Brain Dopamine D 1 Receptors in Twins Discordant for Schizophrenia
Abstract
Objective: It has been suggested that deficits in higher-order cognitive functions serve as intermediate phenotypic indicators of genetic vulnerability to schizophrenia. The dopamine hypothesis of schizophrenia postulates that insufficiency of dopamine transmission in the prefrontal cortex contributes to the cognitive deficits observed in patients with the disease, and there is robust empirical evidence for a central role of prefrontal cortex dopamine D 1 receptors in working memory functions. Method: The authors examined the genetic and nongenetic effects on D 1 receptor binding in schizophrenia by studying monozygotic and dizygotic twin pairs discordant for schizophrenia as well as healthy comparison twins using positron emission tomography (PET) and the D 1 receptor antagonist ligand [ 11 C]SCH 23390. Performance on neuropsychological tests sensitive to frontal lobe functioning was evaluated. Results: High D 1 receptor density in the medial prefrontal cortex, superior temporal gyrus, and heteromodal association cortex (angular gyrus) was associated with increasing genetic risk for schizophrenia (comparison twins < unaffected dizygotic co-twins < unaffected monozygotic co-twins). Medicated schizophrenia patients demonstrated a widespread reduction in D 1 receptor binding when compared with the unaffected co-twin, and higher doses of antipsychotics were associated with lower D 1 receptor binding in the frontotemporal regions. Conclusions: This study demonstrated an association between genetic risk for schizophrenia and alterations in cortical D 1 receptor binding, an observation that has implications for future studies of the molecular genetics of schizophrenia. In addition, the data indicate a widespread reduction of D 1 receptor binding in medicated schizophrenia patients, supporting a link between antipsychotic drug action and dopamine D 1 receptor down-regulation.
Schizophrenia is characterized by positive symptoms (such as hallucinations and delusions), negative symptoms (such as blunt affect), cognitive impairment, and affective symptoms (1) . Cognitive functions involving working memory—the ability to hold and manipulate pieces of information online for subsequent use in planning and acting—are particularly impaired in schizophrenia (2) . Working memory is critically dependent on prefrontal cortical functioning (3) . Patients suffering from schizophrenia show aberrant activation of the prefrontal cortex during working memory tasks, which suggests a role for the prefrontal cortex in the pathophysiology of cognitive deficits in schizophrenia (4) . Animal and human studies have highlighted the significance of dopamine in regulating prefrontal cortical activity related to cognitive processing (5) . Accordingly, the dopamine hypothesis of schizophrenia suggests that reduced dopamine function in the prefrontal cortex is responsible for the cognitive deficits encountered in patients with the disorder (6 , 7) .
At the receptor level, D 1 receptor function is essential for working memory function (8) . It has been suggested that D 1 receptors in the prefrontal cortex, located mostly in apical dendrites and spines of pyramidal neurons (9) , modulate the postsynaptic neuron’s response to incoming depolarizing, mostly glutamatergic, signaling (8) . This modulation seems to be dependent on the state of the target neurons, and it seems to protect task-associated working memory representations from interfering and distracting stimuli by tuning the functional state of the target neurons (10) . Too much or too little stimulation at the D 1 receptor can impair working memory, which suggests that the dependence of working memory on D 1 receptor stimulation may be described by an inverted U-shaped curve (11) .
Schizophrenia is highly heritable (12) , but the molecular basis of its heritability has remained largely unknown. The search for quantifiable mediators of this genetic vulnerability (intermediate phenotypes, or endophenotypes) could facilitate the exploration of the exact pathophysiological mechanisms behind the genetic vulnerability for schizophrenia (13) . Recent evidence suggests that neuropsychological deficits (14) , reduction of prefrontal cortical gray matter (15) , and prefrontal cortical dopaminergic dysfunction (16) are associated with an increased genetic risk of schizophrenia. We recently demonstrated an increased density of dopamine D 2 receptors in the caudate nuclei of unaffected identical co-twins of patients with schizophrenia (17) that was similar in magnitude to the overall increase in density observed in a meta-analysis of studies of patients with schizophrenia (7) . However, as yet, the role of D 1 receptors in the genetic etiology of schizophrenia has remained unaddressed.
We used positron emission tomography (PET) and the well-documented D 1 receptor tracer [ 11 C]SCH 23390 to examine the genetic and nongenetic contributions to D 1 receptor binding in schizophrenia by investigating twin pairs discordant for schizophrenia as well as healthy comparison twins. To explore the effects of genetic proximity to the patient, group comparisons were performed across three levels of genetic loading: comparison twins, dizygotic co-twins, and monozygotic co-twins. Monozygotic co-twins share 100% and dizygotic co-twins on average 50% of their segregating genes with the proband. All subjects also underwent comprehensive neuropsychological testing. Probands were included in the analyses, although they had experienced chronic antipsychotic drug treatment, which has been shown to down-regulate cortical D 1 receptors (18 , 19) .
The aim of the study was twofold: to investigate the effects of genetic liability to schizophrenia on D 1 receptor binding by comparing unaffected co-twins of patients with schizophrenia with matched healthy comparison twin pairs, and to examine disease- and treatment-specific contributions by comparing medicated patients with their own unaffected co-twins.
Method
The study protocol adhered to the principles of the Helsinki Declaration and was reviewed and approved by the institutional review boards or ethical committees of the University of California Los Angeles, the University of Turku, the University of Pennsylvania, and the National Public Health Institute of Finland. All participants provided written informed consent after receiving a complete description of the study.
Sample Ascertainment and Clinical Evaluation
Using a procedure described previously, we drew subjects from a twin cohort consisting of virtually all same-sex twin pairs born in Finland from 1940–1957 (14 , 17 , 20) . Nine discordant pairs were studied (four monozygotic and five dizygotic) along with two additional unaffected monozygotic co-twins and 13 twins from seven comparison twin pairs (four monozygotic and three dizygotic). Each co-twin was given a structured diagnostic interview.
Clinical patient data have been described previously (14) . All patients except one had received chronic antipsychotic treatment with conventional neuroleptics. Use of clozapine or flupenthixol was an exclusion criterion since these drugs have low D 2 /D 1 selectivity (21) . The drugs used by these probands were haloperidol (N=4, dose range=1–8 mg/day), levomepromazine (N=1, dose=25 mg/day), sulpiride (N=1, dose=100 mg/day), thioridazine (N=4, dose range=50–400 mg/day), and chlorprothixene (N=1, dose=50 mg/day). In chlorpromazine equivalents, daily doses ranged from 0 mg to 800 mg. The probands underwent the PET studies despite evidence that antipsychotic drug treatment may down-regulate D 1 receptors in the nonhuman primate cortex (18 , 19) . The duration of illness ranged from 10 years to 36 years.
Exclusion criteria for unaffected co-twins and comparison twins have been described previously (14 , 17) . The study groups were matched for demographic variables. The zygosity of the studied twins was confirmed by genetic markers as described previously (14) .
All subjects were tested with a comprehensive neuropsychological battery, from which a canonical liability variable was constructed to reflect performance on tests of spatial working memory, divided attention, intrusions during recall of a word list, and choice reaction times to visual targets (14) . Deficits on these tests have been shown to be heritable in twins discordant for schizophrenia, increasing in severity with increasing genetic proximity to the proband. Spatial working memory, as measured by the visual span subtest of the Wechsler Memory Scale—Revised, was selected from among these tests to be individually correlated with D 1 receptor binding. Findings on the neuropsychological data in the original twin sample have been previously published (14) .
PET Studies
For the PET studies, a whole-body three-dimensional PET scanner (GE Advance; GE, Milwaukee) was used to acquire 35 slices of 4.25 mm thickness covering the whole brain. The scanning procedure and preparation of [ 11 C]SCH 23390 have been described in detail elsewhere (22) . Briefly, an intravenous rapid bolus of 174–268 MBq of [ 11 C]SCH 23390 (mean=233.01 MBq, SD=24.4) was given to each subject (specific radioactivity, mean=63.5 MBq/nmol [SD=18.3]; amount of radiotracer, mean=1.14 μg [SD=0.31]). No statistically significant differences were observed between study groups in any of these parameters.
Quantification of [ 11 C]SCH 23390 Binding
For the calculation of regional time-activity curves, regions of interest were manually delineated using Imadeus software (version 1.15, Forima, Inc., Turku, Finland) on the putamen, caudate, angular gyrus, anterior cingulate cortex, cerebellum, dorsolateral prefrontal cortex, insular cortex, medial prefrontal cortex, orbitofrontal cortex, posterior cingulate cortex, supramarginal gyrus, subgenual anterior cingulate cortex, and superior, middle, and inferior temporal gyri, as previously described (22) . There were no statistically significant differences between groups in region-of-interest size, except in the insular cortex, which was smaller in probands compared with other subjects (–5.0 to –7.1%, p<0.05), and the putamen, which was smaller in probands compared with monozygotic co-twins (–10.3%, p<0.05). The simplified reference tissue model (23) with cerebellum as a reference region was applied to the time-activity data of each region of interest to calculate binding potential values (f 2 B max /K D ), which represent the product of receptor density (B max ), apparent affinity (1/K D ), and the free fraction of the nondisplaceable tissue compartment (f 2 ). This method has been previously validated for [ 11 C]SCH 23390 (22 , 23) .
Statistical Analyses
SAS version 8.2 (SAS Institute, Cary, N.C.) was used for data analysis. The general linear mixed model with repeated measures was used, correcting for dependency (i.e., correlation) among the healthy co-twins by treating twin pair as a random variable and adjusting the model error terms accordingly (Satterthwaite option). Differences in mean binding potential between groups were tested by modeling risk group (probands, unaffected monozygotic co-twins, unaffected dizygotic co-twins, healthy twin pairs) as a fixed-effect predictor, while covarying for sex and age. To test for laterality, hemisphere was entered into the model as a within-subject repeated-measures factor, and a group-by-hemisphere interaction was entered into the model to test for differences in laterality between the groups. We did not test for sex-by-group interaction because of the small number of one sex or the other in some groups. Whenever one of these terms contributed significantly to the prediction of [ 11 C]SCH 23390 binding potential, contrast analyses were performed comparing conditions within the term collapsing over nonsignificant terms in the model. This approach maintains the hypothesis-wise type I error rate at 0.05 because a predictor’s contribution to particular dependent measures is evaluated only if its effect is found to vary at the multivariate level. The significance of each predictor was tested while accounting for all other model terms simultaneously. Since the SAS Proc Mixed procedure did not allow for the use of multiple repeated-measures factors and a random factor within the same model, a separate model was used for each region of interest. Because of the large number of brain regions studied, we applied a Bonferroni-corrected p value of 0.0033 (0.05/15) to control for multiple comparisons for the main effect of group. The relationships between D 1 binding potential and neuropsychological test scores were examined using mixed-model regression analysis predicting the neuropsychological measurement with D 1 binding potential, while covarying for age and sex and correcting for dependency (i.e., correlation) among the healthy co-twins, as we did in the linear mixed model. The relationship between D 1 binding potential and antipsychotic drug dose in the probands was tested using partial correlation, partialing out the effects of age and sex. Intraclass correlations (ICCs) for healthy comparison monozygotic twin pairs were calculated for the binding potential in each of the total regions. We did not calculate ICCs for the two healthy comparison dizygotic twin pairs. Differences between groups in demographic characteristics were tested using analysis of variance for continuous data and the chi-square test for nominal data with a significance level of p<0.05, correcting for dependency among twin pairs.
Confirmatory Voxel-Based Statistical Analyses
An independent confirmatory voxel-based receptor mapping analysis was performed as described previously (17) . Briefly, parametric binding potential images were calculated using receptor parametric mapping based on the simplified reference tissue model (24) . Preprocessing and statistical analysis of parametric images were performed using SPM99 (25) and Matlab 6.5 for Windows (MathWorks, Natick, Mass.). Parametric binding potential images were spatially normalized using summed PET images and a ligand-specific template for [ 11 C]SCH 23390 and were smoothed using a 12-mm Gaussian filter. To explore the effects of genetic load on [ 11 C]SCH 23390 binding potential, a multiple regression analysis with group indicator variables was performed. The effects of age and sex were eliminated by treating them as nuisance covariates. The search volume of the analysis was restricted to the same regions as the region-of-interest analysis using small-volume correction. A p value of 0.05, corrected for multiple comparisons, was considered the criterion for significance in the voxel-based analyses.
Results
Table 1 summarizes the demographic and clinical characteristics of the study groups, and Table 2 lists the [ 11 C]SCH 23390 binding potential values for each group. Significant group effects were found in various brain regions: the anterior cingulate cortex, angular gyrus, caudate, dorsolateral prefrontal cortex, medial temporal gyrus, superior temporal gyrus, insular cortex, medial frontal cortex, orbitofrontal cortex, putamen, and supramarginal gyrus (all p<0.0033; statistics available on request). We then looked at individual brain regions to see if unaffected co-twins differed from healthy comparison twins. Monozygotic co-twins had higher D 1 binding potential values compared with healthy comparison twins in the medial prefrontal cortex (+35%, F=8.74, df=1, 20.9, p=0.008), superior temporal gyrus (+38%, F=7.51, df=1, 18.2, p=0.013), and angular gyrus (+22%, F=5.90, df=1, 19.1, p=0.025). The independent voxel-based receptor parametric mapping analysis with correction for multiple testing confirmed these findings and showed significant clusters in the inferior parietal and lateral temporal lobes as well as the medial prefrontal cortex ( Figure 1 ). In unaffected co-twins, canonical liability scores were negatively correlated with D 1 binding potential in the putamen (N=9, R=–0.68, p=0.042), whereas spatial working memory performance was negatively associated with D 1 binding potential in the superior temporal gyrus (N=9, R=–0.48, p=0.045), suggesting that high D 1 binding potential was associated with poorer cognitive performance in these regions.
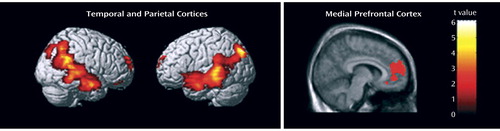
a On the left, results are rendered on an anatomical brain model, illustrating increased D 1 receptor density in the temporal and parietal cortices in monozygotic co-twins of patients with schizophrenia compared with healthy comparison twins (right hemisphere: T max =4.36 at [70, –54, 14], cluster-level corrected p<0.0005; left hemisphere: T max =6.08 at [–34, –84, 40], cluster-level corrected p<0.0005). On the right, results are rendered on an MRI template, illustrating increased D 1 receptor density in the medial prefrontal cortex in monozygotic co-twins of patients with schizophrenia compared with healthy comparison twins (T max =4.31 at [22, 42, –12], cluster-level corrected p<0.0005). The color intensity represents t values at the voxel level.
Patients with schizophrenia showed a significant widespread reduction in D 1 binding potential compared with their unaffected co-twins in almost all studied brain regions (see Table 2 ; statistics available on request). No associations were observed between antipsychotic drug dose (in chlorpromazine equivalents) and D 1 binding potential in individual regions of interest, but a significant negative correlation was observed between drug dose and average D 1 binding potential of the frontal and temporal regions (partial R=–0.86, p=0.012, controlling for the effects of age and sex), indicating that high drug doses were associated with low D 1 binding potential in these regions.
A significant age effect was noted in the whole sample, with D 1 binding potential tending to decline with age in the angular gyrus (F=5.99, df=1, 13.1, p=0.029), caudate (F=7.49, df=1, 13.1, p=0.017), insular cortex (F=4.66, df=1, 14.7, p=0.048), putamen (F=5.20, df=1, 14.6, p=0.038), and supramarginal gyrus (F=6.79, df=1, 14.1, p=0.021). We observed a significant sex difference in the whole sample, with women having higher binding potential values than men in the medial prefrontal cortex (F=7.43, df=1, 12.2, p=0.018) and anterior cingulate cortex (F=5.75, df=1, 10.4, p=0.037). A significant hemisphere effect was observed in the inferior temporal gyrus (F=14.31, df=1, 40.3, p<0.001) and the middle temporal gyrus (F=7.28, df=1, 40.7, p=0.010). Left hemisphere values were higher than right hemisphere values in these regions. No significant group-by-hemisphere interactions were seen.
Moderate to high ICCs for binding potential in the healthy monozygotic twins were noted for both striatal and cortical regions, namely, the inferior temporal gyrus (0.84), putamen (0.80), middle temporal gyrus (0.80), caudate (0.71), supramarginal gyrus (0.66), and superior temporal gyrus (0.63). For other regions studied, ICCs were below 0.50.
Discussion
In this study, we used PET with [ 11 C]SCH 23390 to investigate brain D 1 receptors in monozygotic and dizygotic twin pairs discordant for schizophrenia. The study produced two major findings. First, unaffected monozygotic co-twins of patients with schizophrenia showed increased D 1 receptor binding in the medial prefrontal cortex, superior temporal gyrus, and angular gyrus compared with healthy comparison twins. Monozygotic co-twins displayed the highest values compared with the comparison twins, while dizygotic co-twins had values intermediate between monozygotic co-twins and comparison twins, which suggests an association with increasing genetic loading for schizophrenia. To our knowledge, this is the first report of D 1 receptor alterations related to genetic risk for schizophrenia. The other major finding is that patients with schizophrenia who had experienced chronic antipsychotic treatment (probands in the discordant pairs) had a widespread reduction of D 1 receptor binding in the brain, which was associated with antipsychotic medication dose, a finding consistent with previous preclinical work (18 , 19) .
Binding potential represents a combined estimate of receptor density and apparent affinity, and the latter is affected by both the propensity of the radioligand to bind to the receptor and competition with endogenous dopamine for binding to the receptor. Acute fluctuations of endogenous dopamine do not change the in vivo binding of [ 11 C]SCH 23390 in the striatum or cortex in humans (26) or monkeys (27 , 28) , and we interpret the results of our study as reflecting altered D 1 receptor density. It seems unlikely that the up-regulation of D 1 receptors in unaffected monozygotic co-twins observed in this study would be mediated by primary variations of the gene coding for the D 1 receptor. It is currently unknown whether some polymorphism in the D 1 receptor gene might affect receptor binding in vivo or even carry a risk for schizophrenia. SCH 23390 shows non-negligible in vitro affinity for serotonin 5-HT 2 receptors, and there is preliminary evidence from a recent nonhuman primate study (29) that a small proportion of the cortical specific in vivo binding of both [ 11 C]SCH 23390 (14%) and [ 11 C]NNC 112 (28%) may originate from 5-HT 2A receptors. Human data are currently lacking for the in vivo pharmacological selectivity of these radioligands, but we cannot exclude the possibility that our findings may be due, to some extent, to hypothesized group differences in 5-HT 2A receptor density.
A novel finding of our study is the association of increased D 1 receptor density with increasing genetic risk for schizophrenia in the medial prefrontal cortex, superior temporal gyrus, and angular gyrus. This finding was confirmed with an independent voxel-based receptor mapping analysis. The interpretation of our finding of D 1 receptor binding elevation as an intermediate phenotype for schizophrenia is hindered by the fact that previous PET studies of D 1 receptor binding have produced contradictory findings, with studies showing decreased (30) , unaltered (31) , and increased (32) binding in unmedicated patients compared with comparison subjects. These studies used different radioligands for D 1 receptors, namely [ 11 C]SCH 23390 (30 , 31) and [ 11 C]NNC 112 (32) . The radioligand used may be important, since it has been shown that the cortical binding of [ 3 H]SCH 23390 and [ 11 C]NNC 112 behave differently following chronic dopamine depletion in rodents: [ 11 C]NNC 112 binding increases, whereas [ 3 H]SCH 23390 binding remains unchanged, a difference hypothesized to relate to differential affinity for internalized versus externalized receptors (33) . Clearly, more studies are needed to validate these assumptions and to shed light on the discrepant findings in subjects with unmedicated schizophrenia.
Our second important finding was that patients with schizophrenia who had chronic antipsychotic treatment showed a profound and widespread reduction in D 1 receptor binding throughout the brain, including regions such as the striatum as well as frontal, temporal, and parietal cortices. Studies of nonhuman primates have shown that chronic administration of D 2 receptor antagonist medications down-regulates D 1 receptor binding in the frontal and temporal association areas but not in motor, visual, or somatosensory cortex or striatum (18) , as well as levels of D 1 and D 5 receptor mRNAs in the prefrontal cortex but not in the striatum (19) . Consistent with this preclinical data, we found that high doses of antipsychotic medication were associated with low frontal and temporal D 1 receptor binding potential in the probands. Some of the antipsychotic drugs used by the patients in our study may also directly occupy D 1 receptors and thereby cause a reduction in [ 11 C]SCH 23390 binding potential. Although the use of clozapine and flupenthixol was excluded, other drugs (such as thioridazine, which has only around a 10-fold D 2 /D 1 selectivity and was used by four patients) might contribute to a reduction in binding potential as well. However, we regard direct occupancy unlikely because a previous clinical PET study with medicated patients indicated that a daily dose of 300 mg of thioridazine did not induce detectable D 1 occupancy as assessed with [ 11 C]SCH 23390 (21) , and only one patient in our study had a daily dose over 300 mg. Because of this potential confounder, our results on our patient group are not directly comparable with previous D 1 receptor PET studies of unmedicated schizophrenia (30 – 32) and are most likely reflective of D 1 receptor down-regulation, at least in the frontal and temporal cortices, induced by antipsychotic medication.
Dopaminergic hypofunction in the prefrontal cortex has long been regarded as one of the key neural substrates for deficit aspects of schizophrenia (6) , and a postmortem study has provided direct evidence for abnormal dopaminergic innervation of the medial prefrontal cortex in schizophrenia (34) . The increased D 1 receptor density observed in our study might be viewed as a compensatory reaction to decreased cortical dopamine levels, which has been suggested to confer genetic liability for schizophrenia via polymorphisms in the gene coding for catechol O -methyltransferase (16) . Another hypothesis is that increased D 1 receptor binding is a compensatory phenomenon secondary to reduced neuropil (35) —dendritic processes where D 1 receptors are preferentially located (9) —which may be responsible for genetic vulnerability-related gray matter loss (15) . The functional significance of D 1 receptors in the superior temporal gyrus and inferior parietal areas is currently unknown. Neurons in these regions express D 1 receptors (36) , and they participate in working memory functions in concert with neurons of prefrontal cortex (37 , 38) , suggesting that they may be relevant for the pathophysiological process of schizophrenia. Consistent with the notion of marked inherited contributions, we observed high ICCs for striatal and parietotemporal D 1 receptor densities in the healthy monozygotic twins.
Sex differences in D 1 receptor density in the anterior cingulate cortex and medial prefrontal cortex are novel findings: women had higher receptor densities than men. Women have also been shown to have higher D 2 receptor binding in the anterior cingulate cortex (39) . The significance of the sex difference in D 1 receptor density remains to be established in future studies. The observed significant lateralization in D 1 binding potential in the lateral temporal cortex is in line with the well-documented structural and functional asymmetry in these regions that has been attributed to the specialization of the left hemisphere to language functions (40) .
One limitation of this study is the relatively small sample size. Nevertheless, the effect sizes for the group contrasts of interest were of sufficient magnitude to detect significant differences in cortical regions of theoretical importance in the pathophysiology of schizophrenia. It is important to emphasize, however, that with larger samples, similar effects may be detected in other brain regions.
1. Schultz SK, Andreasen NC: Schizophrenia. Lancet 1999; 353:1425–1430Google Scholar
2. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Google Scholar
3. Goldman-Rakic PS: Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 1996; 93:13473–13480Google Scholar
4. Weinberger DR, Berman KF, Zec RF: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood-flow evidence. Arch Gen Psychiatry 1986; 43:114–124Google Scholar
5. Nieoullon A: Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002; 67:53–83Google Scholar
6. Davis KL, Kahn RS, Ko G, Davidson M: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Google Scholar
7. Laruelle M: Dopamine transmission in the schizophrenic brain, in Schizophrenia: Part 2, Biological Aspects. Edited by Hirsch SR, Weinberger DR. Oxford, Blackwell Scientific Publishing, 2003, pp 365–387Google Scholar
8. Goldman-Rakic PS, Muly EC, Williams GV: D 1 receptors in prefrontal cells and circuits. Brain Res Rev 2000; 31:295–301 Google Scholar
9. Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS: D 1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 1994; 91:5720–5724 Google Scholar
10. Abi-Dargham A, Moore H: Prefrontal DA transmission at D 1 receptors and the pathology of schizophrenia. Neuroscientist 2003; 9:404–416 Google Scholar
11. Lidow MS, Williams GV, Goldman-Rakic PS: The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 1998; 19:136–140Google Scholar
12. Sullivan PF, Kendler KS, Neale MC: Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003; 60:1187–1192Google Scholar
13. Gottesman II, Gould TD: The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003; 160:636–645Google Scholar
14. Cannon TD, Huttunen MO, Lönnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M: The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet 2000; 67:369–382Google Scholar
15. Cannon TD, Thompson PM, van Erp TGM, Toga AW, Poutanen VP, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J: Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA 2002; 99:3228–3233Google Scholar
16. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Google Scholar
17. Hirvonen J, van Erp TGM, Huttunen J, Aalto S, Nagren K, Huttunen M, Lonnqvist J, Kaprio J, Hietala J, Cannon TD: Increased caudate dopamine D 2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 2005; 62:371–378 Google Scholar
18. Lidow MS, Goldman-Rakic PS: A common action of clozapine, haloperidol, and remoxipride on D 1 and D 2 dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci USA 1994; 91:4353–4356 Google Scholar
19. Lidow MS, Elsworth JD, Goldman-Rakic PS: Down-regulation of the D 1 and D 5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther 1997; 281:597–603 Google Scholar
20. Kaprio J, Koskenvuo M: Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res 2002; 5:358–365Google Scholar
21. Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G: Positron emission tomographic analysis of central D 1 and D 2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry 1992; 49:538–544 Google Scholar
22. Hirvonen J, Någren K, Kajander J, Hietala J: Measurement of cortical dopamine D 1 receptor binding with [ 11 C]SCH 23390: a test-retest analysis. J Cereb Blood Flow Metab 2001; 21:1146–1150 Google Scholar
23. Lammertsma AA, Hume SP: Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4:153–158Google Scholar
24. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ: Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6:279–287Google Scholar
25. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith C, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Google Scholar
26. Verhoeff NPLG, Hussey D, Lee M, Tauscher J, Papatheodorou G, Wilson AA, Houle S, Kapur S: Dopamine depletion results in increased neostriatal D 2 , but not D 1 , receptor binding in humans. Mol Psychiatry 2002; 7:322–328 Google Scholar
27. Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van HR, Mann JJ, Foged C, Halldin C, Laruelle M: PET studies of binding competition between endogenous dopamine and the D 1 radiotracer [ 11 C]NNC 756. Synapse 1999; 32:93–109 Google Scholar
28. Chou YH, Karlsson P, Halldin C, Olsson H, Farde L: A PET study of D 1 -like dopamine receptor ligand binding during altered endogenous dopamine levels in the primate brain. Psychopharmacology (Berl) 1999; 146:220–227 Google Scholar
29. Ekelund J, Narendran R, Guillin O, Castrillon J, Hwang DR, Hwang Y, Slifstein M, Abi-Dargham A, Laruelle M: Pharmacological selectivity of the in vivo binding of [ 11 C]NNC 112 and [ 11 C]SCH 23390 in the cortex: a PET study in baboons. J Nucl Med 2005;46:13P–13P Google Scholar
30. Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M: Decreased prefrontal dopamine D 1 receptors in schizophrenia revealed by PET. Nature 1997; 385:634–636 Google Scholar
31. Karlsson P, Farde L, Halldin C, Sedvall G: PET study of D 1 dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 2002; 159:761–767 Google Scholar
32. Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van HR, Gorman JM, Laruelle M: Prefrontal dopamine D 1 receptors and working memory in schizophrenia. J Neurosci 2002; 22:3708–3719 Google Scholar
33. Guo NN, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A: Dopamine depletion and in vivo binding of PET D 1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology 2003; 28:1703–1711 Google Scholar
34. Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA: Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 1999; 156:1580–1589Google Scholar
35. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Google Scholar
36. De Keyser J, Claeys A, Debacker JP, Ebinger G, Roels F, Vauquelin G: Autoradiographic localization of D 1 and D 2 dopamine receptors in the human brain. Neurosci Lett 1988; 91:142–147 Google Scholar
37. Cabeza R, Nyberg L: Imaging cognition, II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000; 12:1–47Google Scholar
38. Chafee MV, Goldman-Rakic PS: Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 1998; 79:2919–2940Google Scholar
39. Kaasinen V, Någren K, Hietala J, Farde L, Rinne JO: Sex differences in extrastriatal dopamine D 2 -like receptors in the human brain. Am J Psychiatry 2001; 158:308–311 Google Scholar
40. Toga AW, Thompson PM: Mapping brain asymmetry. Nat Rev Neurosci 2003; 4:37–48Google Scholar





