzed leg and also as she moved her nonparalyzed right leg. Her attempt to move the paralyzed leg failed to activate the right primary motor cortex, and there were significant activations—not observed under other conditions—of the right anterior cingulate cortex and the right orbitofrontal cortex (16) . With the same experimental design, Halligan and colleagues (17) measured brain activity in a man with hypnotically induced paralysis of the left leg. They found similar activations of the right anterior cingulate cortex and the orbitofrontal cortex and no activation of the motor and premotor cortex. The activations of the anterior cingulate cortex and the orbitofrontal cortex apparently represented inhibition of the subject’s voluntary attempt to move his left leg. Other functional imaging studies of patients with acute conversion paralysis (18 , 19) and astasia-abasia (20) also implicated disruption of striatothalamocortical premotor pathways, with possible pathological inhibition from activation of the anterior cingulate cortex and the orbitofrontal cortex.
Comorbid Conditions
Therapy for nonepileptic seizures must take into account the likelihood that a patient with conversion disorder will also meet criteria for another axis I disorder. Typical comorbid diagnoses include mood disorders, panic disorder, generalized anxiety disorder, posttraumatic stress disorder, dissociative disorders, social or specific phobias, and obsessive-compulsive disorders (21 – 23) . Axis II pathology and having close relatives with psychiatric illness or severe somatic disease are also common (21) . Treatment of the associated psychiatric conditions will benefit overall functioning and recovery. Our patient profited from treatment of comorbid depression, which improved her overall functioning and responsiveness to psychotherapeutic interventions.
Patients with conversion symptoms commonly report a history of physical or sexual abuse. A study comparing 54 patients who had conversion disorder with 50 matched patients who had an affective disorder (24) found a higher incidence and longer duration of physical or sexual abuse and more incestuous experiences in patients with conversion disorder.
Whether some of our patient’s memories were false is unknown. Having false memories may be a form of conversion, with pathophysiological mechanisms similar to those of motor conversion (25) . Although specific details of any abuse may be questionable, many patients undoubtedly experienced substantial family dysfunction, attachment disorders, or impaired object relations. In contrast to patients with motor conversion symptoms, patients with nonepileptic seizures are more likely to have experienced childhood abuse (26) .
In patients with nonepileptic seizures, depression is the most common comorbid diagnosis, occurring in 12%–100%. Also common are anxiety disorders (11%–80%), dissociative disorders (90%), other somatoform disorders (42%–93%), and personality disorders (33%–66%) (27) . The strong overlap of nonepileptic seizures with dissociative disorders has prompted some authors to propose reclassifying conversion disorders within the dissociative disorders spectrum (28) .
Patients with nonepileptic seizures appear to have greater psychopathology and somatization, as measured by personality tests, than healthy comparison subjects or patients with epilepsy. Owczarek (29) found that such patients scored significantly higher on four of five MMPI somatization parameters than patients with mixed epilepsy and nonepileptic seizures or epilepsy alone. A tendency to discount the importance of psychological factors contributing to illness, denial of external stressors, and an external locus of control are additional cognitive factors shown to be more prevalent in patients with nonepileptic seizures than in patients with epilepsy (30) . When Reuber and colleagues (31) compared 85 patients with nonepileptic seizures with 63 with epilepsy and 100 healthy volunteers, they found that the patients with nonepileptic seizures had more personality abnormalities and that outcomes varied by personality profile. Cragar and colleagues (32) further defined three clusters of personality subtypes among patients with nonepileptic seizures and 79 epilepsy patients prospectively evaluated in an epilepsy monitoring unit and noted significant differences between the two groups. Our patient’s relative lack of severe axis II pathology may have contributed to her ability to benefit from treatment.
Treatment
Treatment begins with presentation of the diagnosis. Even before a formal discussion of treatment options, the diagnostic workup and the presentation of the diagnosis offer opportunities to improve the patient’s outcome. Conversely, the use of intravenous saline or placebo patches to induce nonepileptic seizures for diagnostic purposes may be perceived by the patient as dishonest, and therefore, it may risk serious damage to the doctor-patient relationship (33 , 34) . It may even induce unrepresentative nonepileptic seizures in patients with epilepsy. Hypnosis can avoid the pitfall of deception, if its purpose and aims are fully explained in advance. Once in a trance-like state, patients are directed to turn the seizure-like event “on” and “off” (35) , a technique that can be used again for treatment purposes.
Many physicians are uncomfortable presenting a diagnosis of conversion disorder to a patient. Angry reactions from patients may derive from a perceived sense (sometimes based on reality) of abandonment by a physician. A prior experience of abandonment or abuse by authority figures compounds these reactions (36) . Therefore, careful attention to how the diagnosis is presented can often help maintain an ongoing therapeutic relationship. Key points are listed in Appendix 1 .
A discussion of the diagnosis must be timed sensitively to occur after confirmation of the diagnosis but before the patient has been upset by indirect and fragmentary discussions (37) . Discussion ideally should take place after the patient and family have agreed that representative events have been captured by video EEG monitoring. If not all types of events have been characterized, as is often the case, then the clinician should openly admit that other types of episodes may be extant. A standard protocol for presenting the diagnosis (38) can be individualized and updated to conform to the current base of evidence and experience. We also find it useful to give printed educational materials on conversion disorder to patients and their families. Because patients with conversion disorder may be less open to psychological explanations than are patients with defined neurological illness (30) , the groundwork for a discussion of psychological and stress-related factors must be laid carefully.
We start with a recapitulation of the results of the tests, central to which is the observation that brain waves were unremarkable during the episode. Although some brief, focal, and deeply situated seizures show no scalp-recorded EEG changes (39) and may be associated with histrionic behaviors (40) , epilepsy episodes severe enough to alter consciousness, memory, responsiveness, and generalized motor activity should show EEG correlates. We avoid mentioning “real” or “unreal” seizures because patients should know that the medical care team believes that the symptoms are “real” even if the patients are not epileptic and that they have a negative impact on the patient’s functioning and quality of life. We reassure such patients that we know they are not intentionally producing their episodes and that the episodes do not mean that they either are faking it or are “crazy.” The absence of epilepsy is presented as good news. We admit that we do not know what causes nonepileptic seizures, but they result in general from interactions between the subconscious mind and the body.
At this point, we explain that many patients with conversion disorder have a past history of trauma or stress during the critical developmental years and tend to be persons who value being emotionally “strong,” which causes them to discount emotional reactions when coping with difficult situations. Even though the traumatic events may have occurred years ago, the physical symptoms usually begin later in response to new—and often not immediately apparent—triggering events. The physician may also add that prior to their disabling symptoms, patients with conversion disorder are typically highly competent, caring individuals who prefer to focus on others rather than themselves. Patients who relate to these generalizations will find it easier to begin to accept the diagnosis of conversion disorder and understand the need for psychological interventions.
The neurologist may express the view that conversion disorder can coexist with neurological illness (i.e., epilepsy), even when the neurological illness is not detected. Some patients with partial seizures elaborate their symptoms under observation (41) but do have epilepsy. The possibility of a mixed epileptic and nonepileptic pathogenesis should be used as mutual motivation for ongoing vigilance. Offers to continue to follow the patient as long as symptoms persist are usually welcomed, although such visits can span a relatively long interval because the goals of the visits are surveillance and avoidance of abandonment rather than medical therapy. Instead of the automatic and usually futile addition of more medication, the neurologist should work collaboratively with mental health providers. All too often, neurologists and psychiatrists convey differing views on the cause of symptoms and the ways to control them (42) . Therefore, communication between the neurologist and psychiatrist will decrease these mixed messages and set the stage for more successful treatment.
Treatment Options
An overview of the existing medical literature on the treatment of nonepileptic seizures has been presented elsewhere (43) . Although most reports of treatment are anecdotal, there are a growing number of prospective trials.
As a practical matter, we suggest a treatment paradigm for patients with conversion disorder that takes into account the risk factors, perpetuating factors, and triggering events Figure 1 . First, the treatment team should consider the relevant risk factors for any given patient.
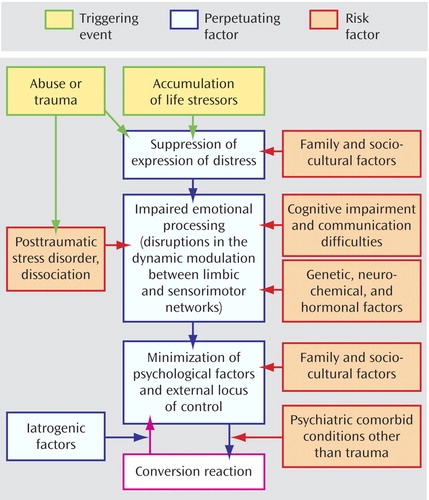
a After a diagnosis is presented, treatment begins by directly addressing relevant risk factors (e.g., psychiatric comorbid conditions and communication difficulties). Next, psychological interventions should focus on minimizing the perpetuating factors and recognizing triggering events. These data are from references 30, 43, 48, and 50.
If the patient has substantial cognitive impairment or communication difficulties, treatment is best focused on simple behavioral interventions, physical therapy (44 – 47) , reassurance, and helping the patient verbalize distress.
Working with the family unit may be necessary when family and sociocultural factors predominate, particularly in children and adolescents. Family therapy interventions help the patient and family recognize and address key issues that may be fueling the symptoms. For example, in an analysis of videotaped family interviews of adolescent patients, an unspeakable dilemma was imposed by family or social circumstances in 13 of 14 cases, leading patients with nonepileptic seizures to suppress emotional distress (48) . An open-label trial of family therapy with a problem-centered systems approach (49) for patients with nonepileptic seizures is in process.
Recognition and treatment of comorbid psychiatric conditions are almost always necessary for symptom resolution. Indeed, it may be sufficient to treat the comorbid condition in conjunction with proper presentation of the conversion disorder diagnosis.
However, if patients continue to be symptomatic after these risk factors have been addressed, then psychological treatments that focus more directly on “perpetuating factors” will be necessary. Patients’ (and physicians’) reactions to their conversion symptoms can serve to unwittingly perpetuate them. Avoidant behaviors, minimization of psychological factors, and suppression of expression of distress reinforce an external locus of control. It is easy to see why cognitive behavior therapy would lend itself well to addressing these issues. It is specifically helpful in addressing illness beliefs and denial of stress and in modifying the locus of control (50) . Psychodynamic psychotherapy can also serve to help patients reframe their world view through empathic interpretations and the development of insight, enabling the process of working through past trauma rather than relying on dissociation as a defense (51) . Both approaches will increase awareness of “triggering events,” ultimately leading to greater sense of control of symptoms.
Psychotherapy
Cognitive behavior therapy for nonepileptic seizures is based on the concept that symptoms occur when a patient is confronted with “intolerable or fearful circumstances” and that such symptoms are maintained by a “vicious circle of behavioral, cognitive, affective, physiological, and social factors” (50) . Specific techniques include graded exposure to feared or avoided situations, use of problem-solving techniques, and the reframing of distorted cognitive beliefs about their illness and powerlessness. An open trial of cognitive behavior therapy decreased the frequency of nonepileptic seizures and improved psychosocial functioning (50) . One ongoing controlled study is evaluating the effectiveness of cognitive behavior therapy for patients with nonepileptic seizures (43) , but more well-controlled clinical trial data are needed.
At least five sessions of “counseling” by a therapist affiliated with a comprehensive epilepsy center proved to be more effective in reducing nonepileptic seizures than therapy administered by a nonaffiliated therapist, as measured by a retrospective telephone follow-up survey (52) . Referral to a therapist knowledgeable about nonepileptic seizures or conversion disorder, as was the case for our patient, may also increase the likelihood of a better outcome.
To our knowledge, there are no prospective controlled trials of psychodynamic psychotherapy for nonepileptic seizures (43) , but Kalogjera-Sackellares’s (51) extensive review of 15 years of psychodynamic psychotherapy experience with patients with nonepileptic seizures provides a good overview of that approach. The primary focus of this therapy is on the role of trauma and dissociation (51) , inadequate attachment, and the patient’s difficulty in coping with intrapsychic conflict and anxiety (53) .
Group therapy, preferably in conjunction with concurrent individual therapy, offers advantages of reinforcing psychoeducational concepts, while providing the opportunity for patients to learn from and help each other. Three noncontrolled studies have reported its benefit for patients with nonepileptic seizures (36 , 54 , 55) . Multidisciplinary inpatient treatment may be preferred for patients with severe and prolonged symptoms (56 – 58) , but such resources are not available for many patients.
Hypnosis
Hypnosis has been advocated for the treatment of conversion symptoms since the time of Charcot, Janet, and Freud. Neuroimaging data reinforce the idea that conversion symptoms and hypnosis involve common neurological pathways, and the high hypnotizability of these patients invites the use of hypnosis in their treatment. A study of 44 outpatients with conversion disorder (59) randomly assigned to hypnosis or a waiting list found greater improvement at 3 months with hypnosis. Another study comparing a comprehensive treatment program comprising intensive group therapy, social skills training, creative therapy, sports therapy, and physical therapy with or without hypnosis (58) showed no added benefit from hypnosis for resolving conversion symptoms and no predictive value of hypnotizability for treatment outcome. Hypnosis can be a useful adjunctive treatment, but it is not essential for improvement. A comprehensive approach is likely to be the most effective. Hypnosis without other forms of psychiatric treatment may decrease conversion symptoms but have less impact on overall psychopathology.
Our patient used hypnosis to reduce head tremor, but she also benefited from individual therapy using insight-oriented and cognitive behavior approaches and from medication treatment for overall improvement in functioning, quality of life, and self-esteem.
Pharmacotherapy
Given the lack of data for controlled trials on the pharmacological treatment of conversion disorder, the current practice is to use medications appropriate for the comorbid psychiatric and somatic symptoms and to withdraw antiepileptic drugs unless they are benefiting the comorbid conditions. Anecdotal studies report improvement with selective serotonin reuptake inhibitors (SSRIs), beta-blockers, analgesics, and benzodiazepines (60) . An open trial of antidepressants in patients with psychogenic movement disorder and recent or current depression also showed that class of medications to be effective in reducing conversion symptoms (61) . An ongoing randomized controlled study is evaluating the effectiveness of sertraline for patients with nonepileptic seizures and comorbid depression and anxiety (43) .
Our patient’s condition improved with an SSRI, but her conversion symptoms fully resolved only after she started taking a low-dose atypical antipsychotic medication. No controlled studies have evaluated atypical antipsychotics for the treatment of conversion reactions, particularly in the absence of frank paranoia or psychosis. Reports of the benefits of antipsychotic medications in conversion reactions (36 , 62 – 64 ) are anecdotal.
No general rule exists about whether to continue taking antiepileptic drugs after establishing a diagnosis of nonepileptic seizures. If nonepileptic seizures seem to be the exclusive diagnosis, and the patient willingly enters into treatment, then antiepileptic drugs usually can be tapered. Even in this setting, it is prudent to discontinue one medication at a time, each over a span of a few weeks or months. Barbiturates and benzodiazepines are habit forming and should be tapered gradually. Where nonepileptic seizures and epilepsy are believed to coexist, at least one antiepileptic drug should be maintained. Many antiepileptic drugs provide concomitant mood-stabilizing actions and are sometimes continued for this reason.
Transcranial Magnetic Stimulation
More recent anecdotal reports about the benefit of transcranial magnetic stimulation in refractory conversion paralysis (65) and somatization associated with posttraumatic stress disorder (66) are of particular interest given the functional imaging data that infer disruption of the frontal-subcortical circuits. If transcranial magnetic stimulation can target the specific frontal-subcortical circuit thought to be involved in the development of conversion symptoms, then perhaps such future procedures will ultimately benefit patients with conversion reactions.
Course and Prognosis
Between 50% and 90% of the patients with conversion disorder exhibit short-term resolution of symptoms after reassurance, but as many as 25% of these responders relapse or develop new conversion symptoms over time (67 , 68) . A longer duration of symptoms, psychiatric comorbidity, subacute presentation, and tremor or nonepileptic seizure subtypes are associated with a worse prognosis.
Among patients with nonepileptic seizures, even those with symptomatic improvement may remain disabled (69) . In one outcome study of 56 such patients, only half of the patients had a resolution of nonepileptic seizures a mean of 1.5 years after diagnosis, and many still exhibited depressive symptoms, suicidal ideation, and suicide attempts. A patient’s perception of good health and occupational functioning is correlated with resolution, which suggests that interventions that focus on improving functioning and self-esteem could aid treatment (70) .
Conclusions
Early recognition of a conversion disorder will limit unnecessary tests and medications. Long-term benefit likely requires a comprehensive treatment approach, recognition of risk factors, and treatment of comorbid conditions, with a focus on cognitive styles that perpetuate symptoms. The quality of the doctor-patient relationship can influence outcome. Hard-to-treat patients may engender feelings of powerlessness, frustration, and mistrust in their treaters, which, if unprocessed, may lead to a poor relationship and excessive use of medications, tests, and procedures.
There are few published reports on prospective studies or controlled trials of treatment for patients with nonepileptic seizures. The existing medical literature supports a multidisciplinary treatment approach, with specific interventions, such as cognitive behavior therapy for cognitive restructuring and psychodynamic therapy for addressing symptom connections to trauma and dissociation. Adjunctive group therapy or family therapy works well for certain patients. Hypnosis can be beneficial, although it is not essential for a good outcome. Judicious medication treatment for comorbid disorders, alone or in combination with psychotherapy, is often needed for sustained recovery.
1. Gates JR, Ramani V, Whalen S, Loewenson R: Ictal characteristics of pseudoseizures. Arch Neurol 1985; 42:1183–1187Google Scholar
2. Benbadis SR, O’Neill E, Tatum WO, Heriaud L: Outcome of prolonged video-EEG monitoring at a typical referral epilepsy center. Epilepsia 2004; 45:1150–1153Google Scholar
3. Chen DK, So YT, Fisher RS, Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology: Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675Google Scholar
4. Bortz JJ, Prigatano GP, Blum D, Fisher RS: Differential response characteristics in nonepileptic and epileptic seizure patients on a test of verbal learning and memory. Neurology 1995; 45:2029–2034Google Scholar
5. Graham JR: MMPI-2: Assessing Personality and Psychopathology, 3rd Ed. New York, Oxford University Press, 2000Google Scholar
6. Spiegel H, Spiegel D: Trance and Treatment: Clinical Uses of Hypnosis. New York, Basic Books, 1978Google Scholar
7. Breuer J, Freud S: Studies on hysteria, in The Standard Edition of the Complete Psychological Works of Sigmund Freud, Vol. II. Edited and translated by Strachey J, Strachey A. London, Hogarth Press and the Institute of Psycho-Analysis, 1955, pp vii–xxxi, 1–311 (original work published 1893–1895)Google Scholar
8. Black DN, Seritan AL, Taber KH, Hurley RA: Conversion hysteria: lessons from functional imaging. J Neuropsychiatry Clin Neurosci 2004; 16:245–251Google Scholar
9. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Google Scholar
10. Athwal BS, Halligan PW, Fink GR, Marshall JC, Frackowiak RSJ: Imaging hysterical paralysis, in Contemporary Approaches to the Study of Hysteria. Edited by Halligan PW, Bass C, Marshall JC. Oxford, UK, Oxford University Press, 2001, pp 216–250Google Scholar
11. Cotterill RMJ: On the unity of conscious experience. J Consciousness Studies 1995; 2:290–312Google Scholar
12. Lane RD, Ahern GL, Schwartz GE, Kaszniak AW: Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry 1997; 42:834–844Google Scholar
13. Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM: Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet 2000; 355:1243–1244Google Scholar
14. Mailis-Gagnon A, Giannoylis I, Downar J, Kwan CL, Mikulis DJ, Crawley AP, Nicholson K, Davis KD: Altered central somatosensory processing in chronic pain patients with “hysterical” anesthesia. Neurology 2003; 60:1501–1507Google Scholar
15. Werring DJ, Weston L, Bullmore ET, Plant GT, Ron MA: Functional magnetic resonance imaging of the cerebral response to visual stimulation in medically unexplained visual loss. Psychol Med 2004; 34:583–589Google Scholar
16. Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS: The functional anatomy of a hysterical paralysis. Cognition 1997; 64:B1–B8Google Scholar
17. Halligan PW, Athwal BS, Oakley DA, Frackowiak RS: Imaging hypnotic paralysis: implications for conversion hysteria (letter). Lancet 2000; 355:986–987Google Scholar
18. Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T: Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain 2001; 124:1077–1090Google Scholar
19. Tiihonen J, Kuikka J, Viinamaki H, Lehtonen J, Partanen J: Altered cerebral blood flow during hysterical paresthesia (letter). Biol Psychiatry 1995; 37:134–135Google Scholar
20. Yazící KM, Kostakoglu L: Cerebral blood flow changes in patients with conversion disorder. Psychiatry Res 1998; 83:163–168Google Scholar
21. Binzer M, Andersen PM, Kullgren G: Clinical characteristics of patients with motor disability due to conversion disorder: a prospective control group study. J Neurol Neurosurg Psychiatry 1997; 63:83–88Google Scholar
22. Ford CV, Folks DG: Conversion disorders: an overview. Psychosomatics 1985; 26:371–374, 380–383Google Scholar
23. Lazare A: Current concepts in psychiatry: conversion symptoms. N Engl J Med 1981; 305:745–748Google Scholar
24. Roelofs K, Keijsers GP, Hoogduin KA, Naring GW, Moene FC: Childhood abuse in patients with conversion disorder. Am J Psychiatry 2002; 159:1908–1913Google Scholar
25. Oakley DA: Hypnosis and conversion hysteria: a unifying model. Cogn Neuropsychiatry 1999; 4:243–265Google Scholar
26. Stone J, Sharpe M, Binzer M: Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics 2004; 45:492–499Google Scholar
27. Bowman ES: Nonepileptic seizures: psychiatric framework, treatment, and outcome. Neurology 1999; 53(suppl 2):S84–S88Google Scholar
28. Bowman ES, Markand ON: Psychodynamics and psychiatric diagnosis of pseudoseizure subjects. Am J Psychiatry 1996; 153:57–63Google Scholar
29. Owczarek K: Somatisation indexes as differential factors in psychogenic pseudoepileptic and epileptic seizures. Seizure 2003; 12:178–181Google Scholar
30. Stone J, Binzer M, Sharpe M: Illness beliefs and locus of control: a comparison of patients with pseudoseizures and epilepsy. J Psychosom Res 2004; 57:541–547Google Scholar
31. Reuber M, Pukrop R, Bauer J, Derfuss R, Elger CE: Multidimensional assessment of personality in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2004; 75:743–748Google Scholar
32. Cragar DE, Berry DT, Schmitt FA, Fakhoury TA: Cluster analysis of normal personality traits in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2005; 6:593–600Google Scholar
33. Devinsky O, Fisher R: Ethical use of placebo and provocative testing in diagnosing nonepileptic seizures. Neurology 1996; 47:866–870Google Scholar
34. Stagno SJ, Smith ML: The use of placebo in diagnosing psychogenic seizures: who is being deceived? Semin Neurol 1997; 17:213–218Google Scholar
35. Barry JJ, Atzman O, Morrell MJ: Discriminating between epileptic and nonepileptic events: the utility of hypnotic seizures induction. Epilepsia 2000; 41:81–84Google Scholar
36. Prigatano GP, Stonnington CM, Fisher RS: Psychological factors in the genesis and management of nonepileptic seizures: clinical observations. Epilepsy Behav 2002; 3:343–349Google Scholar
37. Lesser RP: Treatment and outcome of psychogenic nonepileptic seizures. Epilepsy Curr 2003; 3:198–200Google Scholar
38. Shen W, Bowman ES, Markand ON: Presenting the diagnosis of pseudoseizure. Neurology 1990; 40:756–759Google Scholar
39. Nobili L, Sartori I, Terzaghi M, Tassi L, Mai R, Francione S, Cossu M, Cardinale F, Castana L, Lo Russo G: Intracerebral recordings of minor motor events, paroxysmal arousals and major seizures in nocturnal frontal lobe epilepsy. Neurol Sci 2005; 26 (suppl 3):s215–s219Google Scholar
40. Rowan AJ: Diagnosis of non-epileptic seizures, in Non-Epileptic Seizures. Edited by Gates JR, Rowan AJ. Boston, Butterworth-Heinemann, 2000, pp 15–30Google Scholar
41. Kapur J, Pillai A, Henry TR: Psychogenic elaboration of simple partial seizures. Epilepsia 1995; 36:1126–1130Google Scholar
42. Kanner AM: More controversies on the treatment of psychogenic pseudoseizures: an addendum. Epilepsy Behav 2003; 4:360–364Google Scholar
43. LaFrance WC Jr, Barry JJ: Update on treatments of psychological nonepileptic seizures. Epilepsy Behav 2005; 7:364–374Google Scholar
44. Brazier DK, Venning HE: Conversion disorders in adolescents: a practical approach to rehabilitation. Br J Rheumatol 1997; 36:594–598Google Scholar
45. Calvert P, Jureidini J: Restrained rehabilitation: an approach to children and adolescents with unexplained signs and symptoms. Arch Dis Child 2003; 88:399–402Google Scholar
46. Heruti RJ, Levy A, Adunski A, Ohry A: Conversion motor paralysis disorder: overview and rehabilitation model. Spinal Cord 2002; 40:327–334Google Scholar
47. Speed J: Behavioral management of conversion disorder: retrospective study. Arch Phys Med Rehabil 1996; 77:147–154Google Scholar
48. Griffith JL, Polles A, Griffith ME: Pseudoseizures, families, and unspeakable dilemmas. Psychosomatics 1998; 39:144–153Google Scholar
49. Ryan CE, Epstein NB, Keitner GI, Miller IW, Bishop DS: Evaluating and Treating Families: The McMaster Approach. New York, Routledge, 2005Google Scholar
50. Goldstein LH, Deale AC, Mitchell-O’Malley SJ, Toone BK, Mellers JD: An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures: a pilot study. Cogn Behav Neurol 2004; 17:41–49Google Scholar
51. Kalogjera-Sackellares D: Psychodynamics and Psychotherapy of Pseudoseizures. Carmarthen, Wales, UK, Crown House, 2004Google Scholar
52. Aboukasm A, Mahr G, Gahry BR, Thomas A, Barkley GL: Retrospective analysis of the effects of psychotherapeutic interventions on outcomes of psychogenic nonepileptic seizures. Epilepsia 1998; 39:470–473Google Scholar
53. Gabbard GO: Dissociative disorders, in Psychodynamic Psychiatry in Clinical Practice, 4th ed. Washington, DC, American Psychiatric Publishing, 2005, pp 283–312Google Scholar
54. Zaroff CM, Myers L, Barr WB, Luciano D, Devinsky O: Group psychoeducation as treatment for psychological nonepileptic seizures. Epilepsy Behav 2004; 5:587–592Google Scholar
55. Wittenberg D, Michaels J, Ford C, Bullock K, Barry JJ: Group psychotherapy for patients with non-epileptic seizures: a pilot study (abstract). Epilepsia 2004; 45 (suppl 7):57–58Google Scholar
56. McDade G, Brown SW: Non-epileptic seizures: management and predictive factors of outcome. Seizure 1992; 1:7–10Google Scholar
57. Kim CM, Barry JJ, Zeifert PA: The use of inpatient medical psychiatric treatment for nonepileptic events (abstract). Epilepsia 1998; 39(suppl):242–243Google Scholar
58. Moene FC, Spinhoven P, Hoogduin KA, van Dyck R: A randomised controlled clinical trial on the additional effect of hypnosis in a comprehensive treatment programme for in-patients with conversion disorder of the motor type. Psychother Psychosom 2002; 71:66–76Google Scholar
59. Moene FC, Spinhoven P, Hoogduin KA, van Dyck R: A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn 2003; 51:29–50Google Scholar
60. LaFrance WC Jr, Devinsky O: The treatment of nonepileptic seizures: historical perspectives and future directions. Epilepsia 2004; 2(45 suppl):15–21Google Scholar
61. Voon V, Lang AE: Antidepressant treatment outcomes of psychogenic movement disorder. J Clin Psychiatry 2005; 66:1529–1534Google Scholar
62. Drake ME Jr, Pakalnis A, Phillips BB: Neuropsychological and psychiatric correlates of intractable pseudoseizures. Seizure 1992; 1:11–13Google Scholar
63. Marazziti D, Dell’Osso B: Effectiveness of risperidone in psychogenic stiff neck. CNS Spect 2005; 10:443–444Google Scholar
64. Persinger MA: Seizure suggestibility may not be an exclusive differential indicator between psychogenic and partial complex seizures: the presence of a third factor. Seizure 1994; 3:215–219Google Scholar
65. Schonfeldt-Lecuona C, Connemann BJ, Spitzer M, Herwig U: Transcranial magnetic stimulation in the reversal of motor conversion disorder. Psychotherapy Psychosom 2003; 72:286–288Google Scholar
66. Grisaru N, Amir M, Cohen H, Kaplan Z: Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry 1998; 44:52–55Google Scholar
67. Hafeiz HB: Hysterical conversion: a prognostic study. Br J Psychiatry 1980; 136:548–551Google Scholar
68. Baker JH, Silver JR: Hysterical paraplegia. J Neurol Neurosurg Psychiatry 1987; 50:375–382Google Scholar
69. Pestana EM, Foldvary-Schaefer N, Marsillio D, Morris HH III: Quality of life in patients with psychogenic seizures. Neurology 2003; 60(suppl 1):A355Google Scholar
70. Ettinger AB, Devinsky O, Weisbrot DM, Ramakrishna RK, Goyal A: A comprehensive profile of clinical, psychiatric, and psychosocial characteristics of patients with psychogenic nonepileptic seizures. Epilepsia 1999; 40:1292–1298Google Scholar




