Elevated Cerebrospinal Fluid Substance P Concentrations in Posttraumatic Stress Disorder and Major Depression
Abstract
Objective: The authors tested the hypothesis that concentrations of the pain-transmitting neuropeptide substance P are elevated in the CSF of patients with major depression or posttraumatic stress disorder (PTSD), which have overlapping symptoms. The authors also sought to determine if CNS substance P concentrations change on provocation of symptoms in PTSD patients. Method: The authors measured CSF substance P concentrations in medication-free patients with either major depression or PTSD and in healthy comparison subjects. Next, using a within-subject, crossover design, the authors sampled CSF for 6 hours through an indwelling subarachnoid catheter in PTSD patients before, during, and after exposure to a 60-minute traumatic or neutral videotape stimulus. Results: Both depressed and PTSD patients had significantly elevated basal CSF substance P concentrations. In the challenge study, marked increases in CSF substance P concentrations were found only after precipitation of PTSD symptoms. CSF substance P concentrations increased by 169% and 90.6% of baseline levels at 10 and 70 minutes, respectively, after the start of the traumatic videotape but changed by only 1.1% and –8.1% of baseline levels 10 and 70 minutes after the start of the neutral videotape. Conclusions: These results suggest that elevated CNS substance P concentrations are involved in both major depression and PTSD. The marked increase in CSF substance P concentrations during and after the symptom-provoking stimulus, but not after the neutral stimulus, implicates CNS release of substance P in the mechanism of acute PTSD symptoms. These data also reveal that CNS substance P responds acutely to psychological stress in humans.
In 1931, Von Euler and Gaddum (1) analyed extracts from equine organ systems and identified an active entity from the brain and intestines (but not from 10 other organs tested) that they termed extract or powder P; the substance was found to have potent hypotensive effects in atropinized rabbits anesthetized with ether. Numerous experiments over subsequent decades led to the characterization of an 11-amino acid peptide, substance P (2) , now recognized to be the preferred ligand at the neurokinin-1 receptor (3 , 4) . Human CNS tissue exhibits a heterogeneous distribution of neurokinin-1 receptors that is especially enriched throughout the spinal cord, brainstem, and limbic system (5 – 11) . Among its many actions, substance P has a major role in facilitating or transmitting nociceptive and stressful stimuli in the CNS (12 – 14) .
In depressed humans, the substance P (neurokinin-1) antagonist MK-869 was reported to be an effective antidepressant in a double-blind, placebo-controlled trial (15) , although subsequent clinical trials failed to confirm this finding (16) . However, another substance P antagonist, L-759274, did exhibit antidepressant effects in a controlled clinical trial (17) . In a postmortem study of human brains, Stockmeier et al. (18) observed a decreased density of neurokinin-1 receptors in the orbitofrontal cortex of individuals who died by suicide and individuals with major depression, compared with individuals who were reportedly “psychiatrically normal,” raising the possibility of chronic hypersecretion of substance P and subsequent down-regulation of these neurokinin-1 receptors.
Despite the ongoing testing of substance P antagonists in clinical trials for the treatment of depression, evidence of CNS substance P abnormalities in patients with major depression and/or PTSD is lacking. The measurement of CSF substance P concentrations in depressed patients is a remarkably understudied area, with findings (almost two decades old) of both elevated (19) and diminished (20) CSF concentrations of substance P-like immunoreactivity. Moreoever, until the investigations reported herein, to our knowledge, there have been no studies of CSF substance P in PTSD or in any anxiety disorder.
Major depression and posttraumatic stress disorder (PTSD) are common and severe psychiatric syndromes that share risk factors (such as early life adversity), have overlapping symptoms (such as dysphoria and anxiety), frequently occur together, and are associated with high suicide rates. Pathophysiologically, both melancholic depression and PTSD are associated with elevated CSF concentrations of corticotropin-releasing hormone (CRH) and norepinephrine (21 – 25) , but only the former syndrome is characterized by hypercortisolemia (26) . On the basis of the extant preclinical and clinical literature (12) , the posited shared CNS pathophysiological mechanisms for depression-spectrum disorders and PTSD, and the clinical overlap between the syndromes, we hypothesized that CSF concentrations of the undecapeptide substance P are elevated in both conditions.
To measure CNS concentrations of substance P in depression and PTSD, we performed a series of CSF sampling experiments. First, using standard lumbar puncture, we measured CSF substance P concentrations in drug-free depressed patients (N=40) and healthy comparison subjects (N=47). Next, we collected CSF for 6 hours by means of an indwelling subarachnoid catheter in the spinal canal from eight drug-free, male military veterans with postcombat PTSD and from healthy comparison subjects. We waited approximately 3 hours after the placement of the subarachnoid catheter before beginning to withdraw CSF in order to control for the stress-related effects of needle insertion into the spinal canal (27) . Finally, in a crossover symptom provocation study, an additional seven drug-free combat veterans with chronic PTSD completed 6-hour CSF sampling procedures on two occasions each (6–9 weeks apart) in which patients watched in random order either a 1-hour film containing combat footage from the Vietnam War (traumatic stimulus) or a 1-hour instructional film on oil painting (neutral stimulus).
Method
These studies were approved by the Institutional Review Boards of the University of Cincinnati Medical Center (Cincinnati) and Butler Hospital (Providence, R.I.), the Human Investigations Committee of Yale University School of Medicine (New Haven, Conn.), and the Research and Development Committee of the Cincinnati Veterans Affairs (VA) Medical Center, as appropriate. Written, informed consent was provided by each patient or healthy subject before his or her participation.
The serial CSF sampling studies in PTSD patients were performed in the Psychoneuroendocrinology Research Suite at the Cincinnati VA Medical Center. The lumbar puncture studies of depressed patients were performed at the Clinical Neuroscience Research Center of the Connecticut Mental Health Center, New Haven, and in the Mood Disorders Clinic at Butler Hospital. All CSF samples were assayed for substance P at the Laboratory of Neuropsychopharmacology, Emory University School of Medicine, Atlanta. The PTSD and depression studies were unified when the assay for CSF substance P became available, due to the common interests of the authors of this article.
Patients
PTSD patients and matched healthy volunteers
Eight male combat veterans with chronic PTSD (mean age=42.6 years, SD=7.4) and six healthy comparison subjects (mean age=40.7 years, SD=9.6 years) underwent continuous CSF withdrawal, as previously described (28) , from 11:00 a.m. to 5:00 p.m. Substance P concentrations were measured in samples collected at hourly intervals. All patients and healthy volunteers were medication free. In patients who had received psychotropic medication, the medication had been discontinued for a minimum of five drug disappearance half-lives. Healthy subjects were without personal psychiatric history of any DSM-IV axis I condition. The mean Clinician-Administered PTSD Scale (29) score of the veterans with PTSD was 81 (SD=28). All participants had normal platelet counts, clotting times, hematocrit values, hepatic indices, and serum creatinine, pituitary-thyroid axis hormone, and random glucose concentrations.
PTSD patients in the symptom-provocation study
Seven male Vietnam veterans (mean age=51 years, SD=3, range=49–56) with combat-related, chronic PTSD each completed two 6-hour continuous CSF sampling procedures on separate days 6–9 weeks apart (except for one patient, for whom the interval between procedures was 5 months). The patients’ mean body mass indexes were 31.0 (SD=6.6) and 31.1 (SD=6.4) before the traumatic and neutral videotape sessions, respectively. The patients had no comorbid medical conditions.
All patients met the DSM-IV diagnostic criteria for PTSD, had had extensive combat experience during the Vietnam War while serving in either the Marines (four subjects) or the Army (three subjects), and had witnessed or participated in atrocities. Their mean Clinician-Administered PTSD Scale score was 78 (SD=24) and their mean Mississippi PTSD Scale (30) score was 155 (SD=18). These scores are collectively indicative of severe PTSD symptoms. Two patients had received comorbid clinical diagnoses of major depression, whereas all others had mild to moderate depressive symptoms. The mean Hamilton Depression Rating Scale (HAM-D) (31) score the day before each CSF sampling session was 13 (SD=8).
Six of the seven subjects had a history of alcohol dependence or abuse, but all six had been abstinent from alcohol for an average of 45 months (range=5 to 216 months). Six of the seven subjects also had histories of abuse of other substances, but their histories indicated that this abuse had ceased a minimum of 3 months before they started the study protocol (two subjects claimed to have abused substances only while serving in Vietnam). A history of alcohol dependence or abuse in a first-degree relative was reported by five of the six patients with knowledge of their relatives (the seventh patient was adopted). The results of urine drug screens were negative in all enrolled patients. Two of the patients smoked tobacco and were nicotine dependent at the time of study; two others were former smokers.
All subjects were medication free for a minimum of more than five medication disappearance half-lives before undergoing CSF sampling. The results of screening laboratory tests performed the evening before the film sessions indicated that all participants were euthyroid. All patients had normal platelet counts, clotting times, hematocrit values, hepatic indices, and serum creatinine, pituitary-thyroid hormone, and random glucose concentrations.
Depressed patients and matched healthy volunteers
Forty adult outpatients (mean age=36.7 years, SD=13.8, range=18–66) who fulfilled the DSM–IV criteria for unipolar major depressive disorder and 47 healthy comparison subjects (mean age=32.7 years, SD=10.9, range=19–58) were included in the study. Fifty percent (N=20) of the depressed patients and 55% (N=26) of the healthy subjects were female. All enrolled patients had HAM-D scores greater than 17. Semistructured diagnostic interviews were used to determine the presence of unipolar major depressive disorder in the patients and the absence of any current and lifetime DSM–IV axis I disorder in the healthy volunteers. Patients with any other major axis I comorbidity were excluded. Also excluded were subjects with any clinically significant medical disorder detected by means of a medical history, physical examination, and laboratory studies. All subjects were medication free for at least 2 weeks by the time of participation (at least 5 weeks for patients who had been taking fluoxetine).
Procedures
Serial CSF sampling
For both continuous CSF sampling studies, the patients and healthy volunteers were admitted to the Clinical Psychobiology Unit of the Cincinnati VA Medical Center the day before each procedure. During the afternoon, standardized tests were administered. At 8:00 p.m., the subjects ate a standard 665-calorie meal (20% protein, 24% fat, and 56% carbohydrate) and fasted thereafter, with the exception of water ingestion, until the end of the CSF sampling period at 5:00 p.m. the next day. At 8:00 p.m. the evening before the study, an intravenous line was placed in an arm for continuous infusion of normal saline. At midnight, all subjects were confined to bedrest, and, for participants who smoked , no further smoking was permitted until the end of the experiment.
The next morning, at approximately 8:30 a.m. by using strict sterile technique and 1% intradermal and subcutaneous xylocaine anesthesia, a 20-gauge polyamide catheter was placed in the subarachnoid space (spinal canal) by means of a 17-gauge Tuohy needle inserted through the L3–L4 vertebral interspace. The subarachnoid catheter was capped until 11:00 a.m., at which time continuous CSF withdrawal at a rate of 0.1 ml/minute commenced by means of a peristaltic pump. With subjects supine, CSF was collected hourly (until 5:00 p.m.) into iced test tubes for determination of substance P concentrations; these samples were “flash frozen” on dry ice at bedside then kept at –80˚C until assayed. Physiological saline solution was infused at a rate of 100 ml/hour throughout each procedure to maintain hydration.
Lumbar punctures
Subjects with major depression and matched healthy volunteers underwent a single lumbar puncture procedure, standardized to yield CSF samples at 12:00 noon 15 minutes, as outpatients at either the Clinical Neuroscience Research Unit at Connecticut Mental Health Center or the Mood Disorders Research Program at Butler Hospital. All subjects agreed to comply with instructions for a modified diet and activity schedule in the 24 hours preceding the lumbar puncture. Samples were collected with subjects seated in a forward-leaning position. After intradermal injection of 1% lidocaine, a 20-gauge introducer needle was used to penetrate the skin and superficial tissue. The introducer was used because the Sprotte 24-gauge pencil point spinal needle (B. Braun Medical, Bethlehem, Pa.) is very thin and has a relatively dull tip. The spinal needle was then inserted, by means of the introducer, through the L3–L4 or L4–L5 interspace into the spinal canal. A total of 12 cm 3 of CSF was collected, immediately divided into 0.5-ml aliquots, and frozen at –80˚C until assayed.
Use of videotapes for symptom provocation in PTSD
To examine the effects of symptom provocation on CSF substance P concentrations in patients with PTSD, at 12:00 noon, 1 hour after the start of serial CSF sampling in the second CSF sampling procedure, either a “stress” or neutral videotape was shown at moderately high volume in a random order of assignment. Both the stress and neutral videotapes were 60 minutes in duration. The stress videotape consisted of a 15-minute training film compiled for medics awaiting deployment to Vietnam (provided by Barbara Rounds-Kugler, R.N., a research nurse and decorated Vietnam veteran; the film itself was untitled and without attribution) followed by 45 minutes of the documentary film Letters Home from Vietnam (Home Box Office Studios, 1987). The medic training tape was by far the most graphic and disturbing portion of the stressful film. This tape consisted of serial scenes, with sound but no voice-over or music, of combat and combat casualties both on the battlefield and in a field hospital. It included depictions of several helicopter landings and take-offs, firefights (gun battles), artillery and mortar firing, shots of incoming artillery and mortar rounds, and infantry charges, including one in which an underground enemy tunnel was overrun. Multiple bloody and moaning soldiers were shown. The neutral videotape consisted of an oil painting instructional film.
Vital sign and mood assessment during serial CSF sampling
Mood and anxiety were self-rated with 100-mm visual analogue scales at 10-minute intervals throughout each procedure (32) . The analogue scale anchors were “high” and “low.” An automated vital signs monitor (Dinamap, Critikon, L.L.C., Tampa, Fla.) was used to monitor heart rate and blood pressure from the left leg at hourly intervals.
CSF substance P assay
CSF substance P immunoreactivity was determined with solid phase radioimmunoassay in CSF samples by using a highly specific substance P antibody. The researchers who conducted the assay were blinded to the identity of the groups from which the CSF samples were obtained. Briefly, Nunc-Immuno module plates (Fisher Scientific, Hamilton, N.H.) were incubated for 1 hour at room temperature with 250 ng/well of protein G in a 0.1-M sodium bicarbonate solution with a pH of 9.0. The plates were rinsed three times in wash buffer (150 mM of K 2 HPO 4 , 20 mM of NaH 2 PO 4 , 200 mM of ascorbic acid, 0.2% polysorbate 20, 0.1% sodium azide) with a pH of 7.6. One hundred microliters of substance P antibody (#RAS-7415, 500-reaction lyophilized formulation [Bachem, King of Prussia, Pa.] reconstituted in 60 ml of wash buffer containing 0.1% gelatin) was added to each well, and the plates were incubated at room temperature for 2 hours. The antibody solution was aspirated, and the samples and standards were added to the wells in a volume of 50 ml. Labeled 125 I-substance P (#H-5104, Bachem, King of Prussia, Pa.) was diluted to approximately 7000 counts per minute/25 ml wash buffer, and 25 ml was added to each well. Nonspecific binding was determined in wells containing only protein G (no antibody). Plates were incubated at 4˚C for 24 hours. After this period, the wells were aspirated, separated, placed in 12×75-mm borosilicate glass tubes, and counted on a four-channel gamma counter. The sensitivity of the assay was 1.5 fmol/ml. Intra-assay variability was 8.2%, and interassay variability was 9.7%.
Statistical Analysis
Baseline serial CSF sampling in PTSD patients and healthy volunteers
Substance P concentrations in the patients with PTSD and healthy volunteers were compared by using repeated-measures analysis-of-variance (ANOVA) with the independent variables of group and time. All p values quoted are for a two-sided hypothesis test; p values <0.05 were considered significant for these and all other results.
Symptom provocation in PTSD
In the symptom-provocation study, the following variables were measured for each patient: substance P concentration, heart rate, systolic blood pressure, diastolic blood pressure, and anxiety and mood self-ratings. Each measurement was taken 50 minutes before the subject viewed each videotape (baseline) and at the following six time points: 10, 70, 130, 190, 250, and 310 minutes after the start of the videotape. The percentage change from baseline was computed for each subject and film at each time point by using the following formula: ([measurement at time point] – baseline)/baseline × 100%. Last, the difference in percentage change from baseline was calculated for each subject at each time point for each of the two films. This difference (Δ percentage change from baseline) was equal to the percentage change from baseline during the provocative film minus the percentage change from baseline during the neutral film. For example, if a patient had a heart rate of 75 bpm at baseline and a heart rate of 80 bpm at 130 minutes while viewing the neutral film, that patient’s percentage change from baseline for heart rate for the neutral film at 130 minutes would be calculated as follows: (80–75)/75 × 100%=6.7%. If the patient had heart rates of 77 bpm and 92 bpm at baseline and 130 minutes, respectively, for the provocative film, the patient’s percentage change from baseline for heart rate for the provocative film at 130 minutes would be calculated as follows: (92–77)/77 × 100%=19.5%. The measure of interest is the difference between these two values—the patient’s Δ percentage change from baseline for heart rate at 130 minutes (19.5% minus 6.7%=12.8%). If there is no effect due to the provocative film, these values should be approximately zero on average. For the vital signs and mood self-ratings, the Δ percentage change from baseline was tested for statistical significance versus zero at each of the six time points. Paired t tests were used to conduct these hypothesis tests. If the data appeared nonnormal (Shapiro-Wilk test p value<0.20), then the Wilcoxon signed-ranks test was used.
Comparison of depressed patients and healthy volunteers
Pearson’s product-moment correlations were generated to examine the relationships between CSF substance P concentration, subject age, and CSF specimen storage time in the freezer. CSF substance P concentrations were not significantly correlated with age when the data for healthy comparison subjects and depressed patients were examined separately. However, when a large database of CSF substance P concentrations in patients with mood disorders was explored (unpublished 2004 data of L. Carpenter; available from the author on request), a significant negative correlation was found between age and substance P level (N=113; r=–0.23, p=0.01). Therefore, ANOVA with age as a covariate was used to determine the effect of subject group on CSF substance P concentration. T tests and chi-square tests were used to compare the groups with regard to mean age and gender composition, respectively.
Results
CSF Substance P Concentration in Major Depression
CSF concentrations of substance P were significantly higher in patients with major depression, compared with healthy subjects, after adjustment for age effects (F=25.5, df=1, 84, p<0.0001) ( Figure 1 ). The mean CSF substance P concentration was 51% higher in the depressed group (mean=79.1 fmol/ml, SD=23.5) than in the healthy comparison group (mean=52.5 fmol/ml, SD=25.1).
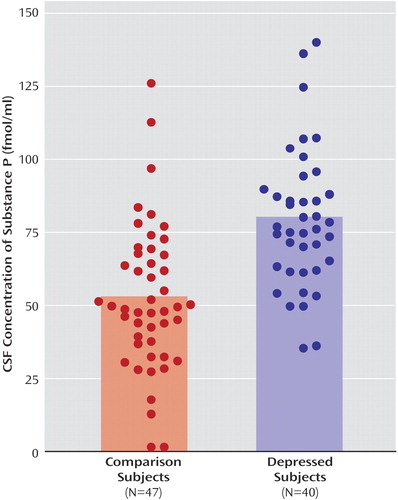
a Bars extend to the mean values. A significant between-group difference in mean values was found (p<0.0001, ANOVA).
Serially Sampled CSF Substance P in PTSD at Baseline
As determined by serial sampling through an indwelling spinal canal catheter, mean CSF substance P concentrations were significantly higher in the patients with PTSD (mean=34.5 fmol/ml, SD=4.8) than in the healthy comparison subjects (mean=22.2 fmol/ml, SD=2.8) (F=6.55, df=1, 12, p<0.03) ( Figure 2 ). Analysis of these data also revealed a time effect of 0.025 that approached significance (F=3.44, df=1, 66, p=0.07). This result may be interpreted as an overall slope (i.e., substance P levels increased slightly for both groups during the study). However, there was no statistically significant interaction between group and time (i.e., the increase in substance P over time was the same for the two groups) (F=2.37, df=1, 66, p=0.13).
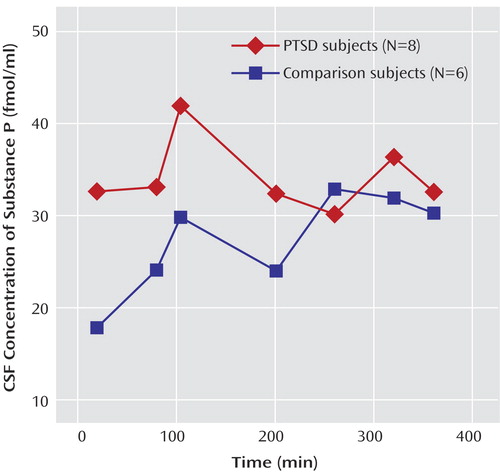
a CSF sampling commenced 3 hours after placement of a flexible polyamide subarachnoid catheter in the spinal canal. Mean CSF substance P concentrations were significantly greater in the patients with PTSD than in the comparison subjects (p<0.03, ANOVA).
Effect of Symptom Provocation in PTSD
During the 1-hour traumatic videotape, the maximum change in subjective anxiety ratings between any two adjacent points was on average 15.3 mm higher than during the neutral videotape (signed rank=14, p<0.02, two-sided, Wilcoxon signed-ranks test). The percentage change from baseline in mood was significantly greater during the traumatic movie than during the neutral film at both 10 minutes (Δ percentage change from baseline=–42.8%; t=–2.54, df=6, p<0.05) and 70 minutes (Δ percentage change from baseline=–57.4%; t=–3.06, df=6, p<0.05).
In the within-subject, crossover serial CSF sampling study (symptom-provoking versus neutral stimulus), hourly substance P concentrations were determined at baseline (–50 minutes before commencement of the audiovisual stimulus) and at 10, 70, 130, 190, 250, and 310 minutes after commencement of the audiovisual stimulus. CSF substance P concentrations increased by 169% and 90.6% of baseline levels at 10 and 70 minutes, respectively, after the start of the traumatic videotape, but changed by only 1.1% and -8.1% of baseline levels 10 and 70 minutes after the start of the neutral videotape (signed rank=12, p<0.05 at 10 minutes and t=3.05, df=6, p<0.03 at 70 minutes for the neutral versus the traumatic videotape [note that the Shapiro-Wilk p value equals 0.87 for substance P levels at 70 minutes, indicating that the data appear Gaussian; using the Wilcoxon nonparametric test, signed rank=11, p<0.07]) ( Figure 3 ). Remarkably, 250 and 310 minutes after the start of the traumatic film, CSF substance P concentrations remained elevated by 146% and 60.7%, respectively, above the premovie baseline, whereas 250 and 310 minutes after the start of the neutral movie, levels were 8% and 2%, respectively, lower than baseline (signed rank=13, p=0.03 at 250 minutes and signed rank=11, p<0.08 at 310 minutes for neutral versus traumatic videotape) ( Figure 3 ). However, the increase in CSF substance P concentrations that followed the traumatic stimuli appeared to be biphasic ( Figure 3 ), as the levels at 130 and 190 minutes were similar to those obtained after the neutral stimuli. Systolic blood pressure increased significantly more from baseline during exposure to the traumatic film, compared with the neutral film, at 10 minutes (14.2% greater increase from baseline during exposure to the traumatic videotape, relative to the neutral videotape, t=3.85, df=6, p<0.02) and at 70 minutes (22.1% greater increase during exposure to the traumatic videotape, relative to the neutral videotape, t=9.02, df=6, p<0.001).
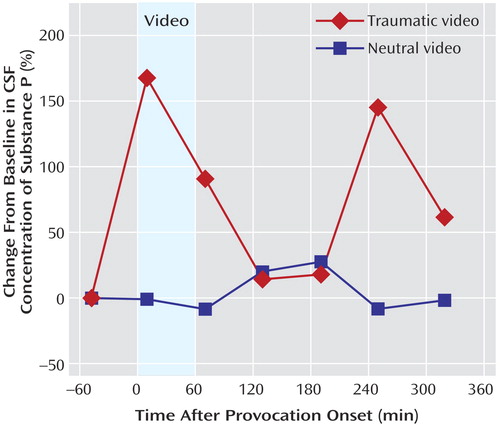
a The videotape stimulus was begun at time 0. CSF substance P concentrations were determined 50 minutes before commencement of the videotape and 10, 70, 130, 190, 250, and 310 minutes after commencement. A within-subject crossover design was used. The traumatic stimulus consisted of a 60-minute videotape showing scenes of combat or related scenes from the Vietnam conflict; the neutral stimulus consisted of a 60-minute instructional videotape on oil painting. CSF substance P concentrations rose significantly after exposure to the traumatic stimulus but not after exposure to the neutral film.
Discussion
The current data, not only demonstrate significantly elevated CSF substance P concentrations in patients with major depression but also demonstrate elevated CSF substance P concentrations in patients with PTSD. Moreover, in patients with PTSD who each underwent continuous CSF sampling on two occasions, we observed a marked increase in CSF substance P concentrations during and after a blood pressure-elevating, anxiogenic, mood-lowering stimulus but not after a neutral stimulus, implicating CNS release of substance P in the mechanism of acute PTSD symptoms.
Taken together, the current results suggest a pathophysiological role for tonically excessive substance P release in major depression and PTSD and, moreover, indicate that CNS substance P is robustly secreted in response to acute psychological stress.
It is of interest that CSF substance P concentrations were higher—about twice as high—in the subjects and patients in whom CSF was obtained immediately after lumbar puncture than in those from whom CSF was obtained by serial sampling by means of a polyamide subarachnoid catheter that had remained in place for approximately 3 hours before CSF collection began. Lumbar puncture and its anticipation are, to varying degrees, stressful experiences, and we have demonstrated in these studies that substance P levels increase in association with stressful psychological stimuli. Therefore, it is likely that the higher substance P concentrations in CSF samples obtained immediately after lumbar puncture are stress-related phenomena. However, we did not assess the study subjects’ level of anxiety in response to the procedure itself. Alternatively, we cannot rule out nonspecific binding of substance P to the polyamide subarachnoid catheter as an explanation for the lower levels in subjects and patients who underwent serial CSF sampling. However, the CSF substance P concentrations obtained by means of the polyamide catheters were in the same range as those previously reported by other investigators (33) . In any event, identical methods were used to study patients and comparison subjects in each situation in which statistical comparisons were made. Nevertheless, it will be important to identify and characterize other factors (besides acute stress and a diagnosis of depression or PTSD) that regulate CSF substance P concentration.
Other preexisting basic and clinical findings are consistent with the elevations in CSF substance P reported here in the patients with PTSD. For example, intrathecal administration of substance P to anesthetized rats has been shown to induce an increased heart rate that is blocked by the β-adrenergic antagonist propanolol (34) . Propanolol has been shown to be effective in preventing the development of PTSD and ameliorating PTSD symptoms (35 , 36) . In addition, various inflammatory cytokines have been shown to stimulate release of substance P (37) , and elevated concentrations of the proinflammatory cytokine interleukin-6 have been reported in postcombat PTSD patients (38) and in depressed patients (39) .
In summary, the present data demonstrate elevated substance P concentrations in the CNS of depressed patients and patients with PTSD. Moreover, our investigations reveal that CSF substance P levels in PTSD patients markedly increase during exposure to traumatic psychological stimuli but not during exposure to neutral psychological stimuli. These data provide the pathophysiological rationale for initiation of clinical trials of substance P antagonists in PTSD. More generally, our observations show that traumatic psychological stimuli can rapidly elicit robust changes in the concentrations of a peptide in the CSF in humans.
1. Von Euler US, Gaddum JH: An unidentified depressor substance in certain tissue extracts. J Physiol 1931; 72:74–87Google Scholar
2. Chang MM, Leeman SE: Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 1970; 245:4784–4790Google Scholar
3. Hokfelt T, Pernow B, Wahren J: Substance P: a pioneer amongst neuropeptides. J Intern Med 2001; 249:27–40Google Scholar
4. Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V: The tachykinin peptide family. Pharmacol Rev 2002; 54:285–322Google Scholar
5. Cuello AC, Polak JM, Pearse AG: Substance P: a naturally occurring transmitter in human spinal cord. Lancet 1976; 2:1054–1056Google Scholar
6. Bennett GW, Nathan PA, Wong KK, Marsden CA: Regional distribution of immunoreactive-thyrotrophin-releasing hormone and substance P, and indoleamines in human spinal cord. J Neurochem 1986; 46:1718–1724Google Scholar
7. Bouras C, Vallet PG, Dobrinov H, de St-Hilaire S, Constantinidis J: Substance P neuronal cell bodies in the human brain: complete mapping by immunohistofluorescence. Neurosci Lett 1986; 69:31–36Google Scholar
8. Ding YQ, Zheng HX, Wang DS, Xu JQ, Gong LW, Lu Y, Qin BZ, Shi J, Li HL, Li JS, Shigemoto R, Kaneko T, Mizuno N: The distribution of substance P receptor (NK1)-like immunoreactive neurons in the newborn and adult human spinal cord. Neurosci Lett 1999; 266:133–136Google Scholar
9. Hargreaves R: Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. J Clin Psychiatry 2002; 63 (suppl 11):18–22Google Scholar
10. Schoenen J, Lotstra F, Vierendeels G, Reznik M, Vanderhaeghen JJ: Substance P, enkephalins, somatostatin, cholecystokinin, oxytocin, and vasopressin in human spinal cord. Neurology 1985; 35:881–890Google Scholar
11. Schwark HD, Petit MJ, Fuchs JL: Distribution of substance P receptor binding in dorsal column nuclei of rat, cat, monkey and human. Brain Res 1998; 786:259–262Google Scholar
12. Stout SC, Owens MJ, Nemeroff CB: Neurokinin(1) receptor antagonists as potential antidepressants. Annu Rev Pharmacol Toxicol 2001; 41:877–906Google Scholar
13. Mantyh PW: Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry 2002; 63(suppl 11):6–10Google Scholar
14. Rupniak NM: New insights into the antidepressant actions of substance P (NK1 receptor) antagonists. Can J Physiol Pharmacol 2002; 80:489–494Google Scholar
15. Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, Hale JJ, Mills SG, MacCoss M, Swain CJ, Harrison T, Hill RG, Hefti F, Scolnick EM, Cascieri MA, Chicchi GG, Sadowski S, Williams AR, Hewson L, Smith D, Carlson EJ, Hargreaves RJ, Rupniak NM: Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998; 281:1640–1645Google Scholar
16. Krishnan KRR: Clinical experience with substance P receptor (NK1) antagonists in depression. J Clin Psychiatry 2002; 63(suppl 11):25–29Google Scholar
17. Kramer MS, Winokur A, Kelsey J, Preskorn SH, Rothschild AJ, Snavely D, Ghosh K, Ball WA, Reines SA, Munjack D, Apter JT, Cunningham L, Kling M, Bari M, Getson A, Lee Y: Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology 2004; 29:385–392Google Scholar
18. Stockmeier CA, Shi X, Konick L, Overholser JC, Jurjus G, Meltzer HY, Friedman L, Blier P, Rajkowska G: Neurokinin-1 receptors are decreased in major depressive disorder. Neuroreport 2002; 13:1223–1227Google Scholar
19. Rimon R, Le Greves P, Nyberg F, Heikkila L, Salmela L, Terenius L: Elevation of substance P-like peptides in the CSF of psychiatric patients. Biol Psychiatry 1984; 19:509–516Google Scholar
20. Berrettini WH, Rubinow DR, Nurnberger JI Jr, Simmons-Alling S, Post RM, Gershon ES: CSF substance P immunoreactivity in affective disorders. Biol Psychiatry 1985; 20:965–970Google Scholar
21. Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W: Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984; 226:1342–1344Google Scholar
22. Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS: Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 54:624–629Google Scholar
23. Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr: Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156:585–588; correction, 1999: 156:986Google Scholar
24. Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW: Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 2000; 97:325–330Google Scholar
25. Geracioti TD Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr, Kasckow JW: CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 2001; 158:1227–1230Google Scholar
26. Kasckow JW, Baker D, Geracioti TD Jr: Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides 2001; 22:845–851Google Scholar
27. Hill KK, West SA, Ekhator NN, Bruce AB, Wortman MD, Baker DG, Geracioti TD Jr: The effect of lumbar puncture stress on dopamine and serotonin metabolites in human cerebrospinal fluid. Neurosci Lett 1999; 276:25–28Google Scholar
28. Geracioti TD Jr, Orth DN, Ekhator NN, Blumenkopf B, Loosen PT: Serial cerebrospinal fluid corticotropin-releasing hormone concentrations in healthy and depressed humans. J Clin Endocrinol Metab 1992; 74:1325–1330Google Scholar
29. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8:75–90Google Scholar
30. Keane TM, Caddell JM, Taylor KL: Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol 1988; 56:85–90Google Scholar
31. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
32. Zealley AK, Aitken RC: Measurement of mood. Proc R Soc Med 1969; 62:993–996Google Scholar
33. Liu Z, Welin M, Bragee B, Nyberg F: A high-recovery extraction procedure for quantitative analysis of substance P and opioid peptides in human cerebrospinal fluid. Peptides 2000; 21:853–860Google Scholar
34. Yashpal K, Henry JL: Neural mediation of the cardiovascular responses to intrathecal administration of substance P in the rat: slowing of the cardioacceleration by an adrenal opioid factor. Neuropeptides 1993; 25:331–342Google Scholar
35. Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002; 51:189–192Google Scholar
36. Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR: Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003; 54:947–949; correction, 2003; 54:1471Google Scholar
37. Cioni C, Renzi D, Calabro A, Annunziata P: Enhanced secretion of substance P by cytokine-stimulated rat brain endothelium cultures. J Neuroimmunol 1998; 84:76–85Google Scholar
38. Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD Jr: Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 2001; 9:209–217Google Scholar
39. Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB: Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001; 158:1252–1257Google Scholar



