In This Issue
Metformin Stabilizes Weight in Children and Teens Taking “Atypical” Antipsychotics
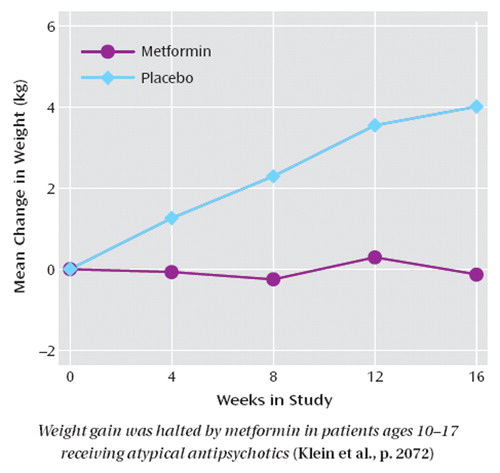
Second-generation, or “atypical, ” antipsychotic medications have benefits for psychiatric illness in children and adolescents but are often accompanied by significant weight gain , possibly resulting in type 2 diabetes. Metformin is a medication used to regulate blood glucose in type 2 diabetes. Klein et al. (p. Original article: 2072 ) compared metformin and placebo in 39 children and adolescents ages 10-17 who had gained at least 10% of their pretreatment weight while taking olanzapine, risperidone, or quetiapine. Metformin halted weight gain and decreased measures of insulin resistance. Over 4 months, the patients taking placebo gained an additional 4.0 kg on average, while weight was stable in the metformin group. However, the metformin group had a decrease in weight relative to height, as measured by body mass index, because the study was conducted in growing children. No serious side effects occurred. Since the substantial weight gain produced by second-generation antipsychotics can decrease compliance with treatment, metformin also has the potential to increase compliance and improve outcome. Dr. Kenneth Towbin discusses the importance of this study in an editorial on p. Original article: 2034 .
Childbearing and Antipsychotics
The risks to offspring from second-generation, or atypical, antipsychotic medications used by pregnant women have not yet been established in blinded or randomized studies. Yaeger et al. (p. Original article: 2064 CME) describe the case of a woman with paranoid schizophrenia to illustrate important considerations in the decision to continue, change, or stop treatment with an atypical antipsychotic during pregnancy. They stress the importance of optimizing the mother’s health and ability to parent. The pregnancies of women with schizophrenia often are unplanned, suffer from complications, and result in loss of custody. Discussions about contraception and pregnancy should therefore begin early in treatment. The medical risks associated with antipsychotics, such as obesity, diabetes, and hypertension, indicate a need to coordinate care during pregnancy with the patient’s obstetrician. After childbirth, dramatic hormonal changes may necessitate an increase in the antipsychotic dose, and additional social support may be helpful in preventing relapse. The benefits of breast-feeding during antipsychotic treatment are likely outweighed by the risks.
Costs and Benefits of Antipsychotics
Cost-effectiveness was analyzed by Rosenheck et al. (p. Original article: 2080 ) for the medications administered in phase 1 of the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE). Perphenazine, a first-generation antipsychotic with intermediate potency, was compared with four second-generation antipsychotics. Perphenazine was associated with a lower total cost of health care and was similar overall in terms of quality-adjusted life years, which were based on symptoms and side effects. The comparison was limited by the 18-month duration of the treatment trial, high dropout rates, and exclusion of patients with tardive dyskinesia at baseline. The results nevertheless encourage consideration of older intermediate-potency antipsychotics. Dr. Freedman comments on the importance of the study and its limitations in an editorial on p. Original article: 2029 . The phase 1 data from the CATIE study were examined from another perspective by Essock et al. (p. Original article: 2090 ). They evaluated the extent to which continuing to take the antipsychotic prescribed before the study, versus switching to that medication, influenced the outcome of that drug treatment. For instance, 23% of the patients randomly assigned to olanzapine in phase 1 were already taking olanzapine at study entry, whereas the other patients in the olanzapine group were switched from other antipsychotics. Comparison of the “stayers” and “switchers” indicated that the rates of treatment discontinuation were lower for the “stayers. ” This advantage was strongest for olanzapine, whereas patients who stayed with quetiapine did less well than those who were switched from quetiapine to olanzapine or risperidone. This distinction between “switchers” and “stayers” attenuates the overall CATIE phase 1 results but does not essentially alter them. Dr. Tamminga provides perspective in an editorial on p. Original article: 2032 . Polsky et al. (p. Original article: 2047 ) identify the essential methodological features of cost-effectiveness analyses of randomized, controlled trials. Applying these standards to eight earlier cost-effectiveness evaluations of second-generation antipsychotics revealed substantial methodological problems, which undermine the findings of those assessments.



