Controlled Trial of Naturalistic Dawn Simulation and Negative Air Ionization for Seasonal Affective Disorder
Abstract
Objective: This trial assessed two novel nonpharmaceutical treatments for winter depression—naturalistic dawn simulation and high-density negative air ionization—delivered during the final hours of sleep. Method: The patients were 99 adults (77 women and 22 men) with the winter seasonal pattern of major depressive disorder (94 cases) and bipolar II disorder (five cases). Five parallel groups received 1) dawn simulation (0.0003–250 lux in the pattern of May 5 at 45° north latitude); 2) a dawn light pulse (13 minutes, 250 lux, with an illuminant dose of 3.25×10 3 lux-minutes matched to the simulated dawn); 3) postawakening bright light (30 minutes, 10,000 lux); 4) negative air ionization at high flow rate (93 minutes, 4.5×10 14 ions/second); or 5) ionization at low flow rate (93 minutes, 1.7×10 11 ions/second). The symptoms were assessed over 3 weeks with the Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version. Results: Posttreatment improvement results were bright light, 57.1%; dawn simulation, 49.5%; dawn pulse, 42.7%; high-density ions, 47.9%; and low-density ions, 22.7% (significantly lower than the others). Contrary to the authors’ hypothesis, analysis of variance failed to find superiority of dawn simulation to the dawn pulse or bright light. However, the dawn pulse led to a pattern of residual or exacerbated depressive symptoms similar to those seen in low-density ion nonresponders. Conclusions: Naturalistic dawn simulation and high-density ionization are active antidepressants that do not require the effort of postawakening bright light therapy. They can be considered candidate alternatives to bright light or medication.
Findings over the last decade have demonstrated that morning bright light exposure ameliorates symptoms of seasonal affective disorder when gauged against nonphotic placebos (1 , 2) . Concurrently, basic biological rhythm research has pointed to dimmer gradual naturalistic dawn and dusk simulation as a potent alternative to bright light exposure. For example, hamsters show stronger circadian entrainment to non-24-hour light-dark cycles under naturalistic twilights than under conventional rectangular transitions (3) . Rats self-select the dimmer twilight signal to maintain circadian entrainment when given the opportunity to escape daylight exposure (4) . The rat retina responds to naturalistic dawn simulation with accelerated shedding of rod outer disk segments compared with sudden light onset (5) . In the human laboratory, naturalistic dawn simulation prevents the delay drift of rhythms under dim light conditions (6) . Bedside administration of a naturalistic dusk-to-dawn signal advances the sleep episode in demented elderly, with a tendency toward reduced sleep latency, longer duration, and decreased nocturnal activity (7) .
In identifying early morning bright light exposure as more effective than later morning or evening light for patients with seasonal affective disorder (8) , our attention was drawn to the early dawn interval, when melatonin wanes, core body temperature begins to rise, and the circadian timing system has the greatest propensity for light-elicited phase advances (9) . In the wintertime at northerly latitudes, this is also the period when it remains dark outdoors, a putative trigger of the depression. Therefore, we set out to explore the antidepressant effects of simulated dawn illumination during the final hours of sleep (10) .
Although we previously demonstrated effective treatment for winter depression by using naturalistic dawn simulation in a case series (11 , 12) , until now it has not been tested against control subjects given placebo or compared directly with postawakening bright light exposure. However, Avery and associates (13 – 15) have investigated a variant of dawn simulation in hypersomnic patients with seasonal affective disorder with a 90-minute sigmoidal illumination ramp with accelerated brief exposures and dim red exposures as controls. They found the sigmoidal simulation superior to postawakening bright light therapy (15) . However, some patients experienced side effects that we had not observed with naturalistic dawns: premature awakening during the initial exposure to the rising signal, occasionally accompanied by hypomania. The sigmoidal signal contrasts with naturalistic dawns, which begin several hours earlier in astronomical twilight and rise more gradually ( Figure 1 ).
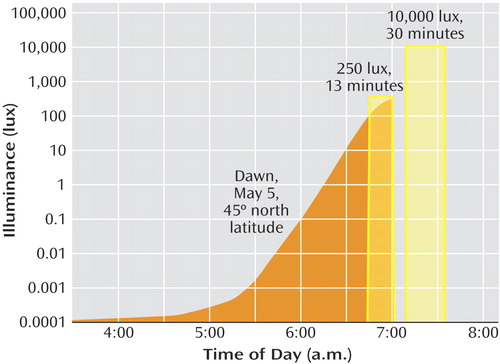
a For illustration, sunrise and wake-up time were anchored at 0700 hours, although they varied according to the individual’s habitual sleep schedule. The gradual dawn signal and dawn pulse were equated for total illuminant dose in lux-minutes. The curved shape of dawn varies with latitude and day of the year (35), as determined by the solar angle, the tilt of the earth and its orbital speed, atmospheric refraction, air mass penetrated, and a set of empirical constants (36). Variations in cloud cover had negligible influence before sunrise. The fastest transitions occur at the equinoxes, and the slowest transitions at the solstices. Accuracy of the simulation has been verified against outdoor measurements (37).
Negative air ionization is another environmental variable with antidepressant properties for patients with seasonal affective disorder (2 , 16) . Our tests of this nonphotic modality followed the use of Eastman and associates (17) of a deactivated ionizer as an inert placebo control for light therapy (1) . With the activated device, we found improvement with 30-minute postawakening exposures to high-density negative air ions, whereas low-density ionization was ineffective. This comparison provided a true double blind—impossible with light—because ambient ion concentration is not perceptible.
In the present study, we examined the efficacy of high-density ion exposure and naturalistic dawn simulation, both presented toward the end of sleep. We compared both methods with low-density negative air ionization (as a placebo) and postawakening bright light (as an established effective treatment). Additionally, as a control for the gradual naturalistic dawn signal, we presented a brief sunrise pulse, matched in total illuminant dose, just before wake-up time.
Method
Subjects
Research volunteers (ages 18–65) were screened for symptoms of winter depression. Diagnoses were based on the Structured Clinical Interview for DSM-III-R (18) or DSM-IV (19) , including major depressive disorder, recurrent, or bipolar disorder not otherwise specified (bipolar II disorder) with a seasonal pattern. The subjects also met the criteria of Rosenthal et al. for seasonal affective disorder (20) , including at least 2 preceding years of depression during the winter and summer remission. The subjects scored at least 20 points on the 29-item Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD) (21) , with a 21-item Hamilton Depression Rating Scale (HAM-D) score of 10 or more and an eight-item atypical symptom score of 5 or more. The subjects were medically healthy, as determined by a physical examination, standard blood work with a thyroid panel, and urinalysis. They were required to abstain from alcohol, psychotropic medication, and recreational drugs (verified by urine toxicology). Exclusion criteria included comorbid axis I disorders, a suicide attempt within 3 years, pregnancy, habitual sleep onset later than 0100 hours or a wake-up time later than 0900 hours, and past treatment with light or negative air ions. Written informed consent was obtained from the subjects after they had been given a full description of the study. The institutional review board of the New York State Psychiatric Institute approved the study protocol.
Protocol
Subjects were randomly assigned to one of five treatment conditions: dawn simulation, dawn pulse, bright light, or high- or low-density ionization. During the baseline phase (7–14 days), the subjects established consistent habitual sleep schedules (within a 30-minute range around the target times for sleep onset and offset) that they would maintain throughout the study. Daily treatment was taken at home for 3 weeks (dawn and ion conditions preceding habitual wake-up time, bright light within 10 minutes of awakening). Compliance was monitored by daily call-ins upon completion of the treatment session.
The subjects rated expectations on a 5-point scale from “no improvement” (1) to “full recovery” (5) based on written descriptions of the rationale for the assigned treatment modality. Ratings were made in three sets, for 1) the dawn signal (with no distinction between dawn simulation and dawn pulse), 2) ionization (without reference to dose), and 3) bright light therapy.
Trained raters who were blind to the treatment assignments evaluated depression severity at baseline and the treatment response after 10 days (midpoint) and 21 days (endpoint). The subjects who showed remission with a SIGH-SAD score reduction to 8 points or lower were monitored for up to 3 weeks of withdrawal for ascertainment of relapse to the minimum entry score of 20. Nonresponders and partial responders did not receive a withdrawal phase. The subjects also rated potential side effects at baseline and endpoint using the Systematic Assessment for Treatment Emergent Effects (22) .
Treatment Apparatus and Procedure
Naturalistic Dawn Simulation
The signal was generated by a microprocessor-based control box (SphereOne, Inc., Silver Plume, Colo.) simulating sunrise on May 5 at 45° north latitude based on our MacLite algorithm (23) . Figure 1 displays the 3.5-hour curvilinear transition from a protracted starlight glow at 0.0003 lux to an attenuated sunrise level of 250 lux (428 μw/cm 2 ; Model J17 photometer/radiometer, Tektronix, Inc., Beaverton, Ore.), as would be experienced outdoors under tree cover.
Calibrated output of the control box powered a glass-shielded 250-watt halogen bulb (#66490 Osram GmbH., Munich) in an overhanging wedge-shaped (1.59×10 3 cm 2 ) indirect light diffuser (SphereOne, Inc.) mounted on a tripod beside the bed. The light projected toward the pillow from a distance of 91 cm. After the simulated sunrise, which was set for wake-up time, the signal terminated with a 90-second logarithmic-linear ramp, and the patient was exposed to uncontrolled bedroom illumination. For late risers, the light of outdoor dawn could precede the scheduled dawn signal and penetrate the bedroom if the shades were open; however, we verified that such spontaneous light exposure did not allay the depression.
The power spectrum of the halogen signal (measured with a fiber optic spectrometer; Model USB2000, Ocean Optics, Inc., Dunedin, Fla.) varied smoothly across the visible range. Increasing irradiance from 0.25 to 250 lux reduced the relative power of short wavelengths (380–500 nm, 39% to 16%), with increases in the midrange (500–625 nm, 19% to 29%) and at long wavelengths (625–740 nm, 41% to 55%). At 250 lux, there was negligible ultraviolet radiation in the ultraviolet A (280–315 nm, 6.8 μw/cm 2 ) and ultraviolet B (315–380 nm, 7.6 μw/cm 2 ) ranges.
Dawn Pulse
As a control for naturalistic dawn simulation, we presented a trapezoidal light pulse of 250 lux (13 minutes) before wake-up time, with 90-sec logarithmic-linear onset and offset ramps, for a total duration of 16 minutes ( Figure 1 ). The illuminant dose, 3.25×10 3 lux-minutes, equaled that of the dawn signal.
Bright Light
The light box (UpLift Technologies, Dartmouth, N.S., Canada) presented 10,000 lux (2600 μw/cm 2 ) white light from three Osram Dulux 40-watt, 3,000-Kelvin fluorescent bulbs mounted behind an ultraviolet-filtered acrylic smooth diffusing screen (58.5×27.9 cm Acrylite OP-3, Piedmont Plastics, Inc., Charlotte, NC; modified with sandblasted diffusion surface by Uplift, Inc.). It was positioned with a downward angle toward the head of the bed at a 31-cm distance on a table stand. The subjects sat at the light box for 30 minutes within 10 minutes of rising, without looking directly at the screen.
The fluorescent power spectrum was composed of seven distinct peaks, with negligible ultraviolet radiation (ultraviolet A, 6.6 μw/cm 2 ; ultraviolet B, 6.8 μw/ cm 2 ). The relative power at short visible wavelengths was 17% (similar to that of the halogen lamp at 250 lux) but was weighted strongly toward the midrange (54%), with a reduction at long wavelengths (29%).
Negative Air Ionization
The negative air ion generator (SphereOne, Inc.) produced ion flow rates of 4.5 ×10 14 ions/second (high-density exposure) or 1.7×10 11 ions/second (low-density exposure). The ionizer was mounted on a tripod at the subject’s bedside, with the ion emitter directed toward the pillow at a distance of 61 cm. Ion flow toward the body was maximized by use of a grounded conductive bed sheet (Charleswater, Inc., Canton, Mass.) and activated by a timer for 93 minutes before wake-up time, corresponding to the dawn simulation interval above 0.001 lux.
Data Analysis
Repeated measures analysis of covariance (ANCOVA) of SIGH-SAD scores tested group differences, with age, gender, and baseline score covariates, by using Fisher’s method of least significant differences for post hoc comparisons. Post hoc ANCOVAs evaluated HDRS and Atypical Symptom Scale scores and percentage improvement. Categorical measures of endpoint response and the relative frequency of residual or exacerbated symptoms were evaluated with the chi-square test or Fisher’s exact test in cases of low expected frequency. Students’ paired and unpaired t tests were used for various between-group comparisons. The correlation between continuous variables was expressed by Pearson’s r. An alpha level of 0.05 was used throughout.
Results
During the 6 years of the study (conducted November to March), 126 subjects entered the study and 118 (93.7%) completed it. The noncompleters all withdrew before the 10-day midpoint evaluation, two for noncompliance (low-density ions, one; bright light, one), with six dropouts (low-density ions, one; high-density ions, three; bright light, one; and dawn simulation, one). Of the completers, 19 who experienced remission (16.1%) did not show relapse during the withdrawal phase; they were distributed across all five groups with no significant differences (Fisher’s exact test). Because such sustained improvement cannot be distinguished from spontaneous seasonal remission, they were excluded according to protocol (2 , 24) from the final data set. The results were analyzed for 99 subjects who either remained depressed at treatment endpoint or showed relapse during the withdrawal phase. The group included 77 (77.8%) women and 22 (22.2%) men, ages 19–63 years (mean=40.4 years, SD=10.4). The distributions of age and gender were closely balanced, with no significant differences by univariate analysis of variance and Fisher’s exact test, respectively. The diagnoses were major depressive disorder in 94 (94.9%) of the cases and bipolar disorder not otherwise specified or bipolar II disorder in five (5.1%) of the cases.
Rating Scale Means and Percentage Improvement
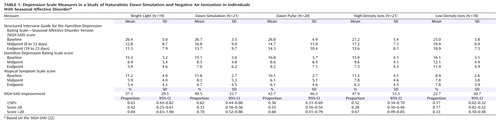
The overall mean SIGH-SAD score at baseline was 26.5 (SD=8.0), with an HAM-D mean score of 15.8 (SD=4.9) and an Atypical Symptom Scale mean score of 10.6 (SD=3.3). Univariate ANOVAs showed no significant baseline differences among the five groups ( Table 1 ). A repeated measures ANCOVA of SIGH-SAD scores across the three assessment points, with baseline score, age, and gender as covariates, revealed the following significant effects: group (F=2.48, df=4, 91, p=0.05), time (F=3.60, df=2, 90, p=0.03), and group-by-time interaction (F=2.51, df=4, 91, p=0.05). There were no significant effects of age (F=0.38, df=1, 91, p=0.94) or gender (F=1.02, df=1, 91, p=0.32). Improvement was greatest between baseline and the 10-day midpoint, with little change between midpoint and endpoint ( Table 1 ). Least significant difference comparisons between groups showed that the percentage improvement for the low-density ion group was significantly lower than for the four alternate groups (p=0.001 to p=0.02), and there were no significant differences among the latter groups. Posttreatment improvement was far lower for the low-density ion group (22.7%) than for the alternate groups (42.7% to 57.1%). The ANCOVA for raw scores also revealed significant effects of the baseline score covariate (F=14.29, df=1, 91, p<0.001) and the baseline score-by-time interaction (F=13.98, df=2, 90, p<0.001), which reflected greater opportunity for score reduction in more severe cases (r=0.28, N=99, p=0.004) (25) .
ANCOVAs on the scores from the HAM-D and Atypical Symptom Scale yielded contrasting results. The HAM-D showed the following significant effects, mirroring results for the SIGH-SAD: group (F=2.97, df=4, 91, p<0.03), time (F=4.19, df=2, 90, p<0.02), group-by-time interaction (F=2.99, df=4, 91, p<0.03), baseline score covariate (F=14.23, df=1, 91, p<0.001), and baseline score-by-time interaction (F=21.09, df=2, 90, p<0.001). On the Atypical Symptom Scale, however, the only significant effects were for the baseline score covariate (F=65.15, df=1, 91, p<0.001) and the baseline score-by-time interaction (F=15.23, df=2, 90, p<0.001). Nonsignificant effects included group (F=1.96, df=4, 91, p=0.11), time (F=1.36, df=2, 90, p=0.26), and the group-by-time interaction (F=2.02, df=4, 91, p=0.10). When we used percentage improvement rather than raw scores, the Atypical Symptom Scale did show a significant group effect (F=2.76, df=4, 91, p=0.03) that isolated low-density ions as less effective treatment, also seen on the HAM-D (F=3.16, df=4, 91, p=0.02). The two scales showed similar magnitudes of improvement except under low-density ions (HAM-D: mean=29.2%, SD=39.7%; Atypical Symptom Scale: mean=3.9%, SD=56.3%) (t=1.95, df=34, p=0.06, two-tailed), which may indicate a blunted placebo response for the symptoms of hypersomnia, hyperphagia, and fatigue. Further analyses focused on the combined SIGH-SAD scale.
Categorical Measures of Treatment Response
The proportion of patients achieving 50% or greater improvement ( Table 1 ) differed significantly between groups (χ 2 =10.55, df=4, p=0.03) and was far lower under low-density ions than under the other conditions (which did not differ significantly from each other). The proportion of patients meeting the remission criterion of a SIGH-SAD score of 8 or lower followed a similar pattern, although the groups did not differ significantly (χ 2 =3.15, df=4, p=0.53), which may reflect the higher remission rate for low-density ions (mean=0.17, SD=0.15) than in our previous parallel group study (mean=0.05, SD=0.09) (2) . The proportion with posttreatment scores below the 20-point baseline entry level, however, varied significantly (χ 2 =11.37, df=4, p=0.02), with the low-density ion group falling lower than the others.
Residual Symptoms, Emergence, and Exacerbation
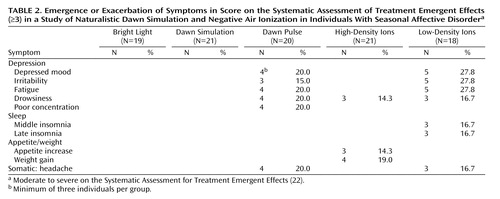
Of 88 potential somatic and psychological side effects tabulated by the Systematic Assessment for Treatment Emergent Effects, the only ones to show posttreatment emergence or exacerbation to moderate or high severity fell into the depression cluster ( Table 2 ). The patients reporting these symptoms were all nonresponders who showed the same symptoms at baseline; thus, none of these symptoms was emergent, and all reflected exacerbation under ineffective treatment. Accordingly, they occurred most often under low-density ions. The dawn pulse produced a profile very similar to that for low-density ions, although poor concentration was reported only by patients under the dawn pulse, and sleep disturbance was reported only under low-density ions. When residual and exacerbated symptoms were combined, a significantly higher proportion of the patients reported disturbance under the dawn pulse than under dawn simulation (mean=0.50, SD=0.19, versus mean=0.19, SD=0.14) (χ 2 =4.36, df=1, p=0.04).
Because the Systematic Assessment for Treatment Emergent Effects does not assess suicidality, we analyzed baseline and endpoint ratings for the HAM-D item. There were only two cases of emergence or exacerbation, both under the dawn pulse. One patient who scored 0 points at baseline scored 2 points (“wishes he were dead or any possible thoughts of death to self”) at the end of treatment, whereas the second patient moved from a score of 2 to 3 points (“suicidal ideas or gesture”).
Expectations
Mean expectation ratings differed across groups by less than 1 point on the 5-point scale: bright light, 3.5 (SD=0.9); dawn signals, 3.3 (SD=0.7); and ions, 2.7 (SD=0.9) (F=8.27, df=2, 96, p<0.001). Comparisons of the least significant difference showed that both the bright light and the dawn groups had higher expectations than the ion group. Of importance, expectations were not significantly different between the dawn simulation and dawn pulse subgroups (mean=3.4, SD=0.7, versus mean=3.1, SD=0.8) or the low- and high-density ion subgroups (mean=2.8, SD=1.0, versus mean=2.5, SD=0.9). Overall, expectation ratings were significantly correlated with endpoint SIGH-SAD percentage improvement (r=0.36, N=99, p<0.001), which was also observed separately in the three light groups (r=0.28, N=60, p=0.03) and the two ion groups (r=0.37, N=39, p=0.02).
Discussion
The trial design provided for several tests of efficacy, following the hypotheses that both dawn simulation and high-density ions would produce greater antidepressant response than low-density ions and that dawn simulation would be superior to both the dawn pulse and bright light treatment. Analysis of raw depression scale scores showed that dawn simulation and high-density ions were superior to low-density ions. However, the responses to bright light therapy and dawn pulse did not differ significantly from the response to dawn simulation. Percentage improvement and categorical measures of response and remission showed similar patterns. On this basis, we concluded that 1) naturalistic dawn simulation provided no advantage (other than convenience of use) over postawakening bright light therapy and 2) the dawn pulse is an effective treatment when we consider its superiority to low-density ions. Thus, it appears that gradual twilight is not necessary for therapeutic action during sleep. There is a partial precedent for such a dawn pulse effect in an uncontrolled trial of remitted depressed patients with residual hypersomnia (26) . When the patients were briefly exposed to 500 lux incandescent light switched on by a timer 10 minutes before the desired wake-up time, they reported easier awakening and a shortened sleep duration.
Despite the superiority of the dawn pulse over low-density ions and lack of difference from the other active treatments, overall response to the pulse was undermined by a distinct group of nonresponders. The Systematic Assessment for Treatment Emergent Effects ratings showed a pattern of exacerbation of depressive symptoms under the dawn pulse similar to that under low-density ions. Furthermore, HAM-D ratings showed two cases of emergent or exacerbated suicidality (both without active intent). By contrast, dawn simulation showed no such problems. We conclude that although the dawn pulse is therapeutically active in some patients, the risk of symptom persistence and emergence and exacerbation in other patients makes it an unfavorable option.
Several potential dosing parameters of naturalistic dawn simulation—dawn pulse, bright light, and negative air ions—might change their relative efficacy. For dawn simulation, there are the choices of day of year (solstices slowest, equinoxes fastest), illuminance anchor at sunrise (unshielded sunrise provides approximately 800 lux), sunrise time anchor relative to habitual wake-up time, spectral composition of the signal, and duration of the signal after sunrise. An additional factor for the dawn pulse is its duration preceding wake-up. Apart from spectral characteristics (27) , the duration and intensity of bright light therapy (24) and its timing relative to the individual’s circadian rhythm phase (28) are known to affect remission rate. As for negative air ions, the effects of flow rate (resulting in proximal ion density) and timing and duration of exposure have yet to be explored.
Expectation ratings for ions in our study were slightly but significantly lower than the ratings for light, which raises the question of the adequacy of low-density ions as a placebo control. Several results mitigate this potential confound. First, the low-density ion group showed no significant correlation of expectation ratings with SIGH-SAD percentage improvement (r=0.16, N=18, p=0.53). Second, the ratings did not differ significantly between the low- and high-density ion groups, yet the response was far greater to the high-density ions. Third, the response to high-density ions did not differ significantly from that for the light groups.
As in our previous trials of light therapy (24) and negative air ionization (2) , we used a strict criterion for data entry into the primary analysis, observation of relapse to the minimum baseline SIGH-SAD score of 20 within 3 weeks of discontinuation for the patients who showed remission during the treatment phase. Two factors support the exclusion of nonrelapsers. First, maintained improvement after treatment discontinuation during the expected period of a major depressive episode is prima facie evidence of a placebo response (29) . Especially in studies with a relatively small group size, higher placebo rates can seriously reduce the power to detect significant differences. This is less of a problem in larger drug trials with hundreds of patients with seasonal affective disorder (30) , which have been economically infeasible for nondrug alternatives (31) . Second, within a time-limited winter episode, it is impossible to know whether maintained remission after discontinuation reflects spontaneous seasonal improvement or a response to active treatment. We have reported universal relapse after treatment early in the winter season (32) . It is possible that prior light therapy trials that failed to find superiority over placebos would have concluded differently if relapse during a withdrawal phase had been ascertained and nonrelapsers excluded. To test this supposition, we conducted a post hoc analysis of SIGH-SAD improvement of 50% or greater, including all patients, regardless of relapse. Perforce, response rates increased, especially for low-density ions. Although the pattern of group contrasts was retained, it fell short of statistical significance: bright light, mean=0.67, SD=0.17; dawn simulation, mean=0.67, SD=0.17; dawn pulse, mean=0.63, SD=0.17; high-density ions, mean=0.57, SD=0.17; low-density ions, mean=0.35, SD=0.17) (χ 2 =6.75, df=4, p=0.15). Similarly, ANCOVAs on raw scores fell short of statistical significance.
The hypothesis that dawn simulation is superior to bright light therapy has been attractive because of the springtime pattern of illumination, which is lacking in winter (10) . However, the hypothesis becomes less attractive given the similarity of response to dawn and postawakening bright light in our study. Like Avery and associates (13 – 15) , we showed that a dawn signal presented toward the end of sleep was superior to that of placebo in comparison subjects (in their case, dim or brief light ramps with lower illuminant dose; in our case, low-density negative air ionization), also presented during sleep. Unlike Avery and associates (15) , however, we did not find dawn simulation superior to postawakening bright light exposure. Furthermore, they found similar responses to bright light and the dim red control. Without the exclusion of nonrelapsers, as determined in a withdrawal phase, their high placebo response rate (approximately 65%) may have impeded detection of a group difference. Alternatively, the reduced efficacy of bright light relative to dawn simulation in their study may have resulted from confinement to hypersomnic patients with standardized wake-up and treatment at 0600 hours, which is likely not individually optimal (28) . In our study, bright light was used shortly after habitual wake-up time, which ranged from 0530 hours to 0900 hours (mean=0705, SD=1.16).
Given the approximate equivalence of naturalistic dawn simulation, high-density ionization, and bright light, the choice between them may depend on convenience and ease of compliance. In this respect, automated exposure to the dawn signal or ions during sleep has an advantage over postawakening bright light therapy. On the other hand, dawn presentation in the bedroom can disturb a sleep partner with a later wake-up time, whereas bright light therapy can be administered privately in a separate room. Negative air ionization during sleep appears to be the most innocuous alternative; thus far, we have received no reports of disturbance in bed partners. Although the antidepressant effect of negative air ionization in seasonal affective disorder recently has been independently replicated using postawakening administration (personal communication, R.K. Flory, May 23, 2006), the result for administration during sleep remains a novel observation.
For open treatment, we recommend starting patients with postawakening bright light therapy, which has seen the most extensive investigation and replication (31) . If it is unsuccessful or proves impractical, given nonresponse, intractable side effects (33) , scheduling inconvenience, or noncompliance, dawn simulation (whether naturalistic or sigmoidal), high-density negative air ionization, and antidepressant drugs (34) provide an armamentarium of alternate treatments.
1.. Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM: Bright light treatment for winter depression: a placebo-controlled trial. Arch Gen Psychiatry 1998; 55:883–889Google Scholar
2.. Terman M, Terman JS, Ross DC: A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry 1998; 55:875–882Google Scholar
3.. Boulos Z, Macchi MM, Terman M: Twilights widen the range of circadian entrainment in hamsters. J Biol Rhythms 2000; 17:353–363Google Scholar
4.. Terman M, Remé CE, Wirz-Justice A: The visual input stage of the mammalian circadian pacemaking system, II: the effect of light and drugs on retinal function. J Biol Rhythms 1991; 6:31–48Google Scholar
5.. Remé CE, Bush R, Hafezi F, Wenzel A, Grimm C: Photostasis and beyond: where adaptation ends, in Photostasis and Related Topics. Edited by Williams TP, Thistle A. New York, Plenum Press, 1998, pp 199–206Google Scholar
6.. Danilenko KV, Wirz-Justice A, Kräuchi K, Weber JM, Terman M: The human circadian pacemaker can see by the dawn’s early light. J Biol Rhythms 2000; 15:437–446Google Scholar
7.. Gasio PF, Kräuchi K, Cajochen C, van Someren E, Amrhein I, Pache M, Savaskan E, Wirz-Justice A: Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp Gerontol 2003; 38:207–216Google Scholar
8.. Terman JS, Terman M, Lo ES, Cooper TB: Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry 2001; 58:69–75Google Scholar
9.. Dijk DJ, Lockley SW: Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol 2002; 92:852–862Google Scholar
10.. Terman M: Light on sleep, in Sleep Science: Integrating Basic Research and Clinical Practice. Edited by Schwartz WJ. Basel, Switzerland, Karger, 1997, pp 229–249Google Scholar
11.. Terman M, Schlager D, Fairhurst S, Perlman B: Dawn and dusk simulation as a therapeutic intervention. Biol Psychiatry 1989; 25:966–970Google Scholar
12.. Terman M, Schlager DS: Twilight therapeutics, winter depression, melatonin, and sleep, in Sleep and Biological Rhythms. Edited by Montplaisir J, Godbout R. New York, Oxford University Press, 1990, pp 113–128Google Scholar
13.. Avery DH, Bolte MA, Dager SR, Wilson LG, Weyer M, Cox GB, Dunner DL: Dawn simulation treatment of winter depression: a controlled study. Am J Psychiatry 1993; 150:113–117Google Scholar
14.. Avery DH, Bolte MA, Wolfson JK, Kazaras AL: Dawn simulation compared with a dim red signal in the treatment of winter depression. Biol Psychiatry 1994; 36:181–188Google Scholar
15.. Avery DH, Eder DN, Bolte MA, Hellekson CJ, Dunner DL, Vitiello MV, Prinz PN: Dawn simulation and bright light in the treatment of SAD: a controlled study. Biol Psychiatry 2001; 50:205–216Google Scholar
16.. Terman M, Terman JS: Treatment of seasonal affective disorder with a high-output negative air ionizer. J Altern Complement Med 1995; 1:87–92Google Scholar
17.. Eastman CI, Lahmeyer HW, Watell LG, Good GD, Young MA: A placebo-controlled trial of light treatment for winter depression. J Affect Disord 1992; 26:211–222Google Scholar
18.. Spitzer RL, Williams JB, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: History, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Google Scholar
19.. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (With Psychotic Screen). New York, New York State Psychiatric Institute, 1995Google Scholar
20.. Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA: Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 1984; 41:72–80Google Scholar
21.. Williams JBW, Link MJ, Rosenthal NE, Terman M: Structured Interview Guide for the Hamilton Depression Rating Scale—Seasonal Affective Disorder Version (SIGH-SAD). New York, New York State Psychiatric Institute, 2002Google Scholar
22.. National Institute of Mental Health: Systematic Assessment for Treatment Emergent Effects (SAFTEE). Rockville, Md, NIMH, 1986Google Scholar
23.. Fairhurst S, Levitt J, Terman M: MacLite Operations Manual. New York, Research Foundation for Mental Hygiene, 2000Google Scholar
24.. Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, Gallin PF, Quitkin FM: Efficacy of brief, intense light exposure for treatment of winter depression. Psychopharmacol Bull 1990; 26:3–11Google Scholar
25.. Lord MF: Elementary models for measuring change, in Problems in Measuring Change. Edited by Harris CW. Madison, Wis, University of Wisconsin Press, 1962, pp 21–38Google Scholar
26.. Jacobsen FM: Waking in a lighted room (letter). Biol Psychiatry 1990; 27:372–374Google Scholar
27.. Oren DA, Brainard GC, Johnston SH, Joseph-Vanderpool JR, Sorek E, Rosenthal NE: Treatment of seasonal affective disorder with green light and red light. Am J Psychiatry 1991; 148:509–511Google Scholar
28.. Terman M, Terman JS: Light therapy, in Principles and Practice of Sleep Medicine, 4th ed. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, Elsevier, 2005, pp 1424–1442Google Scholar
29.. Stewart JW: On placebo effects, SAD assessment, and withdrawal to relapse (letter). Light Treatment Biol Rhythms 1991; 3:19–20Google Scholar
30.. Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, Metz A, Rockett CB, Wightman DS: Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry 2005, 58:658–667Google Scholar
31.. Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Wisner KL, Nemeroff CB: The efficacy of phototherapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 2005; 162:656–662Google Scholar
32.. Terman JS, Terman M, Amira L: One-week light treatment of winter depression at its onset: the time course of relapse. Depression 1994; 2:20–31Google Scholar
33.. Terman M, Terman JS: Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry 1999; 60:799–808Google Scholar
34.. Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, Tam EM: The CAN-SAD study: randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry 2006; 163:805–812Google Scholar
35.. Danilenko KV, Wirz-Justice A, Kräuchi K, Cajochen C, Wever JM, Fairhurst S, Terman M: Phase advance after one or three simulated dawns in humans. Chronobiol Int 2000; 17:659–668Google Scholar
36.. Rozenberg GV: Twilight Study in Atmospheric Optics. New York, Plenum Press, 1966Google Scholar
37.. Terman M, Fairhurst S, Perlman B, Levitt J, McCluney R: Daylight deprivation and replenishment: a psychobiological problem with a naturalistic solution, in Architecture and Natural Light. Atlanta, American Society of Heating, Refrigerating and Air-Conditioning Engineers, 1989, pp 438–445Google Scholar