Psychiatric Morbidity in Dementia With Lewy Bodies: A Prospective Clinical and Neuropathological Comparative Study With Alzheimer’s Disease
Abstract
OBJECTIVE: The literature reports considerable variation in the rates of psychiatric morbidity for patients with dementia with Lewy bodies. The authors intended to clarify the frequency of psychiatric morbidity in dementia with Lewy bodies and how it differs from probable Alzheimer’s disease. METHOD: The study incorporated two groups—a clinical case register cohort (98 with dementia with Lewy bodies; 92 with Alzheimer’s disease) and 80 (40 with dementia with Lewy bodies; 40 with Alzheimer’s disease) prospectively studied, neuropathologically confirmed cases. Diagnoses were made by using the McKeith et al. consensus criteria for dementia with Lewy bodies and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease. Neuropathological diagnoses were made by using the consensus criteria for dementia with Lewy bodies and the Mirra et al. protocol for Alzheimer’s disease. RESULTS: The occurrence of psychiatric symptoms was reported over 1 month. Hallucinations, depression, delusions, and delusional misidentification were all significantly higher for patients with dementia with Lewy bodies. The differences in frequency between dementia with Lewy bodies and Alzheimer’s disease for auditory and visual hallucinations were especially pronounced for patients with mild cognitive impairment. The presence of psychiatric symptoms at presentation was a better discriminator between dementia with Lewy bodies and Alzheimer’s disease than occurrence over the course of dementia. CONCLUSIONS: Delusional misidentification and hallucinations in the early stages of dementia may improve differentiation between patients with dementia with Lewy bodies and those with Alzheimer’s disease and have important treatment implications.
Lewy bodies are intraneuronal eosinophilic inclusion bodies that are seen in the brainstem and cortex of patients with Parkinson’s disease and some patients with dementia. Studies (1–6) have suggested that dementia with Lewy bodies accounts for 10% to 25% of dementia cases in clinical populations. An international meeting developed operationalized clinical diagnostic criteria 7). Key features include persistent or recurrent visual hallucinations, parkinsonism, and fluctuating confusion associated with disturbances of consciousness.
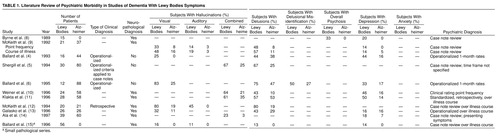
All studies have found visual hallucinations to occur significantly more frequently in dementia with Lewy bodies than in Alzheimer’s disease, although rates have varied greatly, from 25% to 83%, even when smaller studies are excluded from consideration (Table 1) (4–6, 8–15). Delusions are common in patients with dementia with Lewy bodies, with rates varying from 13% to 75%. Some groups have found delusions to have a significantly higher frequency in dementia with Lewy bodies than in Alzheimer’s disease (Table 1) (4–6, 8–15). Auditory hallucinations have received less attention, although they were described as more common in dementia with Lewy bodies than in Alzheimer’s disease by McKeith et al. (9–12). Rates have varied from 11% to 45% (Table 1) (4–6, 8–15). Delusional misidentification has not been studied in postmortem series, although two clinical studies found these symptoms to be common in patients with dementia with Lewy bodies (6, 16).
The rate reported for depression has varied from 14% to 50% in patients with dementia with Lewy bodies. Using a broad clinical definition, Klakta et al. (11) reported depression in 50% of patients with dementia with Lewy bodies. McKeith et al. (9) reported depressive symptoms in 33% of patients with dementia with Lewy bodies, although only 14% met the criteria for major depression, whereas Ballard et al. in a clinical study (6) found that 33% of patients with dementia with Lewy bodies had DSM-III-R major depression. Depressive symptoms are common in patients with dementia with Lewy bodies, although the proportion of patients with major depression requires clarification (Table 1) (4–6, 8–15).
The rates of many important psychiatric symptoms have not been accurately determined for patients with dementia with Lewy bodies. Equally important, it is unclear whether some of these symptoms are more common in patients with dementia with Lewy bodies than in Alzheimer’s disease patients and whether they change with increasing severity. The variability of previous reports is probably accounted for by the small study group sizes, the retrospective design of many studies, the different assessment methods for psychiatric symptoms, the variable time courses of symptom ascertainment, and the biases introduced by clinical selection criteria.
The current study focused on the two largest dementia with Lewy bodies cohorts reported so far. Our aims were to examine the frequency of psychiatric symptoms among patients with dementia with Lewy bodies and to evaluate the differences in symptom profile between patients with dementia with Lewy bodies and those with Alzheimer’s disease.
METHOD
Clinical Cohort
The first cohort was based on patients from the Newcastle dementia case register (337 clinically assessed dementia patients, including 98 with dementia with Lewy bodies and 92 with probable Alzheimer’s disease), incorporating consecutively referred patients with mild or moderate dementia seen by psychiatric services with defined geographical catchment areas who have an informant in regular contact. After a full explanation, written consent was obtained from the patients, with written assent from their nearest relatives. Following death, the next of kin was approached and given a full explanation regarding the aims of the related postmortem study, and written consent was obtained. The study was approved by human subjects ethical committees in both Newcastle and London. Patients from the register who met operationalized clinical criteria for a consensus diagnosis of dementia with Lewy bodies (7) (applied by three experienced clinicians in consensus [C.B., I.M., J.O.]) were compared with patients from the same cohort who met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (17) for probable Alzheimer’s disease. Data from the first 50 patients from within this series for whom there was a postmortem examination showed a positive predictive value of 0.92 for a diagnosis of dementia with Lewy bodies against postmortem diagnosis (18). The corresponding figure for the positive predictive value of a clinical diagnosis of probable Alzheimer’s disease against neuropathological diagnosis was 0.80.
The assessment included a standardized psychiatric history (history and etiology schedule [19]) and an assessment of cognitive function using the cognitive section of the Cambridge Assessment for Mental Disorders of the Elderly (20), a widely used and validated cognitive assessment instrument, scored out of a possible 107 points. A standardized physical examination incorporated the Unified Parkinson’s Disease Rating Scale (21). An assessment of psychosis used the Columbia University Scale for Psychopathology in Alzheimer’s Disease (22), and an evaluation of depression was undertaken by using the Cornell Scale for Depression in Dementia (23). All scales to assess psychiatric morbidity measured the occurrence over 1 month. A computerized tomography scan or magnetic resonance imaging scan and full dementia blood screen were also performed.
The diagnosis of depression was made by using DSM-III-R criteria, ignoring the caveat regarding exclusion if there is evidence of organic causation. Definitions regarding the presence and classification of psychotic features were taken from the criteria of Burns et al. (24). Delusional misidentification included the Capgras delusion and delusional misidentification of television images and mirror images.
Neuropathological Cohort
This cohort’s patients were recruited from two centers (80 prospectively assessed patients with dementia: 40 patients with dementia with Lewy bodies, 40 patients with Alzheimer’s disease)—the Institute for the Health of the Elderly in Newcastle (28 patients with dementia with Lewy bodies, 12 patients with Alzheimer’s disease) and the Institute of Psychiatry in London (12 patients with dementia with Lewy bodies, 28 patients with Alzheimer’s disease). Both centers have a case register of dementia sufferers from defined geographical areas. The Newcastle case register has been described previously. In London, the case register included subjects with dementia known to hospital and social services. All cases of patients with neuropathologically confirmed dementia with Lewy bodies from both centers were included in the current report, together with an equal number of age- or gender-matched patients with neuropathologically confirmed cases of Alzheimer’s disease. At the point of entry into the study, written consent was obtained from the patients, with assent from the next of kin, after a full explanation was given. A separate written consent was obtained from the next of kin after death to permit postmorten examination.
The assessment measures at the Newcastle center are described in the previous section. In London, a standardized psychiatric history was taken by using the Cambridge Assessment for Mental Disorders of the Elderly (20). Both the history and etiology schedule and the Cambridge Assessment for Mental Disorders of the Elderly schedules contain detailed information regarding patient history, medication use, mental state, history of psychiatric symptoms, and fluctuation and disturbances of consciousness. The Mini-Mental State examination (25) (derived from the cognitive section of the Cambridge Assessment for Mental Disorders of the Elderly in Newcastle) was used for cognitive assessment, and the Cornell Scale for Depression in Dementia (23) was used for assessing depression. The Cornell scale was augmented by additional DSM-III-R items pertaining to the duration of the mood disturbance and its effect on social functioning. Psychotic symptoms were evaluated in London by using the Manchester and Oxford Universities Scale for the Psychopathological Assessment of Dementia (26) (all of the Columbia University Scale for Psychopathology in Alzheimer’s Disease items are contained within the Manchester and Oxford Universities Scale for the Psychopathological Assessment of Dementia). All standardized assessments reported the occurrence of psychiatric symptoms over 1 month. A physical examination was performed that included assessment of parkinsonism with the Webster scale (27) and staging through use of the Hoehn and Yahr system (28), with a rating of 1 or more taken as evidence of significant parkinsonism. This detailed operationalized assessment was repeated at annual intervals until the patient’s death.
Psychiatric symptoms were diagnosed by using the same procedures outlined in the clinical section. The symptoms at presentation were recorded from the baseline assessment. Information regarding symptoms over the course of the illness was obtained by combining information from the psychiatric history, the baseline assessment, and subsequent annual assessments.
The neuropathological diagnosis of Alzheimer’s disease was made by using quantitative techniques to determine plaque and tangle densities (6) and according to the criteria of the Consortium to Establish a Registry for Alzheimer’s Disease (29). The quantitative assessment of Lewy body density followed the consensus protocol (7), using ubiquitin and Tau2, Alz50, and AT8 antibodies to detect and distinguish cortical Lewy bodies. The neuropathological diagnosis of dementia with Lewy bodies was made according to the international consensus criteria (7).
The number of patients with dementia with Lewy bodies and those with Alzheimer’s disease experiencing symptoms of interest at presentation were compared by using the chi-square test. The same method was used to compare the rates of symptoms over the course of the illness. The rates of symptoms were described for patients with mild (Mini-Mental State examination score of 20 or more), moderate (Mini-Mental State examination score=10–20), and severe (Mini-Mental State examination score less than 10) cognitive impairment. Overall scores on the Mini-Mental State examination and scores on the cognitive section of the Cambridge Assessment of Mental Disorders of the Elderly were compared between patients with dementia with Lewy bodies and Alzheimer’s disease patients by using the Mann-Whitney U test. All statistics were obtained by using the Statistical Package for the Social Sciences (30).
RESULTS
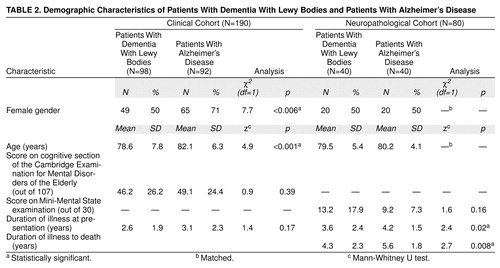
The clinical case register cohort included 337 patients with dementia, 329 of whom met the criteria for dementia with Lewy bodies (N=98), Alzheimer’s disease (92 probable, 74 possible), or vascular dementia (N=65). Only the patients with probable dementia with Lewy bodies or probable Alzheimer’s disease were considered in the present study. The neuropathological series included 40 patients with dementia with Lewy bodies who were compared with 40 Alzheimer’s disease patients matched by age and gender. Their demographic characteristics are described in Table 2. The patients with dementia with Lewy bodies in the clinical cohort were more likely to be male and were significantly younger. The patients in the neuropathological cohort were matched for these variables, but the Alzheimer’s disease patients were significantly more cognitively impaired and had a longer illness duration (Table 2).
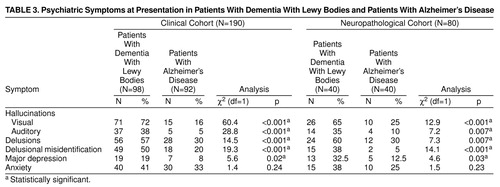
At presentation, visual and auditory hallucinations, delusions, delusional misidentification, and depression were significantly more common in patients with dementia with Lewy bodies, in both the clinical and neuropathological cohorts. The rates of individual symptoms are shown in Table 3 and are similar in the two study groups, apart from the lower frequency of depression in the clinically diagnosed patients. Most psychiatric symptoms had rates exceeding 30% in patients with dementia with Lewy bodies from the clinical cohort, with visual hallucinations occurring in more than 70% of the patients. The two dementias showed the biggest differences regarding visual and auditory hallucinations and delusional misidentification.
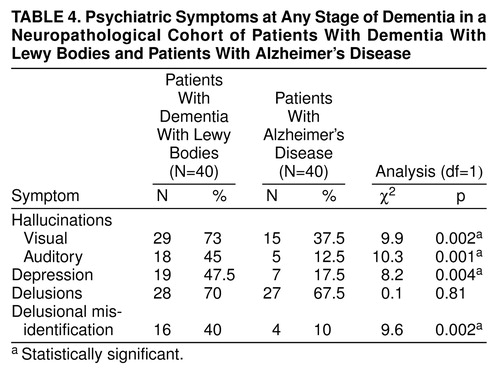
The differences in the frequency of psychiatric symptoms between patients with dementia with Lewy bodies and those with Alzheimer’s disease were less dramatic when observed over the course of the dementia, especially for delusions. That was also true for visual hallucinations, which occurred in more than 30% of Alzheimer’s disease patients over the course of the illness (Table 4).
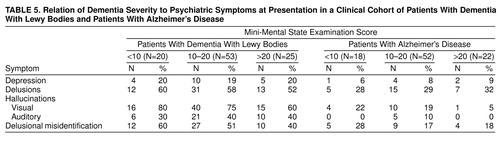
Table 5 shows the rates of key psychiatric symptoms in clinically diagnosed patients with mild, moderate, and severe dementia defined by Mini-Mental State examination cutoff scores. Most important, both auditory (0%) and visual (5%) hallucinations were extremely uncommon among Alzheimer’s disease patients scoring over 20 on the Mini-Mental State examination. Among patients with Mini-Mental State examination scores over 20, all patients with auditory hallucinations and 93% of patients with visual hallucinations had a diagnosis of probable dementia with Lewy bodies.
DISCUSSION
The strength of the current study is the combination of prospective clinical assessment with neuropathological confirmation of diagnoses in two independent but compatible cohorts with dementia with Lewy bodies.
The study confirms the high frequency of visual hallucinations (clinical cohort, 72%; neuropathological cohort, 65%), auditory hallucinations (clinical cohort, 38%; neuropathological cohort, 35%), delusions (clinical cohort, 57%; neuropathological cohort, 60%), and depression (clinical cohort, 19%; neuropathological cohort, 32.5%) at presentation to clinical services in patients with dementia with Lewy bodies. The rates are similar between the clinical and neuropathological cohorts for most symptoms except depression. The last discrepancy is not surprising given the higher mortality rates of depressed dementia patients (31).
Visual and auditory hallucinations, delusions, and depression all occurred at significantly higher rates in patients with dementia with Lewy bodies than in Alzheimer’s disease patients. The presence of visual or auditory hallucinations in patients with Mini-Mental State examination scores greater than 20 was even more suggestive of dementia with Lewy bodies, which may have important implications for early diagnosis. Patients with hallucinations early in the course of their dementia are also a key group for intervention studies aiming to reduce the severity, persistence, and distress of these symptoms as well as preventing the expedited institutionalization that often ensues.
When symptom rates across the course of the dementia were calculated, the magnitude of difference between dementia with Lewy bodies and Alzheimer’s disease patients diminished. This is supported by previous work, where the differences in frequency of psychiatric symptoms for dementia with Lewy bodies and Alzheimer’s disease patients are more substantial in studies evaluating point or 1-month rates (Table 1). Although a good psychiatric history is important and will more accurately identify a particular symptom over the course of an illness, it is suggested that the differential diagnosis of dementia with Lewy bodies can be made more accurately by focusing on symptoms over the month before presentation. This is particularly true for delusions, reflecting the tendency of Alzheimer’s disease patients to experience brief delusional episodes (32).
Alzheimer’s disease patients in the clinical cohort were older and more likely to be female, whereas Alzheimer’s disease patients in the neuropathological cohort had more severe cognitive impairment and a longer duration of illness. Each of these factors may have elevated the relative frequency of some psychiatric symptoms, although the confounding effects would operate in the opposite direction from the current findings. Because patients from the clinical cohort met the clinical diagnostic criteria for dementia with Lewy bodies, it is possible that the reported rates of visual hallucinations may have been elevated by selection biases. However, the magnitude of difference was similar in the neuropathologically diagnosed patients.
Both cohorts reported in the current study showed significantly higher rates of depression with dementia with Lewy bodies than with Alzheimer’s disease. Five out of six previous studies have reported higher rates of depression in patients with dementia with Lewy bodies (Table 1), although many of the study groups have not been large enough to demonstrate statistically significant differences. This is consistent with the current study, where the effect size was less substantial than for psychotic features.
Delusional misidentification and anxiety have received little previous attention. Anxiety was common in both patients with dementia with Lewy bodies and those with Alzheimer’s disease. However, delusional misidentification was substantially more common in patients with dementia with Lewy bodies and may also be of diagnostic importance.
Given the high frequency of psychiatric symptoms in patients with dementia with Lewy bodies, developing safe and effective treatments is a priority but depends on understanding treatment mechanisms. Perry et al. (33) identified a strong association between visual hallucinations in patients with dementia with Lewy bodies and the severity of cholinergic depletion in the temporal cortex, a finding supported by parallel work with Alzheimer’s disease, suggesting that anticholinesterase inhibitors may improve psychosis (34).
The mechanisms underlying other psychiatric symptoms have received less attention. A pilot study (35) suggested a link between the severity of parkinsonism and depression in dementia with Lewy bodies. Given the established link between depression and Parkinson’s disease, the possibility that similar mechanisms are important merits further study.
For Alzheimer’s disease, Förstl et al. (36) suggested that relative preservation of the parahippocampal gyrus is associated with delusions. The relative sparing of medial temporal lobe structures in patients with dementia with Lewy bodies (37) may hence predispose them to delusional phenomena. Further work needs to focus on the neurochemical correlates of preserved temporal lobe structures to inform the development of therapeutic strategies. Förstl et al. (36) suggested that delusional misidentification may be associated with more severe neuronal loss in the hippocampus.
While there is already sufficient evidence to merit trials of treatment intervention with cholinesterase inhibitors for visual hallucinations, further work is required to understand the basis of other psychiatric symptoms.
This report confirms a significantly higher frequency of psychiatric symptoms, especially psychotic features, in patients with dementia with Lewy bodies. The finding that these differences were especially pronounced in patients with mild dementia has implications for early diagnosis and treatment.
Received May 28, 1998; revisions received Sept. 22 and Nov. 6, 1998; accepted Dec. 15, 1998. From the Medical Research Council Neurochemical Pathology Unit, Newcastle General Hospital; the Institute of Psychiatry, London; and the University of Newcastle Upon Tyne, U.K. Address reprint requests to Dr. Ballard, Medical Research Council Neurochemical Pathology Unit, Newcastle General Hospital, Westgate Road, Newcastle Upon Tyne, NE4 6BE U.K.; [email protected] (e-mail). The authors thank the Medical Research Council for supporting this work.
 |
 |
 |
 |
 |
1. Hansen L, Salmon D, Galasko D, Maslish E, Katzman R, de Terese R, Thal L, Pay MM, Hojstetter R, Klauber M: The Lewy body variant of Alzheimer’s disease: a clinical and pathological entity. Neurology 1990; 40:1–8Crossref, Medline, Google Scholar
2. Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK: A clinically and pathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci 1990; 95:119–139Crossref, Medline, Google Scholar
3. Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH: Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology 1991; 41:1402–1409Google Scholar
4. Ballard CG, Mohan RNC, Patel A, Bannister C: Idiopathic clouding of consciousness—do the patients have cortical Lewy body disease? Int J Geriatr Psychiatry 1993; 8:571–576Google Scholar
5. Shergill S, Mullen E, D’Ath P, Katona C: What is the clinical prevalence of Lewy body dementia? Int J Psychiatry 1994; 9:907–912Google Scholar
6. Ballard CG, Saad K, Patel A, Gahir M, Solis M, Coope B, Wilcock G: The prevalence and phenomenology of psychotic symptoms in dementia sufferers. Int J Geriatr Psychiatry 1995; 10:477–486Crossref, Google Scholar
7. McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen ENH, Ballard C, de Vos RAI, Wilcock GK, Jellinger KA, Perry RH: Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 1996; 47:1113–1124Google Scholar
8. Byrne EJ, Lennox G, Lowe J, Goodwin-Austen LB: Lewy body disease, clinical features in 15 cases. J Neurol Neurosurg Psychiatry 1989; 52:709–717Crossref, Medline, Google Scholar
9. McKeith IG, Perry RH, Fairbairn AF, Jabeen S, Perry EK: Operational criteria for senile dementia of Lewy body type. Psychol Med 1992; 22:911–922Crossref, Medline, Google Scholar
10. Weiner WF, Risser RC, Cullum CM, Honig L, White C III, Speciale S, Rosenberg RN: Alzheimer’s disease and its Lewy body variant: a clinical analysis of postmortem verified cases. Am J Psychiatry 1996; 153:1269–1273Google Scholar
11. Klakta LA, Louis ED, Schiffer RB: Psychiatric features in diffuse Lewy body disease: findings in 28 pathologically diagnosed cases. Neurology 1996; 47:1148–1152Google Scholar
12. McKeith IG, Fairbairn AF, Bothwell RA, Moore PB, Ferrier IN, Thompson P, Perry RH: An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for SDLT. Neurology 1994; 44:872–877Crossref, Medline, Google Scholar
13. Galasko D, Katzman R, Salmon DP, Thal LJ, Hansen L: Clinical and neuropathological findings in Lewy body dementias. Brain Cogn 1996; 31:166–175Crossref, Medline, Google Scholar
14. Ala TA, Yang K-H, Sung JH, Frey WH II: Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer’s disease at presentation: a clinicopathological study. J Neurol Neurosurg Psychiatry 1997; 62:16–21Crossref, Medline, Google Scholar
15. Ballard CG, Lowery K, Harrison R, McKeith IG: Non-cognitive symptoms in dementia with Lewy bodies, in Dementia With Lewy Bodies. Edited by Perry RH, McKeith IG, Perry EK. New York, Cambridge University Press, 1996, pp 67–84Google Scholar
16. Ballard CG, O’Brien J, Lowery K, Ayre GA, Harrison R, Perry R, Ince P, Neill D, McKeith IG: A prospective study of dementia with Lewy bodies. Age Ageing 1998; 27:631–636Crossref, Medline, Google Scholar
17. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
18. McKeith IG, Ballard CG, Perry RH, Ince PG, Jaros E, Neill D, O’Brien J: Predictive accuracy of clinical diagnostic criteria for dementia with Lewy bodies—a prospective neuropathological validation study (abstract). Neurology 1998; 50(suppl 4):A181Google Scholar
19. Dewey ME, Copeland JRM, Lobo A, Saz P, Dia J-L: Computerized diagnosis from a standardized history schedule: a preliminary communication about the organic section of the HAS-AGECAT system. Int J Geriatr Psychiatry 1992; 7:443–446Crossref, Google Scholar
20. Roth M, Tym E, Mountjoy C, Huppert F, Hendrie H, Verma S, Goddard R: CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149:698–709Crossref, Medline, Google Scholar
21. Ballard C, McKeith I, Burn D, Harrison R, O’Brien J, Lowery K, Campbell M, Perry R, Ince P: The UPDRS Scale as a means of identifying extrapyramidal signs in patients suffering from dementia with Lewy bodies. Acta Neurol Scand 1997; 96:366–371Crossref, Medline, Google Scholar
22. Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, Stern Y: The Columbia University Scale for Psychopathology in Alzheimer’s Disease. Arch Neurol 1992; 49:371–376Crossref, Medline, Google Scholar
23. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA: Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Crossref, Medline, Google Scholar
24. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, I: disorders of thought content, and II: disorders of perception. Br J Psychiatry 1990; 157:72–81Crossref, Medline, Google Scholar
25. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
26. Allen NH, Gordon S, Hope T, Burns A: Manchester and Oxford Universities Scale for the Psychopathological Assessment of Dementia (MOUSPAD). Br J Psychiatry 1996; 169:293–307Crossref, Medline, Google Scholar
27. Webster DD: Critical analysis of the disability in Parkinson’s disease. Mod Treat 1968; 5:257–282Medline, Google Scholar
28. Hoehn M, Yahr M: Parkinsonism: symptoms, progression and mortality. Neurology 1967; 17:427–442Crossref, Medline, Google Scholar
29. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41:479–486Crossref, Medline, Google Scholar
30. Norusis MJ: SPSS for Windows: Base System User’s Guide, Release 5.0. Chicago, SPSS, 1992Google Scholar
31. Burns A, Lewis G, Jacoby R, Levy R: Factors affecting survival in Alzheimer’s disease. Psychol Med 1991; 21:363–370Crossref, Medline, Google Scholar
32. Ballard C, O’Brien J, Coope B, Fairbairn A, Abid F, Wilcock G: A prospective study of psychotic symptoms in dementia sufferers: psychosis in dementia. Int Psychogeriatr 1997; 9:57–64Crossref, Medline, Google Scholar
33. Perry EK, Marshall E, Kerwin JM, Smith D, Jabeen S, Cheng AV, Perry RH: Evidence of a monoaminergic-cholinergic imbalance related to visual hallucinations in Lewy body dementia. J Neurochem 1990; 55:1454–1456Google Scholar
34. Cummings JL, Cyrus PA, Bieber F, Mas J, Orazem J, Gulanski B, Metrifonate Study Group: Metrifonate treatment of the cognitive deficits of Alzheimer’s disease. Neurology 1998; 50:1214–1221Google Scholar
35. Ballard C, McKeith I, Harrison R, O’Brien J, Adams R, Lowery K, Perry R, Ince P: Depression in dementia with Lewy bodies and Alzheimer’s disease. J Affect Disord (in press)Google Scholar
36. Förstl H, Burns A, Levy R, Cairns N: Neuropathological correlator of psychotic phenomena in confirmed Alzheimer’s disease. Br J Psychiatry 1994; 165:53–59Crossref, Medline, Google Scholar
37. Hashimoto M, Kitagaki H, Imamura T, Hirono N, Shimomura T, Kazui H, Tanimukai S, Hanihara T, Mori E: Medial temporal and whole-brain atrophy in dementia with Lewy bodies: a volumetric MRI study. Neurology 1998; 51:357–362Crossref, Medline, Google Scholar



