Regional Cerebral Blood Flow During Script-Driven Imagery in Childhood Sexual Abuse-Related PTSD: A PET Investigation
Abstract
OBJECTIVE: The purpose of this study was to determine whether anterior limbic and paralimbic regions of the brain are differentially activated during the recollection and imagery of traumatic events in trauma-exposed individuals with and without posttraumatic stress disorder (PTSD). METHOD: Positron emission tomography (PET) was used to measure normalized regional cerebral blood flow (CBF) in 16 women with histories of childhood sexual abuse: eight with current PTSD and eight without current PTSD. In separate script-driven imagery conditions, participants recalled and imagined traumatic and neutral autobiographical events. Psychophysiologic responses and subjective ratings of emotional state were measured for each condition. RESULTS: In the traumatic condition versus the neutral control conditions, both groups exhibited regional CBF increases in orbitofrontal cortex and anterior temporal poles; however, these increases were greater in the PTSD group than in the comparison group. The comparison group exhibited regional CBF increases in insular cortex and anterior cingulate gyrus; increases in anterior cingulate gyrus were greater in the comparison group than in the PTSD group. Regional CBF decreases in bilateral anterior frontal regions were greater in the PTSD group than in the comparison group, and only the PTSD group exhibited regional CBF decreases in left inferior frontal gyrus. CONCLUSIONS: The recollection and imagery of traumatic events versus neutral events was accompanied by regional CBF increases in anterior paralimbic regions of the brain in trauma-exposed individuals with and without PTSD. However, the PTSD group had greater increases in orbitofrontal cortex and anterior temporal pole, whereas the comparison group had greater increases in anterior cingulate gyrus.
Posttraumatic stress disorder (PTSD) is characterized in part by intense psychological distress and physiologic reactivity upon “exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event” (DSM-IV). Several studies have demonstrated that individuals with PTSD are physiologically more responsive to the recollection and imagery of traumatic events than are trauma-exposed individuals without PTSD (1–3). For example, using the script-driven imagery paradigm (2, 4, 5), Orr and colleagues (1) found that women with PTSD linked to histories of childhood sexual abuse had greater heart rate responses while recalling and imagining abuse-related events than did abused women without PTSD.
Little is currently known about the patterns of brain activation associated with the recollection of traumatic events in individuals with PTSD. Rauch and colleagues (6) used positron emission tomography (PET) to examine regional cerebral blood flow (CBF) in eight individuals with PTSD. In separate conditions, subjects (with eyes closed) recalled and imagined neutral and traumatic autobiographical events. Heart rate and ratings of anxiety, fear, sadness, disgust, anger, and guilt were significantly greater in the traumatic condition than in the neutral control condition. In addition, regional CBF increases occurred in anterior limbic and paralimbic structures (i.e., amygdala, anterior cingulate cortex, anterior temporal pole, insular cortex, and orbitofrontal cortex) and in visual cortex; regional CBF decreases occurred in left inferior frontal gyrus (Broca’s area). Whether similar patterns of activation occur in trauma-exposed individuals without PTSD is currently unknown.
Only one published functional neuroimaging study of PTSD has included trauma-exposed individuals without PTSD. Our group (7) studied visual perception and mental imagery of standardized combat-related photographs in seven combat veterans with PTSD and seven combat veterans without PTSD. During imagery of combat-related photographs, only the PTSD group exhibited regional CBF increases in right amygdala and ventral anterior cingulate cortex; furthermore, during perception of combat-related photographs, only the PTSD group exhibited regional CBF decreases in Broca’s area. Fischer et al. (8) examined regional CBF in six bank officials who had witnessed an armed bank robbery but did not meet diagnostic criteria for PTSD (M. Fredrikson, personal communication, September 1997). In separate conditions, the bank officials viewed a videotape of the witnessed robbery and a neutral control videotape. During the robbery video, in comparison with the neutral video, regional CBF increases occurred in orbitofrontal cortex, and regional CBF decreases occurred in Broca’s area.
The purpose of the current study was to use PET and script-driven imagery to determine whether trauma-exposed individuals with and without PTSD related to childhood sexual abuse exhibit differential regional CBF increases in anterior limbic and paralimbic regions of the brain during the recollection and imagery of traumatic events. In the script-driven imagery paradigm, audiotaped narratives (i.e., scripts) describing autobiographical events prompt subjects to recall and imagine those events. Our limbic and paralimbic regions of interest included the amygdala, anterior cingulate gyrus, anterior temporal pole, insular cortex, and orbitofrontal cortex; previous studies have found regional CBF increases in all of these regions in individuals with PTSD (6, 7). These regions appear to be involved in the processing of emotional stimuli and in the modulation of heart rate, blood pressure, and respiration through projections to autonomic centers of the brainstem (9–11); therefore, they should be activated in individuals with PTSD who experience emotional and psychophysiologic responses during the recollection of traumatic events. Because trauma-exposed individuals without PTSD are typically less emotionally and psychophysiologically reactive to the recollection of traumatic events (1–3), they may exhibit smaller or no regional CBF increases in limbic and paralimbic regions.
Several PET studies of psychiatrically healthy individuals (12–15), however, have found activation in some paralimbic structures during the recollection of emotional events. For example, Pardo et al. (14) found regional CBF increases in orbitofrontal cortex in seven healthy subjects while they recalled sad events, and Lane et al. (15) reported regional CBF increases in anterior temporal poles in 12 healthy individuals while they recalled emotional events and viewed emotional film clips. Thus, trauma-exposed individuals without PTSD may also exhibit regional CBF increases in some paralimbic regions. No previous functional neuroimaging studies have used a symptom provocation paradigm to study adult female survivors of childhood sexual abuse with and without PTSD. However, we hypothesized that the regional CBF responses of PTSD subjects with histories of childhood sexual abuse would be similar to those of PTSD subjects with other types of trauma histories.
Additional regions of interest were visual cortex and left inferior frontal gyrus (Broca’s area). Previous research has revealed regional CBF increases in visual cortex during visual mental imagery in healthy individuals (16, 17) and in individuals with PTSD (6); thus, regional CBF increases in visual cortex may occur during traumatic imagery in subjects with and without PTSD. Given the results of two previous PET studies of PTSD (6, 7), subjects with PTSD should exhibit regional CBF decreases in left inferior frontal gyrus (Broca’s area) during the traumatic condition.
METHOD
The subjects of this study were 16 women who had experienced penetrative sexual abuse before the age of 16 by a perpetrator who was at least 5 years older than they were. Fourteen of the subjects were recruited from a group of survivors of childhood sexual abuse who had previously participated in psychophysiologic studies of PTSD at the Manchester, N.H., Veterans Affairs Research Service (1). Two of the subjects were recruited from the greater Boston area through newspaper advertisements. Laboratory tests confirmed that none of the subjects was pregnant at the time of participation. In addition, the subjects had been free of psychoactive medications for at least 2 weeks (and free of selective serotonin reuptake inhibitors for at least 2 months) prior to PET scanning. None of the subjects had a major nonpsychiatric medical illness or a history of clinically significant head injury. All subjects were strongly right-handed (18). After a full explanation of the procedures, subjects’ written informed consent was obtained. All procedures were approved by the Committee on the Use of Human Subjects at Harvard University and the Subcommittee on Human Studies at the Massachusetts General Hospital.
A psychologist (N.B.L.) used the Clinician-Administered PTSD Scale (19, 20) to establish PTSD diagnoses. The PTSD group consisted of eight women who currently met DSM-III-R criteria for PTSD and were psychophysiologically responsive to imagery of autobiographical abuse-related events. The comparison group consisted of eight women who currently did not meet diagnostic criteria for PTSD and were not psychophysiologically responsive to imagery of autobiographical abuse-related events. The mean age at onset of abuse was similar in the PTSD and comparison groups (mean=8.3 years, SD=3.2, range=4–14; and mean=8.9 years, SD=3.1, range=4–12, respectively; F=0.2, df=1, 14, p=0.70). The mean duration of abuse was also similar in the PTSD and comparison groups (mean=4.0 years, SD=3.6, range=1 week to 9 years; and mean=4.3 years, SD=3.6, range=6 months to 12 years, respectively; F=0.02, df=1, 13, p=0.90). Data on duration of abuse were missing for one PTSD subject. The perpetrator was a family member in seven of eight cases in both groups. Thirteen subjects had continuous memories of abuse, and three (PTSD) subjects had recalled memories of abuse after years of not thinking about it; corroboration of abuse was obtained in two of these three cases.
A psychologist (N.B.L.) used the Structured Clinical Interview for DSM-III-R (21) to determine other psychiatric diagnoses. Subjects in the PTSD group met criteria for the following current comorbid disorders: major depression (N=5), dysthymia (N=1), cyclothymia (N=1), panic with agoraphobia (N=1), panic without agoraphobia (N=1), generalized anxiety disorder (N=1), somatoform disorder (N=1), and bulimia (N=1). In the comparison group, one subject had a current diagnosis of simple phobia. One PTSD subject and one comparison subject had met diagnostic criteria for alcohol dependence in the past, and one PTSD subject had met criteria for alcohol abuse in the past. According to the Clinician-Administered PTSD Scale, three subjects in the comparison group had met criteria for PTSD in the past; the current scores of those three comparison subjects on that scale were similar to those of the comparison subjects who had never had PTSD (mean=9.0, SD=4.0, and mean=7.2, SD=2.4, respectively; F=0.17, df=1, 6, p=0.70).
All subjects completed the Beck Depression Inventory (22), the State-Trait Anxiety Inventory (23), and the Vividness of Visual Imagery Questionnaire (24, 25).
The PTSD and comparison groups did not differ in age (mean=37.1 years, SD=13.5, range=19–54; and mean=37.5, SD=8.3, range=30–56, respectively; F=0.004, df=1, 14, p=0.95) or education (mean=14.1 years, SD=1.9, and mean=14.8 years, SD=2.4, respectively; F=0.3, df=1, 14, p=0.58). Not surprisingly, scores on the Clinician-Administered PTSD Scale, which reflect frequency and severity of PTSD symptoms, were significantly higher in the PTSD group than in the comparison group (mean=64.8, SD=20.3, and mean=7.9, SD=5.6, respectively; F=58.2, df=1, 14, p=0.0001). Scores on the Beck inventory tended to be higher in the PTSD group than in the comparison group (mean=15.6, SD=14.5, and mean=5.0, SD=5.3, respectively; F=3.8, df=1, 14, p=0.08). Trait anxiety, as measured by the trait form of the State-Trait Anxiety Inventory, was significantly greater in the PTSD group than in the comparison group (mean score=50.6, SD=18.2, and mean=34.8, SD=10.0, respectively; F=4.7, df=1, 14, p=0.05). The PTSD and comparison groups did not differ with regard to state anxiety, as measured by the state form of the State-Trait Anxiety Inventory (mean score=38.1, SD=10.1, and mean=34.1, SD=8.9, respectively; F=0.7, df=1, 14, p=0.42). The PTSD and comparison groups had similar scores on the Vividness of Visual Imagery Questionnaire (mean=64.0, SD=7.1, and mean=63.0, SD=8.7, respectively; F=0.06, df=1, 14, p=0.81).
Scripts
Prior to the PET scanning session, subjects provided written descriptions of two neutral autobiographical events and their two most stressful sexual abuse-related autobiographical events. After describing each event, subjects examined a list of bodily responses (e.g., “heart races,” “labored breathing”) and circled the responses (if any) that they experienced during each autobiographical event. Later, scripts describing each event were constructed according to established procedures (2) and were written in the second person, present tense. The scripts included up to five of the bodily responses that each subject selected. The mean number of words per script did not differ between groups (F=1.2, df=1, 14, p=0.30) or type of condition (neutral versus traumatic; F=0.03, df=1, 14, p=0.87). The scripts were read and tape recorded in a neutral female voice for playback in the PET scanner.
Imagery Procedures
Each subject participated in three conditions (neutral, teeth-clenching neutral, and traumatic) with two scans (i.e., replicates) per condition, yielding a total of six scans per subject. During each scan in the neutral and traumatic conditions, subjects recalled and imagined the contents of a neutral and a traumatic script, respectively. In the teeth-clenching neutral condition, which was implemented to control for any spontaneous contraction of the jaw muscles during the traumatic condition, subjects recalled and imagined the contents of a neutral script while clenching their teeth. The two scans within a particular condition were always presented sequentially (i.e., neutral scan 2 always immediately followed neutral scan 1). However, the order of conditions was counterbalanced across subjects, and subjects in the PTSD group received the same orders as subjects in the comparison group.
Before each scan, the subject was instructed to close her eyes, listen carefully to the script, and imagine the described event as vividly as possible, as if she were actually participating in the event. The PET camera was turned on when the script started playing. Thirty seconds later, the script ended and [15O]CO2 administration began. During the next 60 seconds, the subject continued to recall and imagine the event while PET data were acquired. Then [15O]CO2 administration and PET data acquisition were terminated, and the subject was instructed to stop imagining the event. After a 2-minute relaxation period, the subject gave ratings of her emotional state and imagery during the scanning period (see Emotional State and Imagery below). PET scans were separated by at least 10 minutes to allow for radiation decay.
Psychophysiology
Subjects’ heart rate and blood pressure were measured with a finger photoplethysmograph (Finapres, Ohmeda 2300). An inflatable cuff was placed on the middle finger of each subject’s left hand. Heart rate and blood pressure readings were recorded every 15 seconds during the following periods: 1 minute before the reading of each script (baseline), 1 minute during each scan (imagery), and 2 minutes following each scan (recovery). Within the baseline and imagery periods (within each scan), readings were averaged. For each scan, the values of the baseline period were subtracted from the values of the imagery period. These change scores (i.e., responses) were averaged across scans within the same condition.
Emotional State and Imagery
After each scan, subjects rated the intensity of their fear, sadness, anger, guilt, disgust, shame, happiness, amusement, and arousal using separate visual analog scales (6) (0=none, 10=the most you can imagine). Subjects also rated the overall valence (–5 to 5) of their emotional state during each scan.
Subjects also rated (on a scale of 0–10) the vividness of their imagery and the amount of imagery experienced in each sensory modality (visual, auditory, tactile, olfactory, and gustatory). In addition, we asked subjects whether they were aware of their surroundings during each scan and whether they felt as though the imaged event was happening again.
PET Procedures
The PET equipment and procedures have been described previously (6, 7, 17). PET data were gathered by a 15-slice, whole-body tomograph (Scanditronix PC 4096, General Electric, Milwaukee, Wis.). The camera produced contiguous slices 6.5 mm apart, with axial resolution at 6.0 mm full width half maximum (axial field=97.5 mm). Images were reconstructed with the use of a measured attenuation correction and a Hanning-weighted reconstruction filter set to allow for 8-mm in-plane spatial resolution (full width at half maximum). Corrections were made for scattered radiation, random coincidences, and counting losses resulting from dead time in the camera electronics.
After entering the scanner, each subject was fitted with a thermoplastic custom-molded face mask, an overlying face mask attached to a vacuum, and nasal cannulae that delivered the [15O]CO2. (The concentration of the [15O]CO2 was 2960 MBq/liter; the flow rate was 2 liters/min.) The subject’s head was aligned in the scanner relative to the canthomeatal line. After the subject was positioned in the scanner, transmission measurements were made with an orbiting pin source.
A total of 15 measurements were made within each data acquisition run: the first three (10 seconds each) occurred immediately before administration of [15O]CO2, and the final 12 (5 seconds each) occurred during [15O]CO2 administration. After reconstruction, measurements 4–15 were summed to form images of CBF. Terminal count rates were between 100,000 and 200,000 events per second. Arterial lines were not used in view of their invasiveness and previous research in our PET laboratory indicating that integrated counts over periods of up to 90 seconds are a linear function over the flow range of 1–130 ml/min per 100 g (N.M. Alpert, unpublished data, 1991).
The PET images were corrected for interscan head movement and were transformed to the coordinate system of Talairach and Tournoux (26). The images were smoothed and scaled with the use of a two-dimensional Gaussian filter of 20-mm width (full width at half maximum).
Statistical Analysis
Statistical analysis of the PET data was conducted following the theory of statistical parametric mapping (27, 28). Data were analyzed with the SPM95 software package (Wellcome Department of Cognitive Neurology, London). At each voxel the PET data were normalized by the global mean and fitted to a linear statistical model by the least squares method. The analysis of variance (ANOVA) (conducted separately within each group) considered scan condition as the main effect and subjects as a block effect. Planned contrasts at each voxel were conducted; this method fits a linear statistical model, voxel by voxel, to the data. Hypotheses were tested as contrasts in which linear compounds of the model parameters were evaluated by means of t tests. Data from all three conditions (including the replicates per condition) were used to compute the contrast error term. The data from both groups together were also analyzed with SPM95 and a linear model with group (PTSD, comparison) and scan condition as main effects and subjects as a block effect. Planned contrasts at each voxel were conducted to examine the interaction of group and condition. Three replicate scans (two in the PTSD group and one in the comparison group) were removed from the dataset before data analysis because of poor image quality (i.e., having fewer than 1 million events per slice).
Regions containing foci of activation with z scores greater than 3.09 are reported. For our a priori regions of interest, a z score threshold of 3.09 (p<0.001, one-tailed, uncorrected for multiple comparisons) was selected because we had strong and directional a priori predictions about regional CBF increases in limbic, paralimbic, and visual areas and regional CBF decreases in Broca’s area in the traumatic condition compared with the neutral conditions. These strong predictions were based on the results of our previous neuroimaging studies of PTSD (6, 7). For the sake of completeness and in order to obviate bias, we also report other (nonpredicted) regions that exhibited regional CBF increases with z scores greater than 3.09. However, because of the post hoc nature of those findings, we advise the reader to use caution in interpreting them. With regard to the condition-by-group interaction, given that no previous functional neuroimaging study of PTSD has reported such an analysis, our hypotheses did not specify the direction of the differential changes between groups. Thus, we used a more conservative z score threshold (z=3.30, p<0.001, two-tailed) for this analysis.
RESULTS
Psychophysiology
Heart rate and blood pressure (systolic and diastolic) responses were submitted to separate 2 (group: PTSD, comparison) × 3 (condition: neutral, teeth-clenching neutral, traumatic) ANOVAs (figure 1). Heart rate responses increased across conditions (F=21.6, df=2, 28, p=0.0001), and the magnitude of this increase differed between the PTSD and comparison groups (F=11.3, df=2, 28, p=0.0003). Planned comparisons revealed that the PTSD group had greater heart rate responses during the traumatic condition than did the comparison group (figure 1); the two groups did not differ with regard to heart rate responses in the neutral condition (F=0.6, df=1, 14, p=0.47; effect size r=0.20) and the teeth-clenching neutral condition (F=0.1, df=1, 14, p=0.72; effect size r=0.08).
The PTSD group had higher systolic blood pressure responses than did the comparison group (F=4.7, df=1, 14, p=0.05), and systolic blood pressure responses differed among conditions (F=4.3, df=2, 28, p=0.03). Planned comparisons revealed nonsignificant differences between groups in systolic blood pressure responses in the traumatic condition (F=3.4, df=1, 14, p=0.09; effect size r=0.44), the neutral condition (F=2.4, df=1, 14, p=0.15; effect size r=0.38), and the teeth-clenching neutral condition (F=1.8, df=1, 14, p=0.21; effect size r=0.34).
Diastolic blood pressure responses increased across conditions (F=5.9, df=2, 28, p=0.008), and the magnitude of this increase differed between the groups (F=4.1, df=2, 28, p=0.03). Planned comparisons revealed nonsignificant differences between groups in diastolic blood pressure responses in the traumatic condition (F=3.9, df=1, 14, p=0.07; effect size r=0.47), the neutral condition (F=0.8, df=1, 14, p=0.40; effect size r=0.23), and the teeth-clenching neutral condition (F=0.6, df=1, 14, p=0.45; effect size r=0.20).
The p values reported above for the between-group contrasts (within each condition) were not adjusted for multiple comparisons because they reflected planned comparisons. If the p values had been Bonferroni adjusted (for three comparisons multiplied by three psychophysiologic variables, or a total of nine comparisons), the between-group difference in heart rate responses during the traumatic condition would have remained significant (alpha=0.05/9=0.0055).
Emotional State
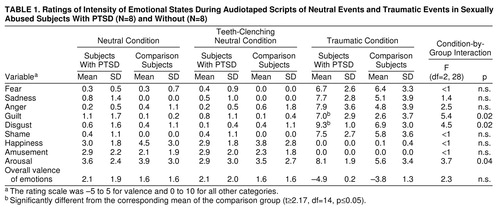
Table 1 shows the mean ratings of emotional states in the PTSD and comparison groups. Group differences in changes in emotional state across conditions were assessed by means of F tests for the condition-by-group interaction. For each dependent variable, if the condition-by-group interaction was significant, then a t test between groups in the traumatic condition was conducted.
Overall, the patterns of ratings for the PTSD and comparison groups appeared quite similar, except that the PTSD group showed greater increases in arousal, guilt, and disgust across conditions than did the comparison group. Within the traumatic condition only, t tests revealed that the PTSD group gave significantly higher ratings of guilt and disgust than did the comparison group. If the p values associated with these t tests were adjusted for multiple comparisons, these two group differences would not remain significant.
Imagery
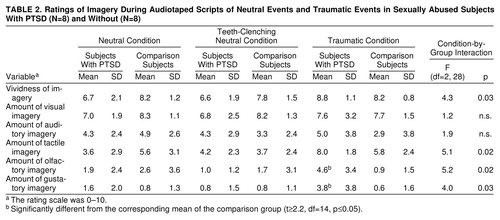
Table 2 shows the mean imagery ratings in the PTSD and comparison groups. Data analysis procedures were similar to those used to examine the data on emotional state. Neither group had significant changes in the reported amount of visual or auditory imagery across conditions. The PTSD group had greater increases in rated vividness and amount of tactile, olfactory, and gustatory imagery across conditions than did the comparison group. Within the traumatic condition only, t tests revealed that the PTSD group reported more olfactory and gustatory imagery than did the comparison group. If the p values associated with these t tests were adjusted for multiple comparisons, these two group differences would not remain significant.
One PTSD subject had a flashback during the traumatic imagery condition. After the scan, she reported that she felt as though the event was happening again and that she had little awareness of her current surroundings.
Regional Cerebral Blood Flow
Within each group, regional CBF images of the traumatic condition were compared with those of the neutral condition, teeth-clenching neutral condition, and both of these neutral control conditions combined. The results of these comparisons were quite similar, and the addition of the teeth-clenching task did not alter regional CBF results in our a priori regions of interest. Because of space limitations, we report only the comparisons between the traumatic condition and both neutral control conditions combined; this type of comparison is the most statistically stable because the neutral images represent data gathered during four scans (two neutral and two teeth-clenching neutral scans). Data from other comparisons are available from the first author on request. In addition, regional CBF images reflecting the interaction of condition and group were examined; this analysis revealed regions in which changes in regional CBF across conditions differed between groups. For the condition-by-group analysis, SPM95 yielded two tables: one listing the regional CBF changes that were greater in the PTSD group and another listing the regional CBF changes that were greater in the comparison group. Because these tables did not indicate which of those changes were regional CBF increases and which were regional CBF decreases, we consulted the contrasts conducted within each group in order to distinguish between the increases and decreases. We used this information to categorize the activations.
Patterns of blood flow results in the comparison group did not change when the three comparison subjects with past PTSD were removed from the analyses; in order to maximize statistical power, all comparison subjects were retained in the analyses reported below.
Traumatic versus neutral control conditions
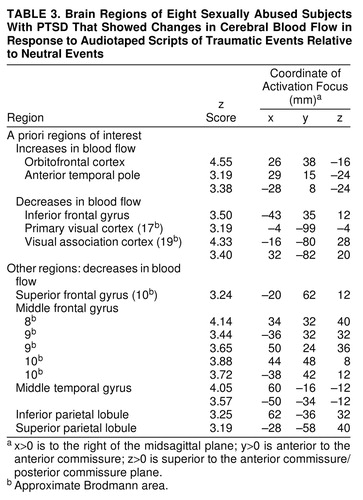
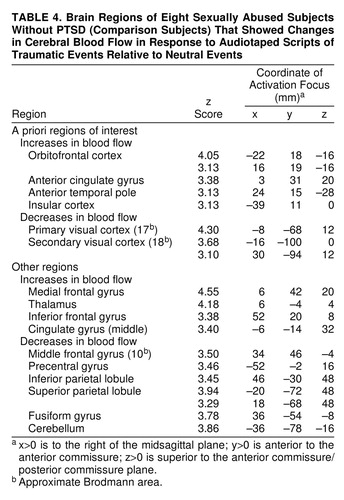
Table 3 displays regions with CBF changes (increases and decreases) in the PTSD group in the traumatic condition relative to the neutral control conditions. Table 4 4 displays the regions with CBF changes in the comparison group. In both groups, regional CBF increases occurred in orbitofrontal cortex and anterior temporal poles. Only the comparison group exhibited regional CBF increases in anterior cingulate gyrus and insular cortex. Both groups exhibited regional CBF decreases in visual cortex and related visual areas. Only the PTSD group exhibited regional CBF decreases in left inferior frontal gyrus.
Teeth-clenching neutral condition versus neutral condition
The PTSD group exhibited no changes in regional CBF between these two neutral control conditions. In the comparison group, regional CBF increases during the teeth-clenching neutral condition occurred in somatosensory cortex (postcentral gyrus) (z score=3.14; x, y, and z coordinates=–58, –20, and 40); regional CBF decreases occurred in orbitofrontal cortex (z score=3.69; x, y, z coordinates=3, 15, and –16).
Condition-by-group interaction
Table 5 shows the regions in which CBF changes in the traumatic condition versus the neutral control conditions differed between groups. Regional CBF increases in both orbitofrontal cortex and anterior temporal pole were greater in the PTSD group than in the comparison group. Regional CBF decreases in widespread regions of frontal, temporal, and parietal cortex were greater in the PTSD group than in the comparison group. Regional CBF increases in anterior cingulate gyrus were greater in the comparison group than in the PTSD group.
DISCUSSION
The PTSD group had larger heart rate responses during the traumatic condition than did the comparison group. In the traumatic condition versus the neutral control conditions, both groups exhibited regional CBF increases in orbitofrontal cortex and anterior temporal poles; however, these increases were greater in the PTSD group than in the comparison group. The comparison group exhibited regional CBF increases in insular cortex and anterior cingulate gyrus; increases in anterior cingulate gyrus were greater in the comparison group than in the PTSD group. Regional CBF decreases in bilateral anterior frontal regions were greater in the PTSD group than in the comparison group, and only the PTSD group exhibited regional CBF decreases in left inferior frontal gyrus. Regional CBF decreases in visual cortex occurred in both groups.
The finding of regional CBF increases in orbitofrontal cortex and anterior temporal poles in the PTSD and comparison groups is consistent with the results of previous functional neuroimaging studies of PTSD (orbitofrontal cortex: x, y, and z coordinates=21, 10, and –16; anterior temporal pole: x, y, and z coordinates=43, –2, and –12 [6]) and studies of normal emotion in nonclinical study groups (13–15, 29, 30) (e.g., anterior temporal pole: x, y, and z coordinates=36, 10, and –20 [15]). In the current study, however, regional CBF increases in orbitofrontal cortex and anterior temporal pole were greater in the PTSD group than in the comparison group. Larger heart rate responses and greater increases in self-reported arousal, guilt, and disgust in the PTSD group may explain these greater regional CBF increases in the PTSD group. However, activation in orbitofrontal cortex and anterior temporal poles per se is not specific to PTSD, since trauma-exposed comparison subjects and patients with other anxiety disorders have exhibited activation in these regions as well (31, 32).
In previous neuroimaging studies of emotion, regional CBF increases in temporal poles were attributed to extracranial artifacts of jaw muscle contraction (33, 34). Several findings suggest that the temporal pole activations in the present study were not attributable to these artifacts. Any jaw muscle contraction in the traumatic condition was controlled for by the teeth-clenching neutral condition; furthermore, the temporopolar activations were within the brain, and their coordinates were very different from those of activations due to extracranial artifacts.
The regional CBF increases in anterior cingulate gyrus and insular cortex in the comparison group are consistent with the results of recent studies of healthy individuals during recollection of sad events (insular cortex: x, y, and z coordinates=–42, 12, and 4; anterior cingulate: x, y, and z coordinates=2, 44, and 12 [13]), performance of the Emotional Counting Stroop task (anterior cingulate: x, y, and z coordinates=–3, 39, and 15 [35]), procaine-induced fear (29), and imagery of aversive stimuli (17). Why significant activations in anterior cingulate gyrus and insular cortex did not occur in the PTSD group is unclear.
No regional CBF increases in the amygdala were observed in either group in any comparison. This result differs from those of previous PET studies of PTSD (6, 7). The reason for the lack of amygdalar activation in the traumatic condition is unclear. However, compared with the subjects with PTSD studied by Rauch et al. (6), the subjects with PTSD in the current study had smaller increases in reported fear and greater increases in disgust, guilt, and anger during the traumatic condition. Activation in the amygdala may be more easily detected during conditions evoking fear rather than these other emotions. Indeed, the amygdala appears to play a central role in fear conditioning (10, 36) and in the processing of fear-related stimuli (37–39).
Contrary to predictions, regional CBF decreases occurred in visual cortex and related visual areas during the traumatic condition versus the neutral control conditions in both groups. This result differs from the findings of previous studies (6, 17). The subjects’ imagery ratings suggest that this finding cannot be accounted for by relatively less visual imagery in the traumatic condition than in the neutral control conditions. However, subjects in this study indicated that their mental imagery during the traumatic condition was most prominent in the tactile modality, whereas subjects in the study by Rauch et al. (6) indicated that their mental imagery was most prominent in the visual modality. Qualitatively different imagery experiences (i.e., tactile versus visual) may be related to different patterns of brain activation and may in part account for the discrepancy between the results of this study and those of Rauch et al. (6).
Previous PET studies (6, 7) have revealed regional CBF decreases in left inferior frontal cortex (Broca’s area) in individuals with PTSD. In the PTSD group of the current study, we found regional CBF decreases in a section of the left inferior frontal gyrus (pars triangularis, Brodmann area 45/46) that corresponds to the anterior portion of Broca’s area. The location of the left inferior frontal gyrus deactivations in the current study (x, y, and z coordinates=–43, 35, and 12) and in that of Rauch et al. (6) (x, y, and z coordinates=–43, 42, and 8) are highly similar. The location of the left inferior frontal gyrus deactivation reported in another previous study of PTSD (7) is more posterior (x, y, and z coordinates=–47, 5, and 16). In general, the left inferior frontal gyrus appears to be involved in speech production (40, 41), subvocal rehearsal (42), and inner speech (43). Although the interpretation of this deactivation remains difficult, regional CBF decreases in left inferior frontal gyrus are consistent with diminished linguistic processing during the recollection of traumatic events versus neutral events.
The finding of greater regional CBF decreases in anterior frontal regions across conditions in the PTSD group than in the comparison group was unexpected. In a study of memory in healthy individuals, some of these frontal regions (Brodmann areas 9 and 10) were activated during conditions that required effortful recall (44). The deactivation of these structures in the current study is consistent with the relatively effortless recollection of traumatic events versus neutral events in the PTSD group. This post hoc explanation is consistent with the “automatic” nature of intrusive recollections.
An important limitation of this study is the relatively small number of subjects tested. Small study groups are common in neuroimaging research, but they may increase the probability of type II errors (i.e., false negatives) (45). The lack of activations in insular cortex and anterior cingulate gyrus in the PTSD group may have reflected such errors. Thus, because of the relatively small size of the study group, the results of this study should be considered preliminary. Another limitation is the presence of current comorbid disorders in some of the subjects with PTSD. Unfortunately, comorbidity is exceedingly common in individuals with PTSD (46, 47), and whether the presence of comorbidity affected the results is unknown. Comorbidity may be less likely to affect the results of PET studies involving within-subject comparisons of different conditions; however, the presence of some comorbid disorders (e.g., depression) in the PTSD group may have differentially affected the blood flow responses to traumatic scripts or may have reduced the range of possible blood flow changes between conditions. Another limitation of this study was our relatively low degree of control over the cognitive task that subjects performed in the PET scanner. The script-driven imagery paradigm does not yield behavioral data (i.e., response times or error rates) that might help to assess subjects’ compliance with task instructions; however, psychophysiologic data suggest that the subjects were indeed performing the task as instructed. There is no convincing way to determine whether the PTSD and comparison groups complied with task instructions to a similar extent. One could argue that the study was further limited by our use of personalized, rather than standardized, stimuli. However, previous research suggests that psychophysiologic responses are more robust with the use of personalized scripts than with standardized scripts (2); hence, we used the former. We did not match the groups on phase of the menstrual cycle, and we do not know whether this affected between-group differences. Limitations common to neuroimaging studies in general also apply; for example, errors in precise neuroanatomical localization can arise from constraints set by the spatial resolution of PET, head movement, or stereotaxic transformation. In addition, normalizing whole-brain blood flow prevented us from detecting any changes in absolute blood flow between conditions. Whole-brain blood flow may have changed from one condition to another, and the normalization process could have resulted in some artifactual changes in regional CBF. We do not know whether the normalization process yielded any such artifacts.
In summary, in the traumatic condition versus the neutral control conditions, both groups exhibited regional CBF increases in orbitofrontal cortex and anterior temporal poles; however, these increases were greater in the PTSD group than in the comparison group. The comparison group exhibited regional CBF increases in insular cortex and anterior cingulate gyrus; increases in anterior cingulate gyrus were greater in the comparison group than in the PTSD group. Regional CBF decreases in bilateral anterior frontal regions were greater in the PTSD group than in the comparison group, and only the PTSD group exhibited regional CBF decreases in left inferior frontal gyrus. The results suggest that the recollection and imagery of traumatic events is accompanied by increased regional CBF in anterior paralimbic regions of the brain in trauma-exposed individuals with and without PTSD; however, the two groups show different patterns of brain activation among these paralimbic regions.
Preliminary results of this study were presented at the New York Academy of Sciences conference on the psychobiology of posttraumatic stress disorder, New York, September 1996. Received March 24, 1998; revision received Sept. 23, 1998; accepted Sept. 29, 1998. From the Department of Psychiatry, the Department of Radiology, and the Department of Neurology, Massachusetts General Hospital, Boston; the Department of Psychology, Tufts University, Medford, Mass.; the Department of Psychology, Harvard University, Cambridge, Mass.; the VA Research Service, Manchester, N.H.; and Harvard Medical School, Boston. Address reprint requests to Dr. Shin, Psychiatric Neuroscience Program, Massachusetts General Hospital–East, Bldg. 149, Room 9147, 13th Street, Charlestown, MA 02129; [email protected] (e-mail). Supported in part by grant N00014-93-1-0720 from the Office of Naval Research (Dr. Kosslyn), grant MH-48559 from NIMH (Dr. Pitman), and a Sackler Scholar Award (Dr. Shin). Dr. Shin was also supported by fellowships from the National Science Foundation and Harvard University (Eliot Fellowship). Dr. Rauch was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, grant MH-01215 from NIMH, and the Pfizer-sponsored Harvard-MIT Clinician Investigator Training Program. The authors thank Avis Loring, Steve Weise, and Sandra Barrow for their technical assistance; Paul Whalen and Darin Dougherty for their comments on an earlier version of this manuscript; and all of the women who participated in this study.
 |
 |
 |
 |
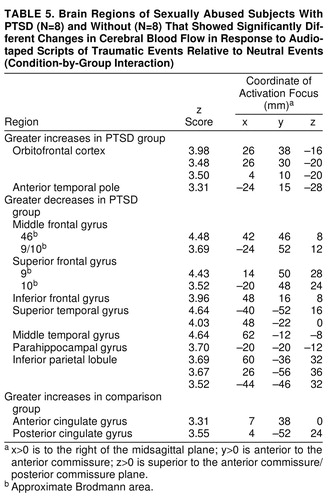 |
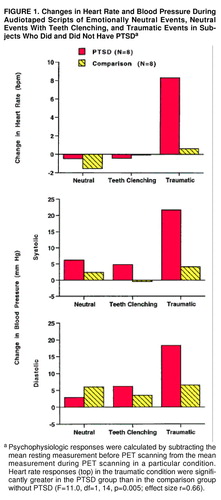
FIGURE 1. Changes in Heart Rate and Blood Pressure During Audiotaped Scripts of Emotionally Neutral Events, Neutral Events With Teeth Clenching, and Traumatic Events in Subjects Who Did and Did Not Have PTSDa
aPsychophysiologic responses were calculated by subtracting the mean resting measurement before PET scanning from the mean measurement during PET scanning in a particular condition. Heart rate responses (top) in the traumatic condition were significantly greater in the PTSD group than in the comparison group without PTSD (F=11.0, df=1, 14, p=0.005; effect size r=0.66).
1. Orr SP, Lasko NB, Metzger LJ, Ahern CE, Berry NJ, Pitman RK: Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J Consult Clin Psychol 1998; 66:906–913Crossref, Medline, Google Scholar
2. Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM: Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry 1987; 44:970–975Crossref, Medline, Google Scholar
3. Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR: Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol 1990; 99:49–54Crossref, Medline, Google Scholar
4. Lang PJ: The cognitive psychophysiology of emotion: fear and anxiety, in Anxiety and the Anxiety Disorders. Edited by Tuma AH, Maser J. Hillsdale, NJ, Lawrence Erlbaum Associates, 1985, pp 131–170Google Scholar
5. Lang PJ, Levin DN, Miller GA, Kozak MJ: Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol 1983; 92:276–306Crossref, Medline, Google Scholar
6. Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK: A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996; 53:380–387Crossref, Medline, Google Scholar
7. Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK: Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry 1997; 54:233–241Crossref, Medline, Google Scholar
8. Fischer H, Wik G, Fredrikson M: Functional neuroanatomy of robbery re-experience: affective memories studied with PET. Neuroreport 1996; 7:2081–2086Crossref, Medline, Google Scholar
9. Kaada BR, Pribram KH, Epstein JA: Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus. J Neurophysiol 1949; 12:347–356Crossref, Medline, Google Scholar
10. LeDoux JE: Emotion and the amygdala, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 339–351Google Scholar
11. Wall PD, Davis GD: Three cerebral cortical systems affecting autonomic function. J Neurophysiol 1951; 14:507–517Crossref, Medline, Google Scholar
12. George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Link, Google Scholar
13. George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM: Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol Psychiatry 1996; 40:859–871Crossref, Medline, Google Scholar
14. Pardo JV, Pardo PJ, Raichle ME: Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150:713–719Link, Google Scholar
15. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ: Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 1997; 154:926–933Link, Google Scholar
16. Kosslyn SM, Alpert NM, Thompson WL, Maljkovic V, Weise SB, Chabris C, Hamilton SE, Rauch SL, Buonanno FS: Visual mental imagery activates topographically organized visual cortex: PET investigations. J Cognitive Neuroscience 1993; 5:263–287Crossref, Medline, Google Scholar
17. Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM: Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport 1996; 7:1569–1576Crossref, Medline, Google Scholar
18. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
19. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzev G, Keane TM: Rating scale for assessing current and lifetime PTSD: the CAPS-I. Behavior Therapist 1990; 13:187–188Google Scholar
20. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM: The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8:75–80Crossref, Medline, Google Scholar
21. Spitzer RL, Williams JBW, Gibbon M, First MB: User’s Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
22. Beck AT, Steer RA: Manual for the Revised Beck Depression Inventory. San Antonio, Tex, Psychological Corp, 1987Google Scholar
23. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
24. Marks DF: Individual differences in the vividness of visual imagery and their effect on function, in The Function and Nature of Imagery. Edited by Sheehan WP. New York, Academic Press, 1972, pp 83–108Google Scholar
25. Marks DF: Visual imagery differences in the recall of pictures. Br J Psychol 1973; 64:17–24Crossref, Medline, Google Scholar
26. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
27. Friston KJ, Frith CD, Liddle PF, Frakowiak RSJ: Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991; 11:690–699Crossref, Medline, Google Scholar
28. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
29. Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, Willis MW, Herscovitch P, Post RM: Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 1996; 53:59–69Crossref, Medline, Google Scholar
30. Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Watkins GL, Boles Ponto LL, Hichwa RD: Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 1997; 154:384–389Link, Google Scholar
31. Cottraux J, Gérard D, Cinotti L, Froment J-C, Deiber M-P, Le Bars D, Galy G, Millet P, Labbé C, Lavenne F, Bouvard M, Mauguiere F: A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res 1996; 60:101–112Crossref, Medline, Google Scholar
32. Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA: A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry 1995; 52:20–28Crossref, Medline, Google Scholar
33. Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, Gjedde A, Evans A: Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry 1995; 152:1180–1184Link, Google Scholar
34. Drevets WC, Videen TO, MacLeod AK, Haller JW, Raichle ME: PET images of blood flow changes during anxiety: correction. Science 1992; 256:1696Crossref, Medline, Google Scholar
35. Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL: The Emotional Counting Stroop Paradigm: a fMRI probe of the anterior cingulate affective division. Biol Psychiatry 1998; 44:1219–1228Crossref, Medline, Google Scholar
36. Davis M: The role of the amygdala in conditioned fear, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 255–306Google Scholar
37. Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ: A differential response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383:812–815Crossref, Medline, Google Scholar
38. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17:875–887Crossref, Medline, Google Scholar
39. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA: Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18:411–418Crossref, Medline, Google Scholar
40. Kertesz A, Lesk D, McCabe P: Isotope localization of infarcts in aphasia. Arch Neurol 1977; 34:590–601Crossref, Medline, Google Scholar
41 Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME: Positron emission tomographic studies of the processing of single words. J Cognitive Neuroscience 1989; 1:153–170Crossref, Medline, Google Scholar
42. Paulesu E, Frith CD, Frackowiak RSJ: The neural correlates of the verbal component of working memory. Nature 1993; 362:342–345Crossref, Medline, Google Scholar
43. McGuire PK, Silbersweig DA, Murray RM, David AS, Frackowiak RSJ, Frith CD: Functional anatomy of inner speech and auditory verbal imagery. Psychol Med 1996; 26:29–38Crossref, Medline, Google Scholar
44. Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS: Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA 1996; 93:321–325Crossref, Medline, Google Scholar
45. Andreasen NC, Arndt S, Cizadlo T, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Sample size and statistical power in [15O]H2O studies of human cognition. J Cereb Blood Flow 1996; 16:804–816Crossref, Medline, Google Scholar
46. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
47. Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS: Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, Brunner/Mazel, 1990Google Scholar



