Contingency Management for the Treatment of Methamphetamine Use Disorders
Abstract
Objective: Theory and some preliminary evidence suggest that contingency management may be an effective treatment strategy or adjunct to psychosocial treatment for methamphetamine use disorders. An experimentally rigorous investigation on the topic was provided by a large multisite trial conducted under the auspices of the Clinical Trials Network of the National Institute on Drug Abuse. Method: The authors report data on 113 participants who were diagnosed with methamphetamine abuse or dependence. They were randomly assigned to receive 12 weeks of either treatment as usual or treatment as usual plus contingency management. Urine samples were tested for illicit drugs, and breath samples were tested for alcohol. The reinforcers for drug-negative samples were plastic chips, some of which could be exchanged for prizes. The number of plastic chips drawn increased with each week of negative samples but was reset to one after a missed or positive sample. Results: The participants in both groups remained in treatment for equivalent times, but those receiving contingency management in addition to usual treatment submitted significantly more negative samples, and they were abstinent for a longer period of time (5 versus 3 weeks). Conclusions: These results suggest that contingency management has promise as a component in treatment strategies for methamphetamine use disorder.
Methamphetamine use and procurement are public health and criminal justice problems throughout much of the world (1) . In the United States, methamphetamine use is most common in the Western and Midwestern United States, but use appears to be increasing in the East (2) . Methamphetamine use occurs in all types of communities, from large cities to rural settings, although the most severe impact is observed most often in rural areas and moderately sized urban communities (2) .
Methamphetamine abuse is associated with a number of medical consequences, including increased HIV risk (3 – 6) and some psychiatric comorbidity (7) . In addition to use, the manufacture and distribution of methamphetamine carry significant medical risks, such as fire and accidental poisoning (8 , 9) . Fortunately, treatment of methamphetamine use disorders appears to ameliorate some of the medical risks associated with its continued use (10) .
Methamphetamine abuse is also associated with neurocognitive impairment, which not only occurs during use but persists even during the early stages of abstinence (11 , 12) . Recent data suggest that prolonged abstinence ameliorates some, but perhaps not all, of this impairment (13 , 14) .
To a large extent, treatment strategies for methamphetamine use disorders have been based on the strategies shown to be effective in treating cocaine use disorders, as the two share many features (15 , 16) . However, some differences between the use of cocaine and methamphetamine do exist. Methamphetamine appears to be used in a more periodic fashion than cocaine (17 , 18) . While cocaine and alcohol are commonly used together, methamphetamine appears to be preferentially used with marijuana (17) .
These differences notwithstanding, some of the psychosocial interventions that have been useful in the treatment of cocaine use disorders also appear to be promising for treating methamphetamine use disorders. These treatments include brief cognitive behavior interventions (19) and the “Matrix model,” the substance abuse treatment developed at the Matrix Institute on Addictions (20) . Other approaches being investigated include pharmacotherapy (for instance, imipramine [21] ) and immunotherapy (22) .
One intervention that has proven useful in treating cocaine and other types of substance use disorders is contingency management. Contingency management interventions are based on an extensive basic science literature supporting the position that drug use is a form of operant behavior (23 – 25) . If so, the availability of salient alternative nondrug reinforcers should decrease drug use (26 – 28) . These observations form the conceptual basis for the contingency management approaches to drug abuse treatment. In a recent meta-analysis it was estimated that participants receiving contingency management interventions for various types of drug abuse averaged a success rate of 61%, compared to 39% for participants in the groups to which contingency management was compared (29) .
Further supporting the use of contingency management in the treatment of methamphetamine use disorders are preliminary data demonstrating the amenability of methamphetamine use to modification through abstinence reinforcement procedures. In an inpatient study using a laboratory model of contingency management, methamphetamine abusers reduced or stopped self-administration of methamphetamine in exchange for small amounts of money (30) . The proclivity to self-administer methamphetamine further decreased with increases in the magnitude of the alternative monetary reinforcers from which participants could choose. In another set of studies, conducted to refine the scheduling variables used in contingency management interventions, we demonstrated that methamphetamine use is amenable to modification through voucher-based contingency management interventions (31 , 32) .
Recent studies have also provided evidence that contingency management may be useful in the treatment of methamphetamine use disorders (33 , 34) . While theory and some preliminary evidence suggest that a contingency management approach may be an effective treatment strategy or adjunct to psychosocial treatment for methamphetamine use disorders (for instance, see reference 2), we know of no results of experimentally rigorous investigations on the topic that have been published to date. A recently completed large clinical trial of contingency management treatment for stimulant abusers (35) provided an opportunity to examine outcomes for a subsample of stimulant abusers whose drug of choice was methamphetamine. Therefore, we sought to examine the effectiveness of contingency management for methamphetamine dependence based on a subanalysis of data from this large randomized, controlled trial conducted as part of the Clinical Trials Network initiative of the National Institute on Drug Abuse.
In the original project sponsored by the Clinical Trials Network, participants who abused stimulants (either cocaine or methamphetamine) were randomly assigned to receive prize-based contingency management (with variable magnitude of reinforcement) (35 – 37) or treatment as usual at substance abuse clinics providing nonpharmacological treatment distributed throughout the country. The overall outcomes of that project have been reported elsewhere and suggest that the contingency management procedure was effective at retaining individuals in treatment and initiating abstinence (35) . The study consisted largely of cocaine-dependent individuals (72%). In the current analyses, we examined the efficacy of the procedure in a subset of 113 individuals with a methamphetamine use disorder (abuse or dependence) who participated in the main trial.
Method
Participants were enrolled between April 30, 2001, and Feb. 28, 2003. Because of concerns about the confidentiality of those who elected not to participate, screening information was not systematically collected, and data are unavailable for clients who did not qualify for or refused participation. In total, 113 participants from four nodes who had diagnoses of either methamphetamine abuse or dependence were randomly assigned to treatments. Of these, 67.3% came from one node, 31.9% came from another node, and the remaining 0.9% came from two additional nodes. Given the small numbers of participants at two of the four sites, analyses at the level of site were not conducted. The majority of participants came from sites located in the western United States.
Study Procedures
The procedures have been previously described (38) . Thus, only brief descriptions are provided here.
Each participant completed a 1.5-hour interview before randomization. The interview covered demographic characteristics, psychosocial problems, and lifetime and current drug use, including DSM-IV substance use diagnoses. Compensation for completing the assessment was provided in the form of a choice of items valued at about $20.
After completion of the assessment, each participant provided a urine sample, which was tested on-site by using procedures described in the following. The results of these samples were used to randomly assign the participants to the two study conditions. The participants were stratified on two variables: 1) presence or absence of a stimulant in the intake sample (cocaine, amphetamine, or methamphetamine) and 2) presence or absence of marijuana or opioids in the sample. The participants were randomly assigned to two study conditions: treatment as usual or treatment as usual plus contingency management. Fifty-one participants were randomly assigned to the combined-treatment group, and 62 participants were assigned to treatment as usual.
The intervention was in effect for 12 weeks. Each participant was expected to provide a urine sample at each of the twice-weekly study visits during the 12-week period, for a total of up to 24 samples. The intake specimen constituted the first of these 24 samples. In many cases, sample collections were observed by a same-sex observer to ensure validity. Additional validity checks included an external temperature strip and adulterant test strip that tested for abnormal levels of pH, creatinine, gluteraldehyde, and nitrite. Samples that failed any of these validity checks were discarded, and these participants were asked to give another specimen. If a participant failed to give a valid sample or attend a scheduled visit, the sample was considered missing. The urine samples were tested with OnTrak TesTcup 5 (Roche Diagnostics, Indianapolis), which tests for amphetamine, methamphetamine, cocaine, tetrahydrocannabinol, and morphine. Each participant also provided a breath sample at each visit that was tested for alcohol by means of a desktop or handheld breath analyzer. Samples with alcohol levels higher than 0.01 g/dl were considered positive.
Study Conditions
Treatment as usual
At the clinic from which the largest proportion of participants was drawn for this subanalysis, the treatment consisted of Matrix model therapy (39) . At the other two clinics, the treatment was largely a mix of cognitive behavior therapy and relapse prevention. All sites encouraged participation in 12-step groups.
Contingency management
Methamphetamine, cocaine, amphetamine, and alcohol were considered the primary target drugs. The participants assigned to contingency management earned the chance to win prizes each time they tested negative for the primary target drugs. Those who tested negative for all four drugs were invited to draw between one and 12 square plastic chips from an opaque container containing 500 chips. Each chip was marked with a value: 250 (50.0%) were marked “Good Job,” 209 (41.8%) were marked “Small,” 40 (8.0%) were marked “Large,” and one (0.2%) was marked “Jumbo.” “Good Job” chips meant no tangible reinforcer was earned. The prizes associated with “Small” chips were worth approximately $1 to $5. When such a chip was drawn, the participant selected from a variety of prizes in the category; popular small items included toiletries, snacks, bus tokens, and gift certificates for fast-food restaurants. Items available as “Large” prizes were worth about $20. Commonly selected ones included kitchen utensils and electronic devices, toys, cordless telephones, portable compact disk players, and gift certificates for retail stores. “Jumbo” prizes were worth $80–$100; popular items were televisions, stereos, and DVD players.
The number of draws earned was determined by a reinforcement schedule that was responsive to test outcomes. Specifically, the number of draws increased by one for each week in which all submitted samples tested negative for the primary target drugs. The number of draws earned was reset to a single draw after an unexcused absence or submission of a sample positive for one or more primary target drugs. This escalating schedule with a reset contingency has been demonstrated to produce more continuous abstinence during treatment than other schedules to which it has been compared (40) .
To offset the low rate of reinforcement early in the study, when the number of draws was low, a single large prize was awarded when a participant first achieved two consecutive weeks of abstinence (i.e., four consecutive urine and breath samples negative for the primary target drugs). In addition, at each study visit the participants testing negative for the primary target drugs earned two bonus draws if their samples tested negative for opioids and marijuana (secondary target drugs). The number of bonus draws did not escalate over time, and bonus draws could not be earned if the sample tested positive for any of the primary or secondary target drugs; that is, bonus draws were dependent on total abstinence.
Participants who provided all scheduled urine and breath samples throughout the study and whose samples were negative for all primary and secondary drugs earned 204 draws, resulting in an average of approximately $400 in prizes, plus one $20 prize after 2 weeks of abstinence.
Outcome Measures
Retention in the study was defined in two ways: 1) as the number of weeks that elapsed between the first and last study urine samples submitted and 2) whether the participant completed the study (i.e., made a study visit in week 12).
Treatment participation was evaluated by examining the number of counseling sessions attended during the 12-week period. It included individual, group, and family counseling sessions.
Drug use was measured in three ways: 1) total number of stimulant- and alcohol-negative samples submitted by each participant, 2) test results (positive/negative/missing) for stimulants and alcohol at each visit and follow-up, and 3) longest documented duration of sustained abstinence from stimulant drugs and alcohol for each participant. This variable was defined as the largest number of consecutive negative samples delivered under the twice-weekly schedule (with each sample representing 2–5 consecutive days of stimulant abstinence since the last test). While the focus of this analysis was on methamphetamine users, the contingency was, in fact, on abstinence from not only methamphetamine but also cocaine and alcohol. However, in only 0.3% of the results was a sample positive for cocaine or alcohol but not methamphetamine. We also assessed marijuana use because of the association between marijuana and methamphetamine previously reported (17) .
Data Imputation and Analysis
Consistent with the parent study on which this subanalysis is based (35) , a single excused absence per week was allowed without penalty. Additionally, a missing visit was coded as negative when at least one sample was collected per week and results for the samples both before and after the missing value were negative. For methamphetamine, which is the focus of the present study, only 3.3% of the missing samples for participants in the incentive condition and 4.0% of the missing samples for subjects receiving treatment as usual were coded as negative. For analysis of urine results over time, missing results not coded as negative under the preceding rule were considered in two ways: 1) coded as missing and not included in the analysis or 2) coded as positive.
Between-group comparisons of baseline measures that were continuous variables were made with t tests, and chi-square tests were used for those that were dichotomous. The durations of study retention (time to last submitted sample) were compared in the two groups by using Cox proportional hazards model. An “event” occurred when the date of the final submitted sample was prior to the last week of the study. Data were censored at week 12 if a submitted sample was provided in the last week of the study. Results are reported as hazard ratios and 95% confidence intervals (CIs). Comparisons of groups on treatment participation, total number of negative urine samples, and longest period of abstinence were performed by means of t tests.
Group results on the urine and breath tests across time were compared by using generalized estimating equations. Results are reported as odds ratios, indicating the likelihood that participants in the incentive condition had outcomes different from those of participants in the usual-care condition, and 95% CIs surrounding the odds ratios. One set of analyses focused on drug use (stimulants and alcohol and, separately, marijuana) during treatment, with results from all treatment visits included. A second analysis focused on posttreatment drug use and included urine and breath results at 3- and 6-month follow-ups, along with the last during-treatment result.
All data analyses were conducted by means of SAS 8.0 for Windows (SAS Institute, Cary, N.C., 2000).
Results
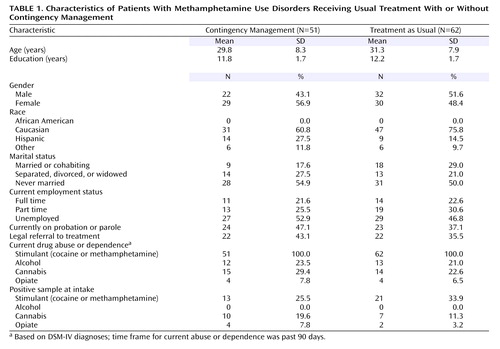
Demographic variables did not differ between conditions and are presented in Table 1 .
Retention, measured in two ways, did not statistically differ between groups. The percentages of participants who were retained for the entire study duration were 54.9% in the contingency management condition and 38.7% in the treatment as usual condition (χ 2 =2.95, df=1, p=0.86). Cox regression analysis showed no significant difference between the two conditions in terms of the number of weeks the subjects remained in the study (hazard ratio=1.51, CI=0.90–2.54; χ 2 =2.46, df=1, p=0.12). Similarly, no significant difference was noted in counseling attendance, with participants in the contingency management condition attending an average of 21.4 sessions (SD=15.8) and those who received treatment as usual attending an average of 19.4 sessions (SD=15.2) (t=0.68, df=111, p=0.50).
With regard to detected drug abstinence during treatment, the participants in the contingency management group submitted significantly more stimulant- and alcohol-negative samples (mean=13.9, SD=8.8) than did participants receiving treatment as usual (mean=9.9, SD=8.0) (t=2.55, df=110, p=0.01) ( Figure 1 ). As only 0.21% of the results were positive for alcohol and 0.07% were positive for cocaine, these results were largely driven by methamphetamine use. Similarly, participants receiving contingency management had a longer mean period of documented continuous abstinence (approximately 4.6 weeks, based on a mean of 9.3 consecutive samples, SD=9.2) than did participants receiving treatment as usual (approximately 2.8 weeks, based on a mean of 5.6 consecutive samples, SD=7.2) (t=2.38, df=110, p=0.02) ( Figure 1 ). Abstinence rates across the treatment visits were analyzed by using generalized estimating equations, which indicated that the contingency management participants were more likely to submit negative urine samples than were the usual-care participants. These results were similar regardless whether we characterized the missing data as positive samples (odds ratio=2.04, 95% CI=1.19–3.49; χ 2 =6.80, df=1, p=0.009) or excluded them from the analysis (odds ratio=2.65, CI=1.50–4.67; χ 2 =11.29, df=1, p<0.001) ( Figure 2 ). Additionally, 17.6% of the individuals in the contingency management condition were abstinent throughout the entire trial, compared to only 6.5% in the treatment as usual condition. This difference approached significance (χ 2 =3.44, df=1, p=0.06).
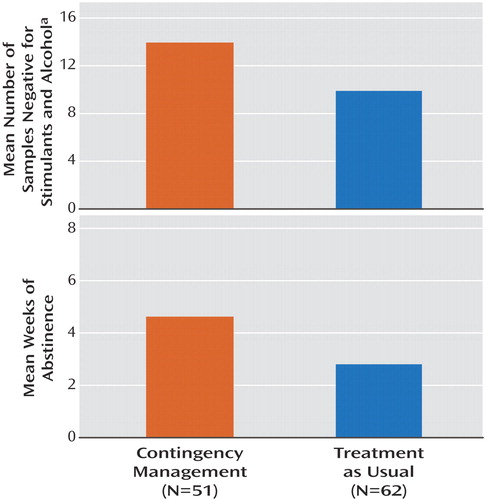
a Urine samples were tested for cocaine, amphetamine, and methamphetamine. Alcohol was sampled by breath tests.
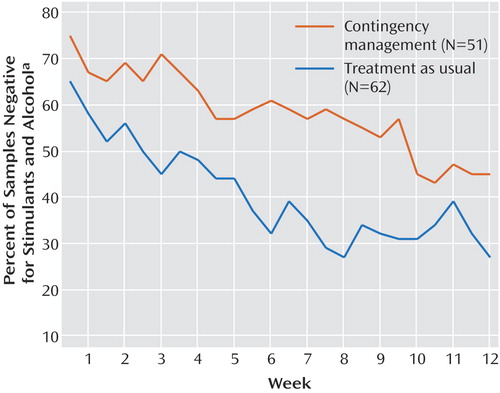
a Urine samples were tested for cocaine, amphetamine, and methamphetamine. Alcohol was sampled by breath tests.
The rates of appearance for the follow-up assessments were low at both 3 months (54.9% for contingency management and 37.1% for treatment as usual) and 6 months (60.8% for contingency management and 58.1% for treatment as usual). Analyses with generalized estimating equations indicated no significant difference between groups with respect to proportion of negative samples, either when the missing data were considered as positive samples (odds ratio=1.52, 95% CI=0.89–2.61; χ 2 =2.36, df=1, p=0.12) or when the missing data were omitted (odds ratio=1.43, 95% CI=0.67–3.06; χ 2 =0.04, df=1, p=0.36). However, regardless of group assignment, providing all negative samples during the last 4 weeks of treatment significantly increased the likelihood of providing a stimulant-negative urine sample at the 3-month follow-up time point (χ 2 =7.80, df=1, p=0.01) and slightly increased the likelihood of providing a stimulant-negative urine test at the 6-month follow-up (χ 2 =3.61, df=1, p=0.06).
Finally, the results for marijuana use during treatment indicated that there was no difference in marijuana use between groups (odds ratio=1.16, 95% CI=0.41–3.32; χ 2 =0.08, df=1, p=0.78). Overall, 5.0% of the submitted urine samples tested positive for marijuana.
Discussion
These results are noteworthy for several reasons. First, to our knowledge they represent the first controlled trial of contingency management as an adjunct to psychosocial treatment of methamphetamine use disorders. The results clearly demonstrate the benefits for methamphetamine abusers of adding prize-based contingency management to standard treatment. Participants receiving a combination of contingency management and treatment as usual were abstinent more often during the 12-week intervention and were abstinent for longer continuous periods during the intervention than participants receiving only treatment as usual. This difference was evident even though the usual treatment was the intervention considered to be the best available treatment by the community treatment providers. Further highlighting the benefit of adding contingency management, 67.3% of the participants received Matrix model psychosocial treatment (39) , which was recently reported to promote greater abstinence during treatment than a variety of other psychosocial models used for treating methamphetamine around the country (20) . Thus, it appears that adding contingency management to the best available psychosocial treatment provides statistically better treatment outcomes.
Another interesting aspect of these data is that the rates of retention during treatment were comparable for the two conditions. This finding contrasts with outcomes in many contingency management studies conducted in outpatient substance abuse clinics providing nonpharmacological treatment, where retention has been greater for the contingency management condition than for control conditions (27) . Further, significant effects on retention were also seen in the parent study from which the methamphetamine data set was drawn (35) . The reasons for the failure to find such a difference in the present study are unclear but may be related to higher than usual retention in the group receiving treatment as usual. A recent study showed that out of 157,701 outpatient admissions for methamphetamine use between 2000 and 2002 in California, only 26% completed treatment (M.L. Brecht et al., unpublished 2006 study). In the present study, the retention rate of 38.7% for the intervention phase of treatment as usual was considerably higher than the rate previously observed in this set of community programs and slightly higher than the retention rate of 35% seen in the main outcome study.
Failure to find a between-group difference in abstinence at either of the follow-up time points is difficult to interpret given the low follow-up rates observed for both groups. However, a relationship between total abstinence during the last month of the intervention and abstinence at follow-up was observed. This finding largely replicates in methamphetamine abusers what has previously been demonstrated for marijuana (41) and cocaine (42) abusers—abstinence during treatment predicts abstinence at follow-up. Because contingency management increases the likelihood of initiating and maintaining abstinence during treatment, it should also increase the likelihood of being abstinent at subsequent follow-up time points. Failure to demonstrate this effect in the present data set may be the result of insufficient power given the low overall follow-up rates.
The results from this study build on a large body of evidence suggesting that most types of substance use disorders are amenable to treatment using contingency management (25) . This study unequivocally adds methamphetamine dependence to the list of substance use disorders for which contingency management is an appropriate intervention. The results extend earlier work suggesting that methamphetamine use would be amenable to contingency management interventions (31 , 32) . Demonstrating the sensitivity of methamphetamine dependence to contingency management further strengthens the position that drug abuse can be usefully characterized as operant behavior. This characterization has obvious treatment implications and may have prevention implications.
Finally, this study was conducted at multiple locales and in community-based treatment centers, as opposed to a single facility designed for research. Thus, these results are likely externally valid. We believe that demonstrating the efficacy of contingency management in a “real world” setting demonstrates the actual clinical utility of the intervention.
1. Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S: History of the methamphetamine problem. J Psychoactive Drugs 2000; 32:137–141Google Scholar
2. Rawson RA, Gonzales R, Brethen P: Treatment of methamphetamine use disorders: an update. J Subst Abuse Treat 2002; 23:145–150Google Scholar
3. Beyrer C, Razak MH, Jittiwutikarn J, Suriyanon V, Vongchak T, Srirak N, Kawichai S, Tovanabutra S, Rungruengthanakit K, Sawanpanyalert P, Sripaipan T, Celentano DD: Methamphetamine users in northern Thailand: changing demographics and risks for HIV and STD among treatment-seeking substance abusers. Int J STD AIDS 2004; 15:697–704Google Scholar
4. Shoptaw S, Reback CJ, Freese T: Patient characteristics, HIV serostatus, and risk behaviors among gay and bisexual males seeking treatment for methamphetamine abuse and dependence in Los Angeles. J Addict Dis 2002; 21:91–105Google Scholar
5. Freese TE, Miotto K, Reback CJ: The effects and consequences of selected club drugs. J Subst Abuse Treat 2002; 23:151–156Google Scholar
6. Tominaga GT, Garcia G, Dzierba A, Wog J: Toll of methamphetamine on the trauma system. Arch Surg 2004; 139:844–847Google Scholar
7. Zweben JE, Cohen JB, Christian D, Galloway GP Salinardi M, Parent D, Iguchi M: Methamphetamine Treatment Project: Psychiatric symptoms in methamphetamine users. Am J Addict 2004; 13:181–190Google Scholar
8. Danks RR, Wibbenmeyer LA, Faucher LD, Sihler KC, Kealey GP, Chang P, Amelon M, Lewis RW: Methamphetamine-associated burn injuries: a retrospective analysis. J Burn Care Rehabil 2004; 25:425–429Google Scholar
9. Kashani J, Ruha AM: Methamphetamine toxicity secondary to intravaginal body stuffing. J Toxicol Clin Toxicol 2004; 42:987–989Google Scholar
10. Reback CJ, Larkins S, Shoptaw S: Changes in the meaning of sexual risk behaviors among gay and bisexual male methamphetamine abusers before and after drug treatment. AIDS Behav 2004; 8:87–98Google Scholar
11. Kalechstein AD, Newton TF, Green M: Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci 2003; 15:215–220Google Scholar
12. Simon SL, Dacey J, Rawson R, Ling W: The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 2004; 27:59–66Google Scholar
13. Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W: Methamphetamine abstinence syndrome: preliminary findings. Am J Addict 2004; 13:248–255Google Scholar
14. Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS: Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 2004; 161:242–248Google Scholar
15. Copeland AL, Sorensen JL: Differences between methamphetamine users and cocaine users in treatment. Drug Alcohol Depend 2001; 62:91–95Google Scholar
16. Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA: Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat 2003; 24:267–277Google Scholar
17. Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W: Methamphetamine and cocaine users: differences in characteristics and treatment retention. J Psychoactive Drugs 2000; 32:233–238Google Scholar
18. Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W: A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 2002; 21:35–44Google Scholar
19. Yen CF, Wu HY, Yen JY, Ko CH: Effects of brief cognitive-behavioral interventions on confidence to resist the urges to use heroin and methamphetamine in relapse-related situations. J Nerv Ment Dis 2004; 192:788–791Google Scholar
20. Rawson RA, Marinelle-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, Galloway G P, Herrell J, Huber A, McCann MJ, Obert J, Penell S, Reiber C, Vandersloot D, Zweben J; Methamphetamine Treatment Project Corporate Authors: A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction 2004; 99:708–717Google Scholar
21. Galloway GP, Newmeyer J, Knapp T, Stalcup SA, Smith D: Imi- pramine for the treatment of cocaine and methamphetamine dependence. J Addict Dis 1994; 13:201–216Google Scholar
22. Haney M, Kposten TR: Therapeutic vaccines for substance dependence. Expert Rev Vaccines 2004; 3:11–18Google Scholar
23. Bigelow GE, Silverman K: Theoretical and empirical foundations of contingency management treatments for drug abuse, in Motivating Behavior Change Among Illicit-Drug Abusers: Research on Contingency Management Interventions. Edited by Higgins ST, Silverman K. Washington, DC, American Psychological Association, 1999, pp 15–31Google Scholar
24. Higgins ST: The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav 1997; 57:419–427Google Scholar
25. Higgins ST, Heil SH, Plebani-Lussier J: Clinical implications of reinforcement as a determinate of substance use disorders. Annu Rev Psychol 2004; 55:431–461Google Scholar
26. Carroll ME, Lac ST, Nygaard SL: A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl) 1989; 97:23–29Google Scholar
27. Higgins ST, Bickel WK, Hughes JR: Influence of an alternative reinforcer on human cocaine self-administration. Life Sci 1994; 55:179–187Google Scholar
28. Nader MA, Woolverton WL: Effects of increasing the magnitude of an alternative reinforcer on drug choice on a discrete-trials choice procedure. Psychopharmacology (Berl) 1991; 105:169–174Google Scholar
29. Prendergast M, Podus D, Finney J, Greenwell L, Roll J: Contingency management for treatment of substance use disorders: a meta-analysis. Addiction (in press)Google Scholar
30. Roll JM, Newton T: Contingency management for the treatment of methamphetamine use disorders, in Contingency Management in the Treatment of Substance Use Disorders: A Science-Based Treatment Innovation. Edited by Higgins ST, Silverman K, Hiel SH. New York, Guilford (in press)Google Scholar
31. Roll JM, Huber A, Sodano R, Chudzynski J, Moynier E, Shoptaw S: A comparison of five reinforcement schedules for use in contingency management-based treatment of methamphetamine abuse. Psychol Rec 2006; 56:67–81Google Scholar
32. Roll JM, Shoptaw S: Contingency management: schedule effects. Psychiatr Res 2006; 144:91–93Google Scholar
33. Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C: Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend 2005; 78:125–134Google Scholar
34. Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff Dang, Roll J, Shapiro B, Rotheram-Fuller E, Ling W: Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend 2006; 85:12–18; Epub 2006, Apr 18Google Scholar
35. Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R: Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006; 63:201–208Google Scholar
36. Petry NM, Martin B: Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol 2002; 70:398–405Google Scholar
37. Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ: Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction 2004; 99:349–360Google Scholar
38. Petry NM, Martin B, Simcic F Jr: Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J Consult Clin Psychol 2005; 73:354–359Google Scholar
39. Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, Brethen PR, Ling W: An intensive outpatient approach for cocaine abuse treatment: the matrix model. J Subst Abuse Treat 1996; 12:117–127Google Scholar
40. Roll JM, Higgins ST: A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend 2000; 58:103–109Google Scholar
41. Moore BA, Budney AJ: Abstinence at intake for marijuana dependence treatment predicts response. Drug Alcohol Depend 2002; 67:249–257Google Scholar
42. Higgins ST, Badger GJ, Budney AJ: Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharmacol 2000; 8:377–386Google Scholar