The Risk of Suicide With Selective Serotonin Reuptake Inhibitors in the Elderly
Abstract
Objective: The authors explored the relationship between the initiation of therapy with selective serotonin reuptake inhibitor (SSRI) antidepressants and completed suicide in older patients. Method: The authors linked population-based coroner’s records with patient-level prescription data, physician billing claims, and hospitalization data for more than 1.2 million Ontario residents 66 years of age and older from 1992 to 2000. For each suicide case, four closely matched comparison subjects were selected using propensity score methods. The authors determined the odds ratio for suicide with SSRIs versus other antidepressant treatment, calculated at discrete monthly intervals from the start of treatment. Results: Of 1,329 suicide cases, 1,138 (86%) were each fully matched to four comparison subjects using propensity scores. During the first month of therapy, SSRI antidepressants were associated with a nearly fivefold higher risk of completed suicide than other antidepressants (adjusted odds ratio: 4.8, 95% confidence interval=1.9–12.2). The risk was independent of a recent diagnosis of depression or the receipt of psychiatric care, and suicides of a violent nature were distinctly more common during SSRI therapy. Numerous sensitivity analyses revealed consistent results. No disproportionate suicide risk was seen during the second and subsequent months of treatment with SSRI antidepressants, and the absolute risk of suicide with all antidepressants was low. Conclusions: Initiation of SSRI therapy is associated with an increased risk of suicide during the first month of therapy compared with other antidepressants. The absolute risk is low, suggesting that an idiosyncratic response to these agents may provoke suicide in a vulnerable subgroup of patients.
Depression is common, affecting about one in five people during their lifetime (1 – 3) . Severe depression is also a major risk factor for suicide, and up to 15% of those hospitalized for depression eventually commit suicide (4) . Most patients who commit suicide suffer from a psychiatric illness, with advancing age, male gender, and medical illness among important predisposing factors (4 , 5) . As the cause of death for approximately 1 million people worldwide each year, suicide can be a devastating event with a complex etiology, and a better understanding of contributing factors is essential to suicide prevention (6 – 8) .
Selective serotonin reuptake inhibitors (SSRIs) have become increasingly popular for the treatment of depression (9) . These drugs are well tolerated by most patients, are safer in overdose than traditional antidepressants, and their availability has encouraged antidepressant prescribing in primary care (9 , 10) . Several anecdotal reports describe the emergence of intense suicidality during the initial period of SSRI therapy (11 – 16) , but it is difficult to separate the role of depression from a possible adverse effect of treatment.
The potential association between SSRI antidepressants and suicide has garnered considerable media attention (17) , prompting multiple editorials (18 – 21) , litigation against the pharmaceutical industry (22) , allegations of corporate malfeasance (23 , 24) , and national public health advisories (25) . Industry-sponsored studies, single case reports, meta-analyses, and practice-based epidemiologic studies have yielded variable findings regarding the association between SSRI antidepressants and suicide (26 – 37) . Moreover, the exclusion of actively suicidal patients from clinical trials of antidepressants renders pooled analyses underpowered to detect differences in mortality, as illustrated by the recent findings of the U.S. Food and Drug Administration (25 , 32 , 38) .
Recent attention has focused on the possible risks of antidepressant treatment in children, yet most cases of SSRI-induced suicidality have been reported in adults (14 , 15 , 39) . No studies have addressed the risk in older patients, despite the high frequency with which antidepressants are used in this population (9 , 40) . In this study, we linked population-based prescription records and coroner’s data to explore the association between the initiation of antidepressant therapy and subsequent risk of suicide in a population of more than 1.2 million elderly patients.
Method
Setting
We conducted a population-based study in Ontario. Ontario is Canada’s largest province, with a population of 11,100,876 at the midpoint of the study period, which included 1,264,686 who were 66 years of age or older. All Ontario residents 65 years and older had universal access to health insurance for prescription drug coverage, physicians’ services, and hospital care. The study was approved by the Chief Coroner for Ontario and the research ethics board of Sunnybrook and Women’s College Health Sciences Centre.
Subjects
Using records from the Office of the Chief Coroner for Ontario, we identified consecutive cases of suicide among Ontario residents aged 66 years and older that occurred over a 9-year period (Jan. 1, 1992, to Dec. 31, 2000). We did not examine the first year of eligibility for prescription drug benefits (age 65) to avoid incomplete medication records, and we excluded patients younger than 65 because prescription records were not available for analysis. The date of suicide served as the index date for all analyses.
Propensity score methods were used to select comparison patients from the general population (41 , 42) . This is an advanced matching technique that involves modeling a large amount of information on each subject to minimize differences between suicide and comparison groups. This included demographic data as well as clinical information regarding specific medical and psychiatric conditions, collectively identified from hospital admission records, physician diagnosis claims, and outpatient prescription claims. A complete list of the various elements of the propensity score is shown in Table 1 .
A structured, iterative process similar to that described by Rosenbaum and Rubin (43) was used to construct a propensity score for each individual that predicted suicide outcome by balancing all characteristics shown in Table 1 between the suicide cases and comparison subjects. To account for changing patterns of antidepressant use in Ontario over the study period (9) , propensity scores were calculated for all possible comparison patients for each case at every index date. Once the final propensity score model was developed and scores calculated for all potential comparison subjects, we identified all eligible comparison patients for each case using calipers of 0.2 standard deviations of the propensity score. From these we randomly selected four comparison subjects for each suicide case. Suicide cases whose propensity scores were too high to permit a match to four comparison subjects were retained for descriptive purposes but excluded from the matched analyses.
Exposure to Antidepressants
We examined prescription records of suicide cases and comparison subjects through the Ontario Drug Benefit program database. The Ontario Drug Benefit program collects detailed records of prescriptions dispensed to all elderly residents of Ontario, contains little (<1%) missing data, and is regularly used to analyze medication use in the community (44 – 47) . SSRI antidepressants included fluoxetine, fluvoxamine, paroxetine, sertraline, and citalopram. Other antidepressants included secondary amine cyclic antidepressants (desipramine, nortriptyline, protriptyline, maprotiline, and amoxapine), tertiary amine cyclic antidepressants (amitriptyline, imipramine, doxepin, trimipramine, and clomipramine), and miscellaneous antidepressants (venlafaxine, trazodone, bupropion, and nefazodone). We did not examine monoamine oxidase inhibitors given their infrequent use, and we did not study mirtazapine because it was not an insured benefit during most of the study period.
In all main analyses, new use of an antidepressant was defined as no previous prescription for a drug from the same class in the previous 6 months. In a secondary analysis, we defined new use as no prescription for any other antidepressant in the preceding 6 months.
Statistical Analysis
Databases were linked in an anonymous fashion using an encrypted version of each patient’s health card number. The primary analysis used conditional logistic regression to estimate the odds ratio and 95% confidence interval (CI) for suicide associated with new use of an antidepressant at discrete monthly intervals from the start of treatment.
Multivariable analysis adjusted for rural place of residence (imputed from home postal code) (48) , estimated residential income quintile, previous suicide attempt, the number of prescription medications dispensed in the previous year (49) , and any evidence (from prescription records, physician diagnosis codes, or hospital discharge records during the preceding year) of alcohol abuse, malignancy, anxiety or sleep disorder, bipolar disorder, depression or other mood disorder, agitation or psychosis, poisoning or other injury, provision of care by a psychiatrist, or admission to a psychiatric facility. All tests of significance used a two-tailed p value of 0.05 for statistical significance and were conducted using SAS version 8.2 (SAS Institute, Cary, N.C.)
Results
Overview
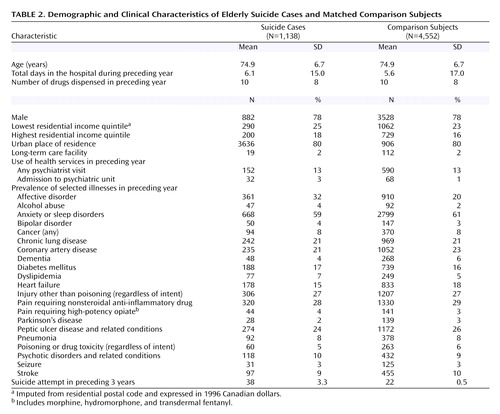
During the study period, we identified 1,354 cases of suicide among individuals 66 years or older. Of these, 25 (2%) were excluded because of an invalid health card number, erroneous identifying data, or principal residence outside Ontario. Of the remaining 1,329 cases, the propensity scores of 191 (14%) were too high to permit propensity-based matching with four comparison subjects. Therefore, the matched analyses included 1,138 suicide cases and 4,552 comparison subjects with comparable demographic characteristics and antecedent patterns of illness ( Table 2 ). The majority of patients who died of suicide were men living in an urban setting, and few had seen a psychiatrist in the year before death. The most common mechanisms of suicide were death by firearm (N=370), hanging (N=318), and self-poisoning (N=285).
Use of Antidepressants
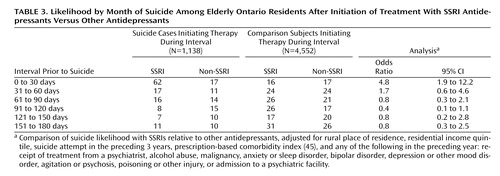
Of the 1,329 suicide cases, 907 (68%) had received no antidepressant therapy in the 6 months before death. The risk of suicide during the first month of treatment with SSRI antidepressants was about fivefold higher than that with other antidepressants ( Table 3 ). In contrast, no differential risk of suicide was evident during the second and subsequent months of SSRI therapy. The temporal relationship between initiation of antidepressant therapy and risk of suicide for SSRI and other antidepressants is depicted in Figure 1 .
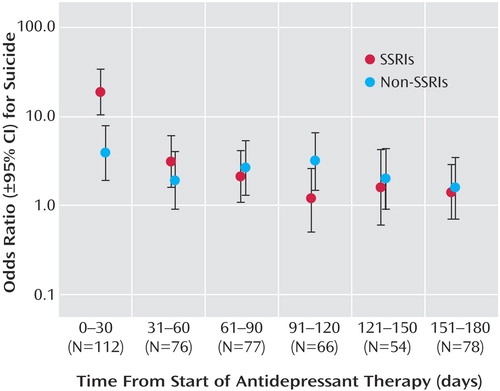
a During the first month of treatment, the risk of suicide with SSRI antidepressants is approximately 5-fold higher than that with other antidepressants (p=0.0009), but no difference in risk is seen with continued therapy.
Antidepressant Subgroups
Some antidepressants are used for illnesses other than depression. This is particularly true for tertiary amine cyclic antidepressants such as amitriptyline and doxepin (often prescribed for conditions such as migraine, pruritus, and neuropathic pain) and for clomipramine (often used for obsessional disorders). Our findings did not change significantly when we excluded all tertiary amine cyclic antidepressants from the group of non-SSRI antidepressants.
Several antidepressants have distinguishing characteristics from others in the same class. We found consistent results when we categorized venlafaxine (which blocks both serotonin and norepinephrine reuptake at higher doses) (50) as an SSRI antidepressant, despite evidence that venlafaxine may be prescribed to patients with a greater burden of risk factors for suicide (51) . Similarly, our findings persisted when we excluded amoxapine and maprotiline (which are structurally dissimilar from other secondary amine cyclic antidepressants) from the analysis, and when we excluded clomipramine (which selectively interferes with serotonin transport and is often used for obsessive disorders) from the group of tertiary amine cyclic antidepressants.
Additional Analyses
Our original findings persisted when we defined new use of antidepressants as no use of any other antidepressant in the preceding 6 months, and also when we replicated our analysis without the propensity score-based matching process by studying all 1,329 cases and 5,315 randomly selected community-dwelling controls matched only on age, gender, and residential income quintile. Some previous studies of the association between antidepressants and suicidal behavior have been confined to patients receiving treatment (31 , 32 , 52) , and we therefore also conducted a nested case-control study of patients treated with antidepressants within 6 months of the index date. These findings also mirrored our original analysis. (An appendix presenting these additional analyses accompanies the online version of this article.) Finally, we found consistent results in a series of analyses stratified by demographic characteristics, mental health history, and patterns of medical illness ( Figure 2 ). The only exception was that the first month of treatment with SSRI antidepressants was not associated with a disproportionate increase in suicide among women.
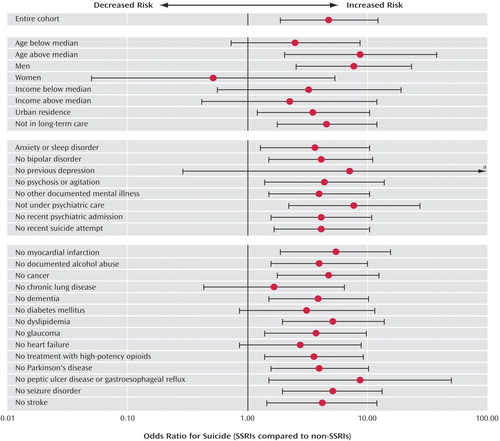
a Extends to 170.3.
Spectrum of Suicide
We examined the association between antidepressant use and method of suicide, since some reports have linked SSRI antidepressants with especially violent suicidal ideation (15 , 39) . Relative to other antidepressants, SSRIs were more strongly associated with suicides of a violent nature (hanging, gunshot, jumping, stabbing, vehicle collision, blunt trauma, explosion, electrocution, and self-immolation) than other antidepressants ( Figure 3 ). A tendency toward violent suicide was apparent only during early therapy with SSRIs, whereas nonviolent suicide was equally common among patients treated with SSRIs and other antidepressants.
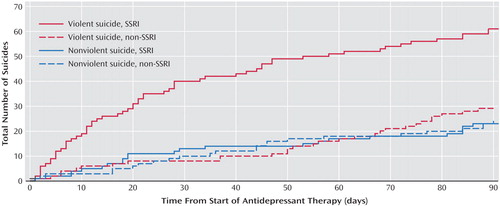
a Violent suicides were distinctly more common among those who recently initiated treatment with SSRI antidepressants (p=0.0016 by Kruskal-Wallis test of median interarrival time).
Absolute Risk of Suicide
We estimated the absolute risk of suicide during the first month of treatment with SSRI antidepressants by dividing the total number of suicides in such patients (N=73 of 1,329 total cases) by the total number of patients who received an SSRI antidepressant during the study period (N=244,749). The same calculation was performed for other antidepressants. Using this approach, we found that the absolute risk of suicide during the first month of treatment was low in both groups (approximately 1 in 3,353 SSRI-treated patients and about 1 in 16,037 patients receiving other antidepressants). Because many suicides during the first month of treatment likely result from depression itself rather than an adverse effect of treatment, the actual risk of suicide due to antidepressant therapy is probably far lower.
To place the absolute risk of suicide in context, we found that 907 of 1,329 suicide cases (68%) received no antidepressant in the 6 months before suicide, yet many were likely depressed (53 , 54) . Although no studies have proven that antidepressants prevent suicide, recent evidence suggests that treating depression reduces suicidal ideation in older patients (55) . If treatment with SSRI antidepressants reduces the risk of suicide by as little as 2% in patients with major depression, we speculate that the number of suicide deaths that might be prevented through increased use of SSRI antidepressants among older patients with major depression would exceed the number of deaths attributable to these drugs.
Discussion
Previous research on SSRI antidepressants and suicide has been limited by an absence of suitable controls, small study group sizes, use of surrogate endpoints, inefficient study designs, and a lack of population-based data (15 , 26 , 31 , 32 , 38, 52, 56–59). Using 9 years of comprehensive coroner’s records and prescription data in a population of more than 1 million older patients, we found a substantial increase in the relative risk of suicide following the initiation of SSRI treatment. The differential risk compared with other antidepressants was confined to the initial month of therapy, after which time no heightened risk was evident. It is interesting that we found SSRI antidepressants to be not associated with an increased suicide risk among women; however, as with all post-hoc analyses, this observation may be due to chance and should be interpreted cautiously.
Although case/control studies cannot generally yield estimates of excess risk, the population-based nature of our data indicates that the absolute risk of suicide during initial treatment with SSRI antidepressants is very low. In contrast, more than two-thirds of cases committed suicide while not receiving an antidepressant, highlighting the undertreatment of depression in older patients (60 , 61) .
Several mechanisms may underlie the association between SSRI antidepressants and suicide (18 , 19) . During initial therapy, the risk of suicide may increase as some aspects of depression resolve (e.g., psychomotor retardation), thereby energizing the patient to suicide (62) . Patients may also develop akathisia-like symptoms during treatment with SSRI antidepressants, which may increase the risk of suicide (4, 14, 63–65). In addition, emerging evidence suggests that genetic differences in drug metabolism or serotonin receptor polymorphisms influence the safety and tolerability of these drugs (66, 66–69). Our findings mirror the clinical observation that the vast majority of patients treated with SSRI antidepressants do not attempt suicide, but that in rare instances these drugs appear to incite suicidal ideation during the first weeks of therapy (39, 70, 71). We speculate that treatment-emergent agitation or dysphoria can provoke suicidal ideation in some patients (72). Like other rare adverse drug events, this idiosyncratic response may have a pharmacogenetic basis (73–76), and future research may provide a means of identifying such individuals before commencing treatment (77, 78).
An alternative explanation for our findings might be that physicians preferentially prescribe SSRI antidepressants to patients at higher risk for suicide because these drugs are safer in overdose. However, this is unlikely to explain our findings for several reasons. Physicians frequently cannot identify patients at increased risk of suicide, particularly among the elderly (79, 80). Moreover, we selected comparison patients matched to cases on important characteristics ( Table 1 ), many of which are independently associated with suicide (54, 81, 82). In addition, we found consistent results regardless of a previous history of depression or psychiatric care, and in a separate nested case/control analysis restricted to patients receiving antidepressant therapy. Notably, no heightened risk of suicide was evident beyond the first month of treatment with SSRIs compared with other antidepressants. Had depression (rather than drug treatment) explained our findings, a more persistent risk should have been identified with SSRI therapy, since depressive symptoms rarely abate completely during the first month of therapy. Finally, the distinctly violent pattern of suicides during early SSRI therapy is consistent with previous reports and argues strongly against selection bias (15, 39), which would have yielded an increase in both violent and nonviolent suicide among patients treated with SSRI antidepressants.
Some limitations of our research merit emphasis. We used administrative data and had no direct measure of antidepressant doses or adherence, and the applicability of our findings to younger patients is not known. These limitations are particularly important given recent warnings regarding antidepressant use in adolescents (21 , 83) . We could not directly measure important risk factors such as bereavement and social isolation. Although administrative data are an imperfect means of identifying certain medical problems such as malignancy and alcoholism, differential detection of these conditions with SSRIs versus other antidepressant treatment is not likely to explain our findings. Although we used a population-based registry to identify suicides, some cases of nonviolent suicide in older patients may have been misattributed to natural causes (84) . Finally, we cannot exclude the possibility that SSRI antidepressants merely reduce the risk of suicide less effectively than other treatments.
Our study does not address the benefits of SSRI antidepressants and cannot establish the number of suicides prevented by treatment (85 – 87) . The findings should not serve to anathematize SSRIs as a class, since they represent an important therapeutic option for patients with depression (85) . Patients responding well to SSRI antidepressants should not discontinue therapy, and individuals with depression must not be deterred from seeking treatment based upon our findings (55) . Indeed, in patients with major depression, the hazards of undertreatment almost certainly outweigh the risks of therapy, which our study suggests are low and transient. Further research is needed to explore the basis of our findings, including the possible role of genetic variability in drug response (66 , 68) . In the interim, our findings reaffirm the need for clinicians to reserve SSRI antidepressants for patients with established indications, monitor them closely after commencing treatment, and inform patients and their families of the possible emergence of suicidality during the first month of therapy.
1. Blazer DG, Kessler RC, McGonagle KA, Swartz MS: The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry 1994; 151:979–986Google Scholar
2. Kringlen E, Torgersen S, Cramer V: A Norwegian psychiatric epidemiological study. Am J Psychiatry 2001; 158:1091–1098Google Scholar
3. Sullivan PF, Kessler RC, Kendler KS: Latent class analysis of lifetime depressive symptoms in the National Comorbidity Survey. Am J Psychiatry 1998; 155:1398–1406Google Scholar
4. Maris RW: Suicide. Lancet 2002; 360:319–326Google Scholar
5. Conwell Y: Suicide among elderly persons. Psychiatr Serv 1995; 46:563–564Google Scholar
6. Althaus D, Hegerl U: The evaluation of suicide prevention activities: state of the art. World J Biol Psychiatry 2003; 4:156–165Google Scholar
7. Conwell Y, Duberstein PR, Caine ED: Risk factors for suicide in later life. Biol Psychiatry 2002; 52:193–204Google Scholar
8. Knox KL, Conwell Y, Caine ED: If suicide is a public health problem, what are we doing to prevent it? Am J Public Health 2004; 94:37–45Google Scholar
9. Mamdani MM, Parikh SV, Austin PC, Upshur RE: Use of antidepressants among elderly subjects: trends and contributing factors. Am J Psychiatry 2000; 157:360–367Google Scholar
10. Gilsa HD, Sondergaard J, Vach W, Freng GL, Rosholm JU, Kragstrup J: Antidepressant drug use in general practice: inter-practice variation and association with practice characteristics. Eur J Clin Pharmacol 2003; 59:143–149Google Scholar
11. Dasgupta K; Hoover CE: Additional cases of suicidal ideation associated with fluoxetine (letters). Am J Psychiatry 1990; 147:1570–1571Google Scholar
12. King RA, Riddle MA, Chappell PB, Hardin MT, Anderson GM, Lombroso P, Scahill L: Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. J Am Acad Child Adolesc Psychiatry 1991; 30:179–186Google Scholar
13. Masand P, Gupta S, Dewan M: Suicidal ideation related to fluoxetine treatment. N Engl J Med 1991; 324:420Google Scholar
14. Rothschild AJ, Locke CA: Reexposure to fluoxetine after serious suicide attempts by three patients: the role of akathisia. J Clin Psychiatry 1991; 52:491–493Google Scholar
15. Teicher MH, Glod C, Cole JO: Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry 1990; 147:207–210Google Scholar
16. Wirshing WC, Van Putten T, Rosenberg J, Marder S, Ames D, Hicks-Gray T: Fluoxetine, akathisia, and suicidality: is there a causal connection? Arch Gen Psychiatry 1992; 49:580–581Google Scholar
17. Mahler J: The antidepressant dilemma. The New York Times Magazine. Nov 21, 2004Google Scholar
18. Healy D: Lines of evidence on the risks of suicide with selective serotonin reuptake inhibitors. Psychother Psychosom 2003; 72:71–79Google Scholar
19. Healy D, Whitaker C: Antidepressants and suicide: risk-benefit conundrums. J Psychiatry Neurosci 2003; 28:331–337Google Scholar
20. Lapierre YD: Suicidality with selective serotonin reuptake inhibitors: valid claim? J Psychiatry Neurosci 2003; 28:340–347Google Scholar
21. Vitiello B, Swedo S: Antidepressant medications in children. N Engl J Med 2004; 350:1489–1491Google Scholar
22. Whitehead PD: Causality and collateral estoppel: process and content of recent SSRI litigation. J Am Acad Psychiatry Law 2003; 31:377–382Google Scholar
23. Gibson L: GlaxoSmithKline to publish clinical trials after US lawsuit. BMJ 2004; 328:1513Google Scholar
24. Kondro W, Sibbald B: Drug company experts advised staff to withhold data about SSRI use in children. CMAJ 2004; 170:783Google Scholar
25. FDA Public Health Advisory: Suicidality in Children and Adolescents Being Treated With Antidepressant Medications. Washington, DC, FDA, Oct 15, 2004 (http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm)Google Scholar
26. Donovan S, Clayton A, Beeharry M, Jones S, Kirk C, Waters K, Gardner D, Faulding J, Madeley R: Deliberate self-harm and antidepressant drugs: investigation of a possible link. Br J Psychiatry 2000; 177:551–556Google Scholar
27. Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P, Hutton B: Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005; 330:396Google Scholar
28. Gunnell D, Ashby D: Antidepressants and suicide: what is the balance of benefit and harm. BMJ 2004; 329:34–38Google Scholar
29. Gunnell D, Saperia J, Ashby D: Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005; 330:385Google Scholar
30. Isacsson G, Holmgren P, Ahlner J: Selective serotonin reuptake inhibitor antidepressants and the risk of suicide: a controlled forensic database study of 14,857 suicides. Acta Psychiatr Scand 2005; 111:286–290Google Scholar
31. Jick H, Kaye JA, Jick SS: Antidepressants and the risk of suicidal behaviors. JAMA 2004; 292:338–343Google Scholar
32. Khan A, Khan S, Kolts R, Brown WA: Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry 2003; 160:790–792Google Scholar
33. Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J, Evans S, Gunnell D: Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005; 330:389Google Scholar
34. Simon GE, VonKorff M: Suicide mortality among patients treated for depression in an insured population. Am J Epidemiol 1998; 147:155–160Google Scholar
35. Storosum JG, van Zwieten BJ, Wohlfarth T, de Haan L, Khan A, van den Brink W: Suicide risk in placebo vs active treatment in placebo-controlled trials for schizophrenia. Arch Gen Psychiatry 2003; 60:365–368Google Scholar
36. Tollefson GD, Fawcett J, Winokur G, Beasley CM Jr, Potvin JH, Faries DE, Rampey AH Jr, Sayler ME: Evaluation of suicidality during pharmacologic treatment of mood and nonmood disorders. Ann Clin Psychiatry 1993; 5:209–224Google Scholar
37. Tollefson GD, Rampey AH Jr, Beasley CM Jr, Enas GG, Potvin JH: Absence of a relationship between adverse events and suicidality during pharmacotherapy for depression. J Clin Psychopharmacol 1994; 14:163–169Google Scholar
38. Beasley CM, Jr, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH Jr, Heiligenstein JH, Thompson VL, Murphy DJ, Masica DN: Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ 1991; 303:685–692Google Scholar
39. Hawthorne ME, Lacey JH: Severe disturbance occurring during treatment for depression of a bulimic patient with fluoxetine. J Affect Disord 1992; 26:205–207Google Scholar
40. Sambamoorthi U, Olfson M, Walkup JT, Crystal S: Diffusion of new generation antidepressant treatment among elderly diagnosed with depression. Med Care 2003; 41:180–194Google Scholar
41. Braitman LE, Rosenbaum PR: Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med 2002; 137:693–695Google Scholar
42. D’Agostino RB Jr: Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281Google Scholar
43. Rosenbaum PR, Rubin DB: Reducing bias in observational studies using subclassification on the propensity score. J Am Statistical Assoc 1984; 79:516–524Google Scholar
44. Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA: Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 2003; 289:1652–1658Google Scholar
45. Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D: Coding accuracy of administrative drug claims in the Ontario drug benefit database. Can J Clin Pharmacol 2003; 10:67–71Google Scholar
46. Mamdani M, Rochon PA, Juurlink DN, Kopp A, Anderson GM, Naglie G, Austin PC, Laupacis A: Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 2002; 325:624Google Scholar
47. Redelmeier DA, Tan SH, Booth GL: The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med 1998; 338:1516–1520Google Scholar
48. Bancroft JH, Skrimshire AM, Reynolds F, Simkin S, Smith J: Self-poisoning and self-injury in the Oxford area: epidemiological aspects 1969–73. Br J Prev Soc Med 1975; 29:170–177Google Scholar
49. Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ: Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154:854–864Google Scholar
50. Tatsumi M, Groshan K, Blakely RD, Richelson E: Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 1997; 340:249–258Google Scholar
51. Mines D, Hill D, Yu H, Novelli L: Prevalence of risk factors for suicide in patients prescribed venlafaxine, fluoxetine, and citalopram. Pharmacoepidemiol Drug Saf 2005; 14:367–372Google Scholar
52. Jick SS, Dean AD, Jick H: Antidepressants and suicide. BMJ 1995; 310:215–218Google Scholar
53. Conwell Y, Lyness JM, Duberstein P, Cox C, Seidlitz L, DiGiorgio A, Caine ED: Completed suicide among older patients in primary care practices: a controlled study. J Am Geriatr Soc 2000; 48:23–29Google Scholar
54. Waern M, Runeson BS, Allebeck P, Beskow J, Rubenowitz E, Skoog I, Wilhelmsson K: Mental disorder in elderly suicides: a case-control study. Am J Psychiatry 2002; 159:450–455Google Scholar
55. Bruce ML, Ten Have TR, Reynolds CF III, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS: Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA 2004; 291:1081–1091Google Scholar
56. Carlsten A, Waern M, Ekedahl A, Ranstam J: Antidepressant medication and suicide in Sweden. Pharmacoepidemiol Drug Saf 2001; 10:525–530Google Scholar
57. Goldstein DJ, Rampey AH Jr, Potvin JH, Masica DN, Beasley CM Jr: Analyses of suicidality in double-blind, placebo-controlled trials of pharmacotherapy for weight reduction. J Clin Psychiatry 1993; 54:309–316Google Scholar
58. Warshaw MG, Keller MB: The relationship between fluoxetine use and suicidal behavior in 654 subjects with anxiety disorders. J Clin Psychiatry 1996; 57:158–166Google Scholar
59. Wheadon DE, Rampey AH Jr, Thompson VL, Potvin JH, Masica DN, Beasley CM Jr: Lack of association between fluoxetine and suicidality in bulimia nervosa. J Clin Psychiatry 1992; 53:235–241Google Scholar
60. Matthews JD, Fava M: Risk of suicidality in depression with serotonergic antidepressants. Ann Clin Psychiatry 2000; 12:43–50Google Scholar
61. Uncapher H, Arean PA: Physicians are less willing to treat suicidal ideation in older patients. J Am Geriatr Soc 2000; 48:188–192Google Scholar
62. Nutt DJ: Death and dependence: current controversies over the selective serotonin reuptake inhibitors. J Psychopharmacol 2003; 17:355–364Google Scholar
63. Baldassano CF, Truman CJ, Nierenberg A, Ghaemi SN, Sachs GS: Akathisia: a review and case report following paroxetine treatment. Compr Psychiatry 1996; 37:122–124Google Scholar
64. Kasantikul D: Drug-induced akathisia and suicidal tendencies in psychotic patients. J Med Assoc Thai 1998; 81:551–554Google Scholar
65. Lipinski JF Jr, Mallya G, Zimmerman P, Pope HG Jr: Fluoxetine-induced akathisia: clinical and theoretical implications. J Clin Psychiatry 1989; 50:339–342Google Scholar
66. Charlier C, Broly F, Lhermitte M, Pinto E, Ansseau M, Plomteux G: Polymorphisms in the CYP 2D6 gene: association with plasma concentrations of fluoxetine and paroxetine. Ther Drug Monit 2003; 25:738–742Google Scholar
67. Murphy GM, Kremer C, Rodrigues H, Schatzberg AF: The apolipoprotein E epsilon4 allele and antidepressant efficacy in cognitively intact elderly depressed patients. Biol Psychiatry 2003; 54:665–673Google Scholar
68. Murphy GM Jr, Kremer C, Rodrigues HE, Schatzberg AF: Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160:1830–1835Google Scholar
69. Steimer W, Muller B, Leucht S, Kissling W: Pharmacogenetics: a new diagnostic tool in the management of antidepressive drug therapy. Clin Chim Acta 2001; 308:33–41Google Scholar
70. Fux M, Taub M, Zohar J: Emergence of depressive symptoms during treatment for panic disorder with specific 5-hydroxytryptophan reuptake inhibitors. Acta Psychiatr Scand 1993; 88:235–237Google Scholar
71. Teicher MH, Glod CA, Cole JO: Antidepressant drugs and the emergence of suicidal tendencies. Drug Saf 1993; 8:186–212Google Scholar
72. Mann JJ, Kapur S: The emergence of suicidal ideation and behavior during antidepressant pharmacotherapy. Arch Gen Psychiatry 1991; 48:1027–1033Google Scholar
73. Chan YC, Valenti D, Mansfield AO, Stansby G: Warfarin induced skin necrosis. Br J Surg 2000; 87:266–272Google Scholar
74. Evans WE, Hon YY, Bomgaars L, Coutre S, Holdsworth M, Janco R, Kalwinsky D, Keller F, Khatib Z, Margolin J, Murray J, Quinn J, Ravindranath Y, Ritchey K, Roberts W, Rogers ZR, Schiff D, Steuber C, Tucci F, Kornegay N, Krynetski EY, Relling MV: Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 2001; 19:2293–2301Google Scholar
75. Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M: Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics 2004; 14:335–342Google Scholar
76. Leeder JS: Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia 1998; 39(suppl 7):S8–S16Google Scholar
77. Guzey C, Spigset O: Genotyping as a tool to predict adverse drug reactions. Curr Top Med Chem 2004; 4:1411–1421Google Scholar
78. Hosford DA, Lai EH, Riley JH, Xu CF, Danoff TM, Roses AD: Pharmacogenetics to predict drug-related adverse events. Toxicol Pathol 2004; 32(suppl 1):9–12Google Scholar
79. Harwood DM, Hawton K, Hope T, Jacoby R: Suicide in older people: mode of death, demographic factors, and medical contact before death. Int J Geriatr Psychiatry 2000; 15:736–743Google Scholar
80. Milton J, Ferguson B, Mills T: Risk assessment and suicide prevention in primary care. Crisis 1999; 20:171–177Google Scholar
81. Conwell Y, Duberstein PR: Suicide in elders. Ann N Y Acad Sci 2001; 932:132–147Google Scholar
82. Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA: Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004; 164:1179–1184Google Scholar
83. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E: Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004; 363:1341–1345Google Scholar
84. Cooper PN, Milroy CM: The coroner’s system and under-reporting of suicide. Med Sci Law 1995; 35:319–326Google Scholar
85. Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM: Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003; 361:653–661Google Scholar
86. Isacsson G: Suicide prevention–a medical breakthrough? Acta Psychiatr Scand 2000; 102:113–117Google Scholar
87. Isacsson G, Bergman U, Rich CL: Epidemiological data suggest antidepressants reduce suicide risk among depressives. J Affect Disord 1996; 41:1–8Google Scholar