Catechol O-Methyltransferase Polymorphism and Eye Tracking in Schizophrenia: A Preliminary Report
Abstract
OBJECTIVE: This study examined associations between functional polymorphism (Val108/158 Met) in the catechol O-methyltransferase (COMT) gene and eye tracking measures in schizophrenia. METHOD: Predictive pursuit and closed-loop gains of 62 patients with schizophrenia and 53 healthy comparison subjects with Val-Val, Val-Met, and Met-Met genotypes were compared. RESULTS: There was a significant diagnosis-by-genotype interaction: patients with the Met-Met genotype showed poor predictive pursuit. The Met-Met genotype in healthy subjects was associated with significantly higher predictive pursuit gain values than the Val-Val genotype in healthy subjects. The COMT genotype explained about 10% of the variance in each group’s predictive pursuit performance. DISCUSSION: These preliminary data suggest that the COMT gene is associated with predictive eye tracking performance in healthy subjects. Predictive pursuit abnormality in schizophrenia is not attributable to the Val allele. These findings suggest a complex interaction with other etiological factors (e.g., another gene), and/or with prefrontal cortical dopaminergic activity.
A functional polymorphism (Val108/158 Met) in the catechol O-methyltransferase (COMT) gene is implicated in the etiology of schizophrenia (see Wonodi et al. [1] for review). The COMT gene affects the level of prefrontal dopamine, which modulates the way information is processed by prefrontal neurons during the performance of a number of important cognitive operations (2). The purpose of the current study was to examine the relationship between COMT polymorphism and smooth pursuit eye movements in subjects with schizophrenia and healthy subjects.
Abnormalities of smooth pursuit eye movements are thought to mark liability for schizophrenia. Smooth pursuit eye movement is a highly complex behavior served by a widely distributed neuronal network. Investigators have divided smooth pursuit eye movements broadly into two components: 1) responses based on retinal motion processing and 2) predictive pursuit responses based on internal representation of the target motion (see Fukushima [3] for review). Together, these two aspects of smooth pursuit eye movements serve to capture and maintain the image of a target on the fovea (4). Each component is served by distinct neural circuits that are only partly overlapping. Frontal cortical neurons play an important role in the modulation of predictive smooth pursuit eye movements (3). The construct of predictive pursuit based on internal representation of the target motion is similar to the construct of working memory. Studies in monkeys (5) suggest a critical role of dopamine in visuospatial working memory.
Method
A total of 62 subjects with schizophrenia (mean age=41 years, SD=10) and 53 healthy comparison subjects (mean age=41, SD=15) gave written informed consent and underwent extensive structured clinical evaluations (details given elsewhere [4]). Interrater reliabilities above 0.85 (kappa) were maintained. Healthy comparison subjects did not have any axis I diagnosis, schizophrenia spectrum personality disorders, or family history of psychosis.
An infrared method (Eye Trac Model 210, Applied Science Laboratories, Waltham, Mass.) was used to monitor eye position (details given elsewhere [4]). Subjects were asked to follow a moving target with their eyes (constant target speed=18.7°/second; amplitude=±12°) while their heads were stabilized with chin and head rests. Twelve trials were administered, each consisting of 1.5 to 2.5 cycles of back-and-forth target motion. Each trial included a mask that briefly (for 500 msec) concealed the target. Subjects were made aware of the fact that the target became invisible briefly and were instructed to continue to follow the target. The mask occurred either during a ramp or at the change in ramp direction. Pursuit gain measures from the mask period from these two types of trials were averaged to obtain a measure of predictive pursuit. Closed-loop gain, a measure of overall pursuit performance, was obtained (details given elsewhere [4]).
Genotyping methods are described elsewhere (1). Briefly, the COMT Val108/158 Met genotype was determined as a restriction fragment length polymorphism after polymerase chain reaction amplification and digested with NlaIII according to the method of Egan et al. (2). Genotyping was repeated in 16 subjects, and in each case the repeat testing showed the same findings.
In our statistical analysis, first we confirmed that the observed distribution of genotypes was not different from the distribution expected under the Hardy-Weinberg equilibrium by chi-square test (χ2=3.0, df=1, p>0.05). The allele and genotype frequencies of subjects with schizophrenia and comparison subjects were then compared by using a chi-square test. Analysis of covariance with age as a covariate and genotype and subject diagnosis as two between-subject factors was carried out to examine the effects of COMT genotype on smooth pursuit measures in the two subject groups.
Results
The 62 subjects with schizophrenia had genotypes with nonsignificantly more Val alleles than the 53 healthy comparison subjects (χ2=3.12, df=1, p=0.08, Mantel-Haenszel chi-square for difference in number of alleles). Val-Val, Val-Met, and Met-Met genotypes were present in 29 (47%), 22 (35%), and 11 (18%) subjects with schizophrenia and in 16 (30%), 23 (43%), and 14 (26%) healthy comparison subjects, respectively.
Analysis of predictive pursuit gain showed a significant interaction between genotype and diagnosis (Figure 1 and Table 1). Planned contrasts suggested that healthy subjects with the Met-Met genotype performed significantly better than healthy subjects with the Val-Val genotype (t=2.3, df=114, p<0.03), whereas the performance of Met-Met subjects with schizophrenia was nonsignificantly worse than that of Val-Val subjects with schizophrenia (t=1.9, df=11, p<0.07). Within each diagnostic group, the COMT genotype explained about 10% of the variance in predictive pursuit performance. Differences between healthy subjects and those with schizophrenia emerged only in the presence of the Met-Met genotype (Figure 1 and Table 1). Analysis of closed-loop gain showed that there were no significant effects of genotype or genotype-by-diagnosis interaction (F<1.12, df=2, 114, p>0.3) but that there was a significant main effect of diagnosis (Table 1).
Discussion
Schizophrenia is now recognized as a multifactorial disorder encompassing several intermediate phenotypes, each likely affected by one or more genes. Defining and applying these endophenotypes in genetic studies is important for identifying schizophrenia-related genes. For this it is critical that physiologically specific or “elemental” deficits are identified that are associated with schizophrenia liability. In this context, note that the traditional measures of smooth pursuit, such as closed-loop gain, are generally global and evaluate the final output of a highly complex and widely distributed system. Recent efforts have identified several distinct aspects of the smooth pursuit eye movement response that are affected in schizophrenia. Data from the current study suggest that the predictive component is influenced by COMT genotype in healthy subjects, explaining about 10% of the variance. Consistent with the effects of the Met-Met genotype on other prefrontal cortical functions, healthy subjects with this genotype performed better than those with the Val-Val genotype. Such an effect of COMT genotype was not observed in patients with schizophrenia. If anything there was an opposite, nonsignificant effect of the Met-Met genotype in subjects with schizophrenia: patients with schizophrenia who had the Met-Met genotype showed significantly poorer predictive pursuit than healthy subjects who had the Met-Met genotype.
Based on these findings, we hypothesize that predictive pursuit deficits in schizophrenia are attributable to the joint effects of the Met-Met genotype of COMT and other etiological factors. For instance, these factors may increase dopamine activity, making the Met homozygous genotype detrimental to performance. This is consistent with the inverted-U model of prefrontal cortical function and dopamine described by Mattay et al. (6), who suggested that, if dopamine signaling is high, Met homozygotes would experience deterioration in prefrontal function and that, in the absence of excessive dopamine signaling, Met homozygotes would experience improved functioning. A recent report (7) noted an aberrant stress-related response associated with Met-Met genotype in healthy subjects, suggesting another possible interaction of COMT genotype and predictive pursuit abnormality involving stress-related etiological factors in schizophrenia.
 |
Presented at the 11th World Congress on Psychiatric Genetics, Quebec City, Que., Canada, Oct. 4–8, 2003. Received Jan. 16, 2004; revision received April 8, 2004; accepted May 14, 2004. From the Maryland Psychiatric Research Center and the General Clinical Research Center Genomics Core Laboratory in the Department of Epidemiology and Preventive Medicine, University of Maryland School of Medicine, Baltimore. Address reprint requests to Dr. Thaker, MPRC, PO Box 21247, Baltimore, MD 21228; [email protected] (e-mail). Supported by NIMH grants MH-49826, MH-67014, and MH-68580.
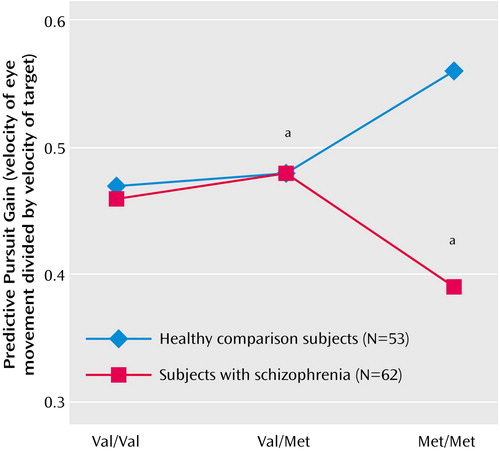
Figure 1. Eye Tracking Performance and Functional Polymorphism of Patients With Schizophrenia and Healthy Subjectsa
aHealthy subjects with the Met-Met genotype showed better performance than healthy subjects with the Val-Val genotype (t=2.3, df=114, p<0.03). The performance of patients with schizophrenia who had the Met-Met genotype was significantly different from that of healthy subjects with the Met-Met genotype (F=22.1, df=2, 24, p<0001).
1. Wonodi I, Stine C, Mitchell BD, Buchanan R, Thaker G: Association between Val108/158Met polymorphisms of the COMT gene and schizophrenia. Am J Med Genet 2003; 120B:47–50Google Scholar
2. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Crossref, Medline, Google Scholar
3. Fukushima T, Hasegawa I, Miyashita Y: Prefrontal neuronal activity encodes spatial target representations sequentially updated after nonspatial target-shift cues. J Neurophysiol 2004; 91:1367–1380Crossref, Medline, Google Scholar
4. Thaker GK, Avila MT, Hong E, Medoff DR, Ross DE, Adami HM: A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology 2003; 40:277–284Crossref, Medline, Google Scholar
5. Sawaguchi T, Goldman-Rakic PS: D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 1991; 251:947–950Crossref, Medline, Google Scholar
6. Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR: Catechol O–methyltransferase val158–met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 2003; 100:6186–6191Crossref, Medline, Google Scholar
7. Oswald LM, McCaul M, Choi L, Yang X, Wand GS: Catechol-O–methyltransferase polymorphism alters hypothalamic-pituitary-adrenal axis responses to naloxone: a preliminary report. Biol Psychiatry 2004; 55:102–105Crossref, Medline, Google Scholar



