High Midbrain [18F]DOPA Accumulation in Children With Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Attention deficit hyperactivity disorder (ADHD) is a highly prevalent childhood psychiatric disorder characterized by impaired attention, excessive motor activity, and impulsivity. Despite extensive investigation of the neuropathophysiology of ADHD by a wide array of methodologies, the neurobiochemical substrate of this disorder is still unknown. Converging evidence, however, suggests a primary role of the dopaminergic system. METHOD: This study examined the integrity of presynaptic dopaminergic function in children with ADHD through use of positron emission tomography and the tracer [18F]fluorodopa ([18F]DOPA). Accumulation of [18F]DOPA in synaptic terminals, a measure of dopa decarboxylase activity, was quantified in regions rich in dopaminergic innervation, including caudate nucleus, putamen, frontal cortex, and midbrain (i.e., substantia nigra and ventral tegmentum). RESULTS: Accumulation of [18F]DOPA in the right midbrain was higher by 48% in 10 children with ADHD than in 10 normal children. Despite its magnitude, this difference would not have reached statistical significance if corrected by the Bonferroni test for multiple comparisons. However, [18F]DOPA in the right midbrain was correlated with symptom severity. No other dopamine-rich regions significantly differed between groups. CONCLUSIONS: These findings are suggestive of dopaminergic dysfunction at the level of the dopaminergic nuclei in children with ADHD. Abnormality in dopa decarboxylase activity may be primary or secondary to deficits in other functional units of the dopamine pathway (e.g., receptor, uptake transporter, vesicular transporter, degradation enzymes). Efforts toward defining the origin of this abnormality should help delineate mechanisms of midbrain control of attention and motor behavior important for the understanding of the causes and treatment of ADHD.
Attention deficit hyperactivity disorder (ADHD) is a highly prevalent disabling psychiatric disorder of childhood characterized by hyperactivity, impulsivity and impairments in attention (DSM-IV). These symptoms, as well as the therapeutic efficacy of stimulants, suggest dopaminergic dysfunction. Yet, the existence and nature of the cerebral dopaminergic abnormality remain in doubt. For example, investigators have been unable to agree on whether there is an association between levels of the dopamine metabolite homovanillic acid in body fluids and symptom severity, and, if present, on the direction of that association (1–7). The inability to detect dopaminergic abnormalities through plasma or CSF measures would be expected were the dopaminergic abnormality in ADHD regionally localized. Such localized changes can be explored by means of imaging techniques. Through the use of magnetic resonance imaging, abnormally small caudate nuclei on either the left or right side have been found (8–10). Although functional neuroimaging (positron emission tomography [PET]) studies with [18F]fluorodeoxyglucose (FDG) have demonstrated abnormally low cerebral glucose metabolism in adults (11), but not in adolescents (12, 13), they have been unable to detect consistently selective abnormalities in the caudate nuclei or other structures dependent on dopamine input. Whereas FDG PET is a useful measure of integrated regional functional brain activity, it may be relatively insensitive to detecting group differences in specific biochemical systems. For example, FDG PET studies of patients at rest or performing an attention task were relatively insensitive to the effects of stimulants, drugs that enhance dopamine release (14–17).
The limited success of biochemical and imaging approaches, coupled with recent progress in molecular biology, has led some investigators to study the role of genetic variation in ADHD with respect to dopamine candidate genes. Whereas studies of the association of ADHD with a unique polymorphism of the dopamine transporter (18) and a specific D4 receptor allele are consistent with dopamine pathway involvement in ADHD (19), the findings can account only for a small amount of the overall genetic risk and cannot as yet be directly related to a specific neural mechanism.
We elected to refine the functional neuroimaging approach by studying the dopaminergic pathways of children with ADHD by using PET with the dopaminergic tracer [18fluorine]fluorodopa ([18F]DOPA) (20). The tracer [18F]DOPA is an analogue of DOPA. It is transported into presynaptic neurons, where it is converted to [18F]fluorodopamine by the enzyme dopa decarboxylase and then stored in catecholamine storage vesicles. Hence, data obtained through use of [18F]DOPA and PET reflect dopa decarboxylase activity and dopamine storage processes.
METHOD
Subjects
This study was approved by the Human Subjects Protection Committee of the National Institute of Mental Health (NIMH), and written informed consent and assent were obtained from all parents and subjects after complete description of the study.
Female and male adolescents were recruited through the office of the National Institutes of Health (NIH) Normal Volunteers, through advertisement in local newspapers, and with the help of an ADHD advocacy and support organization. A child psychiatrist and a child psychologist conducted psychiatric evaluations. Adolescents and their parents completed a structured diagnostic psychiatric interview, the Diagnostic Interview for Children and Adolescents-R-A and Diagnostic Interview for Children and Adolescents-R-P, respectively (21). None of the first-degree relatives of the comparison and ADHD children enrolled in the study presented with axis I psychiatric disorders, except for a history of ADHD. All comparison children had a sibling with a diagnosis of ADHD, except for one child who had a sibling with a diagnosis of autism. The inclusion of comparison children who were siblings of children with ADHD (who themselves were not part of the ADHD group) was mandated by the NIH Institutional Review Board. Such a mandate reflected the difficult ethical concerns that are raised by this type of research (22). After having convened a special committee composed of an outstanding panel of experts in ethical and radiation exposure issues, the NIMH Institutional Review Board decided to allow a limited number of comparison children (N=10), between the ages of 12 and 17 years, to participate in the study. In addition, because siblings of children with the disorders under investigation have some prospect of benefit (better understanding of the disorder that affects their family life might lead to improved treatment intervention), it was decided that unaffected siblings of children with these disorders should be used as the comparison group. Part of the decision of the NIMH Institutional Review Board was based on the fact that there is no evidence of untoward health effects at low-level radiation (for a review, see reference 23).
All children received a physical examination and routine blood tests. Diagnosis of ADHD was based on DSM-III-R criteria. Exclusion criteria included any other axis I or axis II psychiatric disorders (except for learning disorders) and any medical problems, including neurological deficits or history of head trauma with loss of consciousness. Reading disorder was evaluated by the Woodcock Achievement Battery (24) and the Wide-Range Achievement Test (25). IQ was estimated by the vocabulary and block design subtests of the Wechsler Intelligence Scale for Children—Revised (26). Behavioral ratings included the Child Behavior Checklist (27), the 48-item Conners’ Parent Rating Scale for parents, the 39-item Conners’ Teacher Questionnaire (28), and the 10-item Attention Deficit Disorder-Hyperactivity: Comprehensive Teacher’s Rating Scale (29). Subjects were medication free for at least 2 weeks before PET scanning. Stimulants were the only medication used in this group. None of the adolescents reported a history of tobacco use. Psychiatric family history in both ADHD and comparison groups was obtained from one of the parents by structured interviews (30). All patients and comparison subjects had an MRI scan that was read as clinically normal by a neuroradiologist.
Procedure
The tracer [18F]DOPA was administered in a 1-minute intravenous infusion at a dose of 1.0 mCi. The signal to noise ratio was significantly improved by the following strategy. To increase the availability of [18F]DOPA in plasma to the brain, the peripheral decarboxylation of [18F]DOPA was blocked by the administration of 100 mg of carbidopa (l-aromatic amino acid decarboxylase inhibitor) 1 hour before injection of the tracer (31). In addition, to minimize the accumulation of nonspecific cerebral radioactivity, which originates mostly from the peripheral metabolite 3-O-methyl-6-[18F]DOPA, the blood brain barrier transport system for large neutral amino acids was saturated by the intravenous infusion of a solution of large neutral unlabeled amino acids (travasol 5%) starting 60 minutes after injection of the tracer and maintained at a rate of 40 mg/kg per hour throughout the scanning period (32). During the first 80 minutes of tracer uptake, subjects were awake and watched a videotape of their choice. A custom-fitted plastic head holder was used to immobilize the head during the following 30 minutes of scanning time (90 to 120 minutes after injection of the tracer).
A seven-slice brain PET from Scanditronix (Uppsala, Sweden) was used. The in-plane and axial resolutions were 5.2 mm and 11.8 mm, respectively. Four transverse levels of seven slices each were collected, i.e., a total of 28 slices, at 3.5-mm intervals. Transmission scans were employed to correct for attenuation at all four transverse levels. Thirty-two circular regions of interest of 37 pixels each (pixel size=4 mm2) were placed onto PET images so as to match a standard template based on the atlas of Matsui and Hirano (33). The placement of regions of interest was performed by a single rater who was unaware of the identity and diagnosis of the subjects. The regions of interest were placed according to a predetermined algorithm. The slice with the highest striatal [18F]DOPA signal was the slice of reference (at about the level of the canthomeatal line). Striatal regions of interest (caudate nucleus and putamen) were placed first, on the slice of reference and then on both slices directly above and below, respectively. The occipital regions of interest were placed on the same slices as those containing the striatal regions of interest. The frontal regions of interest were placed on the fourth and fifth slices above the slice of reference (about 15 to 20 mm above the striatal plane, at the level of Brodmann’s area 10). Midbrain regions of interest were placed two and three slices lower than the slice of reference (about 7 mm and 10 mm below the striatum). A high level of interrater reliability is achieved with this type of procedure (intraclass correlation coefficients >0.86) (34).
The ratio of specific to nonspecific radioactivity was chosen as the method of analysis. This method has been shown to provide accurate and reliable data and to be sensitive to changes in dopaminergic function (35, 36). Presynaptic accumulation of [18F]DOPA was measured in anatomical regions of interest drawn on five brain areas rich in dopamine (four lateralized pairs: head of caudate nucleus, putamen, midbrain, and lateral prefrontal cortex; one medial: medial prefrontal cortex) and one region poor in dopamine (occipital cortex) (Figure 1). The midbrain region included the mesencephalic dopamine-rich cell bodies of the substantia nigra and of the ventral tegmentum. To minimize the effects of methodological factors that can cause interindividual variability (e.g., amount of tracer injected, amount of tracer crossing the blood brain barrier, scanner detection efficacy), the [18F] activity from the occipital cortex served as the measure of nonspecific activity and was used to normalize [18F] activity of the dopamine-rich areas. These normalized values or ratios, obtained from the formula (region of interest [18F]–occipital [18F])/(occipital [18F]), were the variables used for analysis and are referred to as [18F]DOPA ratio.
Statistical Analysis
The Mann-Whitney U test and Student’s t tests were used to compare the clinical variables between groups. The [18F]DOPA ratios of specific to nonspecific activity (region of interest–occipital)/(occipital) were compared between the two study groups by Student’s t tests. Statistical significance was set at p<0.05. All tests were two-tailed. Because of the preliminary nature of this study, the results are presented with and without correction for multiple comparisons. The Bonferroni test was used for this correction. We corrected for four tests, since four independent regions (frontal, caudate nucleus, putamen, and midbrain) were tested. As the risk of committing a type I error (false positive) increases, the risk of committing a type II error (false negative) decreases. In an initial study, committing a type II error is more deleterious to the scientific purpose because it leads to premature closure of potentially important avenues for research. Replication studies as well as corroborating evidence are essential. The association of clinical measures with regional [18F]DOPA ratios was assessed by means of Pearson product-moment correlation coefficients. Only those measures that significantly differed between groups were entered in the analysis (eight clinical variables).
RESULTS
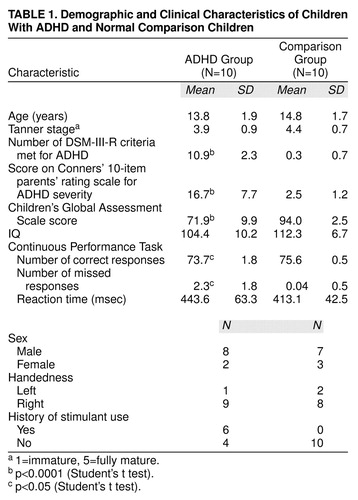
Ten children with ADHD and 10 healthy comparison children matched on age, gender, and sexual maturation completed the study. Table 1 presents the characteristics of the study group.
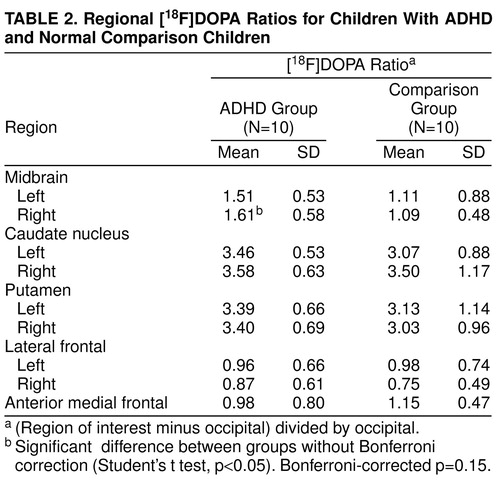
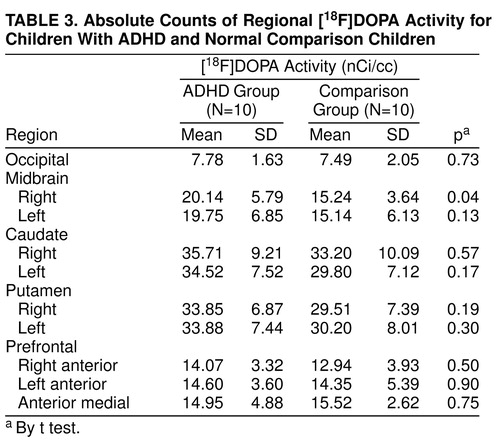
Regional [18F]DOPA ratios are presented in Table 2, and for the sake of completeness, absolute regional radioactivity counts are presented in Table 3. As illustrated in Table 2, the right midbrain [18F]DOPA ratio was higher by 48% in ADHD than in comparison subjects (Student’s t test: t=2.16; df=18; uncorrected p=0.04; Bonferroni-corrected p=0.15) (Figure 2). No other [18F]DOPA ratios differed between groups (Table 2). Regional [18F]DOPA ratio of the left midbrain was also higher in the ADHD than in the comparison group (by 36%), but this difference was not statistically significant (Student’s t test: t=1.2, df=18, uncorrected p=0.24). Within the ADHD group, children with a history of stimulant treatment (N=6) were similar to those never treated with stimulants (N=4) on age, sexual maturation, socioeconomic status, and all regional [18F]DOPA ratios. The effect of diagnosis on [18F]DOPA ratio of the right midbrain was further supported by the finding of significant relationships between severity of ADHD symptoms and [18F]DOPA ratios in the ADHD group (Table 4). The higher the [18F]DOPA ratio was in the right midbrain, the more DSM-III-R criteria for ADHD were met (Pearson product-moment correlation: r=0.71, uncorrected p=0.02, Bonferroni-corrected p=0.16; N=10), and the greater were the sum of scores on the Conners’ hyperactivity problem subscale (Pearson product-moment correlation: r=0.81, uncorrected p=0.01, Bonferroni-corrected p=0.08; N=10). Similar correlations were found with the regional [18F]DOPA ratio in the left midbrain (Pearson product-moment correlation: DSM-III-R criteria—r=0.78, uncorrected p=0.008, Bonferroni-corrected p=0.06; N=10), as could be expected from the correlation between right and left midbrain [18F]DOPA ratios (Pearson product-moment correlation: r=0.62; p=0.05; N=10). None of the regional [18F]DOPA ratios correlated with age or stage of sexual maturation (Tanner stage).
DISCUSSION
Abnormally high accumulation of [18F]DOPA in the right midbrain of children with ADHD indicates an elevated level of dopa decarboxylase activity (high level of synthesis). This finding must be interpreted with caution, given the lack of statistical significance after correction for multiple comparisons. However, because of the preliminary nature of this study, we decided to report the results uncorrected as well. The choice was to favor the risk of making a type I error (false positive) over the risk of making a type II error (false negative). The risk of failing to identify abnormalities that can be further explored in larger studies has worse consequences than the risk of detecting abnormalities that are chance findings, particularly in research that restricts groups to small sizes because of ethical concerns. A strategy to maximize the validity of findings in low-power studies is to investigate independent additional support within the studies. For example, in the present work, the associations of midbrain [18F]DOPA with severity of symptoms independently support the abnormality within the midbrain dopamine region. Replication of the results of this study not only is warranted, but may be the only alternative to confirm results of this type of research, in which the study of large groups is prohibited by both ethical and technical concerns.
Keeping this caveat in mind, elevated accumulation of [18F]DOPA indicates an enhanced level of dopa decarboxylase activity (high level of dopamine synthesis). An elevation in dopa decarboxylase activity could arise from higher enzyme activity in the absence of structural changes, from increased density of dopaminergic cell bodies and terminals, or both. Elevations in dopa decarboxylase activity occur in response to abnormally low extracellular levels of dopamine (37, 38)and to the blockade of dopaminergic receptors (39, 40). In turn, reduction in extracellular dopamine could result from alterations in one or more of the processes involved in the release, reuptake, or catabolism of the neurotransmitter. Blunted response of receptors to dopamine binding may involve abnormal intracellular signal transmission mechanisms.
The dopaminergic midbrain abnormality could be the primary problem in ADHD. As a result, the cerebral regions functionally dependent on midbrain dopamine input (striatum, prefrontal cortex, limbic structures) may be affected during their development (41–45), when fully developed, or both. Alternatively, the midbrain abnormality could be secondary to dysfunction in regions or systems that regulate the midbrain dopaminergic activity (46, 47). Structural abnormalities reported in the caudate nucleus of children with ADHD (8–10) support a more extensive involvement of the dopaminergic pathways. In either instance, treatment with stimulants or monoamine oxidase inhibitors (48, 49) could normalize extracellular dopamine concentration in the midbrain, which would explain the therapeutic efficacy of these agents. The right side lateralization of the [18F]DOPA abnormality may be important, since it is consistent with the proposed right-sided neural substrates of attention (50–53). However, the involvement of the left side cannot be ruled out, considering the significant association of the [18F]DOPA ratio of left midbrain with measures of ADHD severity. Similarly, the negative findings in other brain regions do not rule out abnormality; the sensitivity of the PET methodology is lower in regions other than the basal ganglia, such as in limbic structures or other cortical areas, because of small size or low dopaminergic innervation. Note that a 48% mean difference in regional [18F]DOPA signal is only significant at a p level of 0.04, which, in addition to the relatively small group size, reflects the high variability of the [18F]DOPA measurements. It is also likely that the percent change may be underestimated because of partial volume effects that occur in PET measurements of small brain structures, which lead to an underestimate of localized differences.
If the findings are replicated, the next logical steps would be to systematically examine the various functional units of the dopamine system (e.g., density of dopamine transporter and dopamine receptors, levels of dopamine degradation enzymes, levels of extracellular dopamine) within the dopaminergic network and identify the mechanism that leads to excessive dopa decarboxylase activity in the midbrain of children with ADHD. Delineating these abnormalities is likely to also clarify how the midbrain regulates attention and motor behavior in normal conditions.
Received Dec. 11, 1997; revisions received July 9 and Oct. 28, 1998, and Jan. 5, 1999; accepted Feb. 5, 1999. From the Laboratory of Cerebral Metabolism and the Laboratory of Psychology and Psychopathology, NIMH, Bethesda, Md.; and the Neuroimaging Branch, National Institute on Drug Abuse, Baltimore. Address reprint requests to Dr. Ernst and Dr. Cohen, Bldg. 36, Rm. 1A05, NIH, 36 Convent Dr., MSC 4030, Bethesda, MD 20892-4030. Address correspondence to Dr. Ernst, National Institute on Drug Abuse, Brain Imaging Center, 5500 Nathan Shock Dr., Baltimore, MD 21224; [email protected] (e-mail) The authors thank Dr. Doris Doudet for her help in designing the methodology and the positron emission tomography technologists and Chemistry Department of Nuclear Medicine, NIH Clinical Center, for their assistance in performing the study.
 |
 |
 |
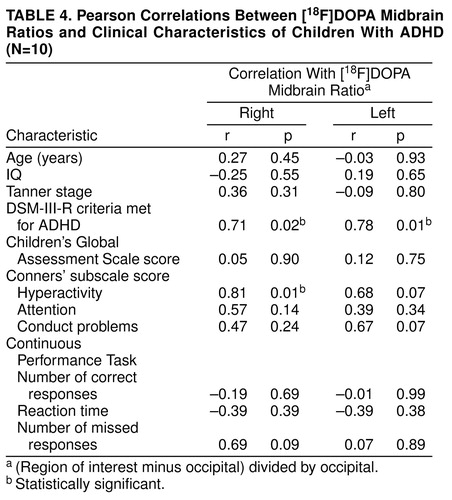 |
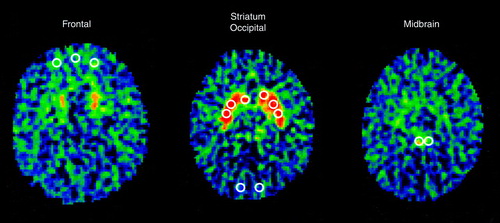
FIGURE 1. Template of Regions of Interesta
aRegions of interest were placed according to a predetermined algorithm. The slice with the highest striatal [18F]DOPA signal was the slice of reference (at about the level of the canthomeatal line). Striatal regions of interest (caudate nucleus and putamen) were first placed, on the slice of reference and then on both slices directly above and below, respectively. Occipital regions of interest were placed on the same slices as those containing the striatal regions of interest. Frontal regions of interest were placed on the fourth and fifth slices above the slice of reference (about 15 to 20 mm above the striatal plane, at the level of Brodmann’s area 10). Midbrain regions of interest were placed two and three slices lower than the slice of reference (about 7 mm and 10 mm below the striatum).
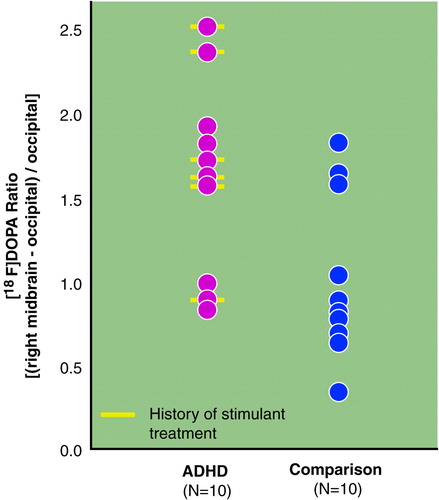
FIGURE 2. Individual Distribution of [18F]DOPA Ratios in the Right Midbrain of 10 Children With ADHD and 10 Comparison Children
1. Castellanos FX, Elia J, Kruesi MJP, Marsh WL, Gulotta CS, Potter WZ, Ritchie GF, Hamburger SD, Rapoport JL: Cerebrospinal fluid homovanillic acid predicts behavioral response to stimulants in 45 boys with attention-deficit/hyperactivity disorder. Neuropsychopharmacology 1996; 14:125–137Crossref, Medline, Google Scholar
2. Shetty T, Chase TN: Central monoamines and hyperkinesis of childhood. Neurology 1976; 26:1000–1002Google Scholar
3. Shaywitz BA, Cohen DJ, Bowers MBJ: CSF monoamine metabolites in children with minimal brain dysfunction: evidence for alteration of brain dopamine: a preliminary report. J Pediatr 1977; 90:67–71Crossref, Medline, Google Scholar
4. Cohen DJ, Caparulo BK, Shaywitz BA, Bowers MB: Dopamine and serotonin metabolism in neuropsychiatrically disturbed children: CSF homovanillic acid and 5-hydroxyindoleacetic acid. Arch Gen Psychiatry 1977; 34:545–550Crossref, Medline, Google Scholar
5. Shekim WO, Javaid J, Davis JM, Bylund DB: Urinary MHPG and HVA excretion in boys with attention deficit disorder and hyperactivity treated with d-amphetamine. Biol Psychiatry 1983; 18:707–714Medline, Google Scholar
6. Ernst M, Liebenauer LL, Tebeka D, Jons PH, Eisenhofer G, Murphy DL, Zametkin AJ: Selegiline in ADHD adults: plasma monoamines and monoamine metabolites. Neuropsychopharmacology 1997; 16:276–284Crossref, Medline, Google Scholar
7. Reimherr FW, Wender PH, Ebert MH, Wood DR: Cerebrospinal fluid homovanillic acid and 5-hydroxyindolacetic acid in adults with attention deficit hyperactivity disorder, residual type. Psychiatry Res 1984; 11:71–78Crossref, Medline, Google Scholar
8. Aylward EH, Reiss AL, Reader MJ: Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol 1996; 11:112–115Crossref, Medline, Google Scholar
9. Castellanos FX, Giedd JN, Marsh WL: Quantitative brain magnetic resonance imaging in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1996; 53:607–616Crossref, Medline, Google Scholar
10. Hynd GW, Hern KL, Novey ES: Attention deficit hyperactivity disorder and asymmetry of the caudate nucleus. J Child Neurol 1993; 8:339–347Crossref, Medline, Google Scholar
11. Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM: Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323:1361–1366Google Scholar
12. Zametkin A, Liebenauer L, Fitzgerald G, King A, Minkunas D, Herscovitch P, Yamada E, Cohen R: Brain metabolism in teenagers with attention deficit hyperactivity disorder. Arch Gen Psychiatry 1993; 50:333–340Crossref, Medline, Google Scholar
13. Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ: Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 1994; 33:858–868Crossref, Medline, Google Scholar
14. Ernst M, Zametkin AJ, Matochik JA, Liebenauer L, Fitzgerald GA, Cohen RM: Effects of intravenous dextroamphetamine on brain metabolism in adults with attention-deficit hyperactivity disorder (ADHD): preliminary findings. Psychopharmacol Bull 1994; 30:219–225Medline, Google Scholar
15. Matochik JA, Nordahl TE, Gross M, Semple WE, King AC, Cohen RM, Zametkin AJ: Effects of acute stimulant medication on cerebral metabolism in adults with hyperactivity. Neuropsychopharmacology 1993; 8:377–386Crossref, Medline, Google Scholar
16. Matochik JA, Liebenauer LL, King C, Szymanski HV, Cohen RM, Zametkin AJ: Cerebral glucose metabolism in adults with attention deficit hyperactivity disorder after chronic stimulant treatment. Am J Psychiatry 1994; 151:658–664Link, Google Scholar
17. Ernst M, Zametkin AJ, Matochik JA, Schmidt M, Liebenauer LL, Jons PH, Hardy K, Cohen RM: Intravenous dextroamphetamine and brain glucose metabolism. Neuropsychopharmacology 1997; 17:391–401Crossref, Medline, Google Scholar
18. Cook EH, Stein MA, Krasowski MD: Association of attention deficit disorder and the dopamine transporter gene. Am J Hum Genet 1995; 56:993–998Medline, Google Scholar
19. LaHoste GJ, Swanson JM, Wigal SB: Dopamine D4 receptor gene polymorphisms associated with attention deficit hyperactivity disorder. Mol Psychiatry 1996; 1:121–124Medline, Google Scholar
20. Cumming P, Gjedde A: Compartmental analysis of dopa decarboxylation in living brain from dynamic positron emission tomograms. Synapse 1998; 29:37–61Crossref, Medline, Google Scholar
21. Reich W, Welner Z: Diagnostic Interview for Children, Adolescents, and Parents. St Louis, Washington University, 1988Google Scholar
22. Zametkin AJ, Schwartz DJ, Ernst ME, Cohen RM: Is research in normal and ill children involving radiation exposure ethical? (letter). Arch Gen Psychiatry 1996; 53:1060–1061Google Scholar
23. Ernst M, Freed ME, Zametkin AJ: Health hazards of radiation exposure in the context of brain imaging research: special consideration for children. J Nucl Med 1998; 39:689–698Medline, Google Scholar
24. Woodcock RW, Johnson BB: Woodcock-Johnson Psychoeducational Battery Examiner’s Manual. Allen, Tex, DLM Teaching Resources, 1977Google Scholar
25. Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
26. Wechsler D: WISC-R Manual: Wechsler Intelligence Scale for Children—Revised. New York, Psychological Corp, 1974Google Scholar
27. Achenbach TM, Edelbrock C: Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, University of Vermont, Department of Psychiatry, 1983Google Scholar
28. Goyette CH, Conners CK, Ulrich RF: Normative data on revised Conners parent and teacher rating scales. J Abnorm Child Psychol 1978; 6:221–236Crossref, Medline, Google Scholar
29. Ullmann RK, Sleator EK, Sprague RL: Introduction to the use of ACTeRS. Psychopharmacol Bull 1985; 21:915–920Google Scholar
30. Gershon ES, DeLisi LE, Hamovit J, Nurnberger JIJ, Maxell ME, Schreiber J, Dauphinais D, Dingman CWI, Guroff JJ: A controlled family study of chronic psychosis. Arch Gen Psychiatry 1988; 45:328–336Crossref, Medline, Google Scholar
31. McLellan C, Doudet D, Brucke T, Aigner T, Cohen R: New rapid analysis method demonstrates differences in 6-[18-F]Fluoro-L-DOPA plasma input curves with and without carbidopa in hemi-MPTP lesioned monkeys. Appl Radiat Isot 1991; 42:847–854Crossref, Google Scholar
32. Doudet DJ, McLellan CA, Aigner TG, Wyatt RJ, Cohen RM: Delayed L-phenylalanine infusion allows for simultaneous kinetic analysis and improved evaluation of specific-to-nonspecific fluorine-18-DOPA uptake in brain. J Nucl Med 1992; 33:1383–1389Google Scholar
33. Matsui T, Hirano A: An Atlas of the Human Brain for Computerized Tomography. New York, Igaku-Shoin, 1978Google Scholar
34. Semple WE, Johnson JL, Cohen RM: Reliability in positron emission tomographym, in Imaging Drug Action in the Brain. Edited by London ED. Boca Raton, Fla, CRC Press, 1993, pp 297–316Google Scholar
35. Doudet DJ, Aigner TG, McLellan CA, Cohen RM: Positron emission tomography with 18-F-DOPA: interpretation and biological correlates in non-human primates. Psychiatry Res 1992; 45:153–168Crossref, Medline, Google Scholar
36. Ernst M, Zametkin A, Matochik J, Pascualvaca D, Jons P, Hardy K, Hankerson J, Doudet D, Cohen R: Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med 1996; 334:1568–1604Google Scholar
37. Torstenson R, Hartvig P, Langstrom B, Westerberg G, Tedroff J: Differential effects of levodopa on dopaminergic function in early and advanced Parkinson’s disease. Ann Neurol 1997; 41:334–440Crossref, Medline, Google Scholar
38. Abercrombie ED, Bonatz AE, Zigmond MJ: Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res 1990; 525:36–44Crossref, Medline, Google Scholar
39. Zhu MY, Jurio AV, Paterson I, Boulton AA: Regulation of striatal L-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol 1993; 238:157–164Crossref, Medline, Google Scholar
40. Hadjiconstantinou M, Wemlinger TA, Sylvia CP, Hubble JP, Neff NH: Aromatic L-amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem 1993; 60:2175–2180Google Scholar
41. Sivam SP: Dopaminergic regulation of postnatal development of dynorphin neurons in rat striatum. Neuropeptides 1996; 30:103–107Crossref, Medline, Google Scholar
42. Schmidt U, Beyer C, Oestreicher AB, Reisert I, Schilling K: Pilgrim C activation of dopaminergic D1 receptors promotes morphogenesis of developing striatal neurons. Neuroscience 1996; 74:453–460Crossref, Medline, Google Scholar
43. Penit-Soria J, Durand C, Herve D, Besson MJ: Morphological and biochemical adaptations to unilateral dopamine denervation of the neostriatum in newborn rats. Neuroscience 1997; 77:753–766Crossref, Medline, Google Scholar
44. Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH: New evidence for neurotransmitter influences on brain development. Trends Neurosci 1997; 20:269–274Crossref, Medline, Google Scholar
45. Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R: Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res 1996; 19:319–325Crossref, Google Scholar
46. Alexander GE, Crutcher MD: Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990; 13:266–271Crossref, Medline, Google Scholar
47. Gerfen CR: The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci 1992; 15:133–139Crossref, Medline, Google Scholar
48. Zametkin A, Rapoport JL, Murphy DL, Linnoila M, Karoum F, Potter WZ, Ismond D: Treatment of hyperactive children with monoamine oxidase inhibitors. Arch Gen Psychiatry 1985; 42:969–973Crossref, Medline, Google Scholar
49. Trott GE, Friese HJ, Menzel M, Nissen G: Use of moclobemide in children with attention deficit hyperactivity disorder. Psychopharmacology (Berl) 1992; 196:134–136Crossref, Google Scholar
50. Voeller KKS, Heilman KM: Attention deficit disorder in children: a neglect syndrome? Neurology 1988; 38:806–808Google Scholar
51. Malone MA, Kershner JR, Swanson JM: Hemispheric processing and methylphenidate effects in attention-deficit hyperactivity disorder. J Child Neurol 1994; 9:181–189Crossref, Medline, Google Scholar
52. Gross-Tsur V, Shalev RS, Manor O, Amir N: Developmental right-hemisphere syndrome: clinical spectrum of the nonverbal learning disability. J Learn Disabil 1995; 28:80–86Crossref, Medline, Google Scholar
53. Campbell L, Malone MA, Kershner JR, Roberts W, Humphries T, Logan WJ: Methylphenidate slows right hemisphere processing in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 1996; 6:229–239Crossref, Medline, Google Scholar



