Association Between Brain Functional Failure and Dementia Severity in Alzheimer’s Disease: Resting Versus Stimulation PET Study
Abstract
OBJECTIVE: This study tested the hypothesis that regional cerebral glucose metabolism during neuronal activation is a more sensitive index of neuronal dysfunction and clinical severity in Alzheimer’s disease than is glucose metabolism at rest. METHOD: The subjects were 15 Alzheimer’s disease patients with a wide range of Mattis Dementia Rating Scale scores (23–128). By using positron emission tomography, absolute glucose metabolism was measured in the parietal, occipital (visual areas), and temporal (auditory areas) cortical regions during rest (eyes/ears covered) and audiovisual stimulation. RESULTS: In the parietal cortex, glucose metabolism correlated with dementia severity in both conditions. In contrast, in the relatively preserved visual and auditory cortical regions, glucose metabolism predicted dementia severity during stimulation but not at rest. CONCLUSIONS: These findings suggest that regional cerebral glucose metabolism during stimulation is a more sensitive index of the functional/metabolic failure of neuronal systems than is metabolism at rest. (Am J Psychiatry 1999; 156:470–473)
Regional cerebral glucose metabolism, measured by using positron emission tomography (PET) with [18F]fluorodeoxyglucose (FDG), is a reliable index of neuronal/synaptic activity (1, 2). Patients with Alzheimer’s disease show heterogeneous low glucose metabolic rates at rest (eyes and ears covered, no sensory stimulation), particularly in the parietal and temporal neocortical association areas (3), which worsen with dementia progression. In the preclinical and initial stages of Alzheimer’s disease, however, plastic compensatory mechanisms, including synaptic hypertrophy (4), may help to maintain relatively stable glucose metabolism at rest, suggesting that neurons can perform biochemical activities related to cell survival despite development of Alzheimer’s disease neuropathology (5, 6) up to a threshold where a dramatic disruption of brain functions is triggered (5). However, even before the decline of cognitive performance and regional glucose metabolism at rest begins, the accumulation of Alzheimer’s disease neuropathology should impair the metabolic capability of neurons to respond to functional activation (6, 7). In older Down’s syndrome subjects, who have a 100% risk of developing Alzheimer’s disease, increasing the functional demand on the brain through audiovisual stimulation revealed abnormalities in glucose metabolism before the onset of dementia and despite normal metabolism at rest (7).
In this study we measured regional cerebral glucose metabolism at rest and during passive audiovisual stimulation in a group of Alzheimer’s disease patients across a wide range of dementia severity. We hypothesized that, as a result of progressive neuronal dysfunction occurring in Alzheimer’s disease, regional cerebral glucose metabolism during neuronal activation would be a more reliable index of dementia severity than metabolism at rest. We focused our analysis on parietal neocortical association areas, which usually are the first and most affected regions in Alzheimer’s disease, and on the auditory and visual cortical areas, which, in contrast, are relatively spared by the disease process (3, 6, 8) and which have the greatest metabolic increases in response to audiovisual stimulation in older healthy comparison subjects (9). We expected that, despite the relative preservation of the latter areas, an audiovisual stimulation paradigm that maximizes metabolic demand would reveal a severity-related functional failure in these areas that is not evident in the resting state.
METHOD
The subjects were 15 otherwise healthy Alzheimer’s disease patients, nine men and six women, with a mean age of 70 years (SD=10), a mean education level of 15 years (SD=4), and a mean score on the Mattis Dementia Rating Scale (10) of 91 (SD=33, range=23–128). They all met the criteria of the National Institute of Neurological Disorders and Stroke and the Alzheimer Disease and Related Disorders Association for “probable” Alzheimer’s disease (11), and three subjects had histologically confirmed Alzheimer’s disease. They were given neuropsychological tests and PET scans (3). After complete description of the study, written informed consent was obtained from the patients or their legal guardians (according to protocol 93-AG-193 of the National Institute on Aging institutional review board).
Absolute values for regional cerebral glucose metabolism (in milligrams per 100 g of brain tissue per minute) were determined at rest (with eyes and ears covered) and during audiovisual stimulation by using a Scanditronix PC2048-15B PET scanner, with two sequential injections of FDG during the same PET session, as previously described (7). During the audiovisual stimulation condition, the patients were required to watch and listen to a colorful movie intended to elicit both sensory and cognitive activation. All of the subjects were presented with the same movie for identical periods of time and were constantly observed to ensure that they complied with the task requirements and did not fall asleep or engage in other cognitive activities(7).
Regional brain radioactivity levels were determined by using a template of circular regions of interest 8 mm in diameter (48 mm2), derived from a PET scan of a healthy normal subject. Regions of interest were spaced evenly throughout the cortical ribbon and then grouped into larger anatomical areas within the frontal, limbic, temporal, sensorimotor, parietal, and occipital cortices for a total of 29 regions (7).
Correlations between dementia severity (as measured by the Mattis Dementia Rating Scale) and cerebral glucose metabolism at rest and during audiovisual stimulation were tested by using regression analysis. Differences between correlation coefficients were assessed by means of t tests for dependent correlations (12). Statistical significance was taken at p<0.05. To reduce the number of overall statistical tests, we restricted the analysis to the parietal, visual, and auditory cortical regions.
RESULTS
Correlations between dementia severity and resting state glucose metabolism were found bilaterally in the inferior (right: r=0.69, df=13, p<0.004; left: r=0.71, df=13, p<0.003), medial (right: r=0.65, df=13, p<0.009; left: r=0.68, df=13, p<0.005), and superior (right: r=0.63, df=13, p<0.01; left: r=0.82, df=13, p<0.0002) parietal areas (figure 1). No significant correlation was found between dementia severity and resting metabolic rate in any primary or association visual cortical area or in the superior temporal auditory cortex.
During audiovisual stimulation, dementia severity correlated with glucose metabolism bilaterally in the inferior (right: r=0.75, df=13, p<0.001; left: r=0.68, df=13, p<0.006), medial (right: r=0.53, df=13, p<0.04; left: r=0.62, df=13, p<0.01), and superior (right: r=0.77, df=13, p<0.0007; left: r=0.73, df=13, p<0.002) parietal cortex. In contrast to the resting state, during audiovisual stimulation a significant correlation between dementia severity and metabolic rate also was found in the right (r=0.56, df=13, p<0.03) and left (r=0.61, df=13, p<0.02) visual association cortex and in the left calcarine (r=0.61, df=13, p<0.02) and in the right (r=0.54, df=13, p<0.04) and left (r=0.62, df=13, p<0.02) superior temporal auditory cortical areas (figure 1). There was no significant relation between age and either test condition for the regions examined.
Correlations with dementia severity were greater during stimulation than at rest for the left calcarine (t=4.6, df=12, p<0.01) and occipital association (t=3.3, df=12, p<0.01) cortical areas but did not differ significantly for other regions.
DISCUSSION
Glucose metabolism in parietal neocortical areas, which are the first and the most affected regions in Alzheimer’s disease (3, 7), correlated with dementia severity both at rest and during audiovisual stimulation. In contrast, metabolism in the visual and auditory regions, which remain relatively spared by the disease process until its later stages (3, 6, 8), was correlated with dementia severity during stimulation but not at rest. As regional cerebral glucose metabolic rate is an index of synaptic activity and increases with increasing neuronal firing and rate of stimulation (2, 13), these findings suggest that the capability of neurons to respond to stimulation is progressively compromised by Alzheimer’s disease (6). Thus, even in areas with less neuropathological involvement, such as the calcarine cortex, neuronal failure can be demonstrated by increasing the functional demand on the brain. In healthy subjects, stimulation conditions, as compared to resting, also are associated with less metabolic variability, potentially because of more organized brain activity during a specific task (14).
As the passive audiovisual stimulation in this study required minimal compliance and no task performance (7), we could study Alzheimer’s disease patients across a wide range of dementia severity. Furthermore, this stimulation was intended to elicit widespread activation of the visual and auditory cortical areas (9), in order to overcome potential compensatory mechanisms that occur in the plastic reorganization of the brain, at least in the mild to moderate stages of Alzheimer’s disease (4, 6, 15). Indeed, we recently demonstrated (6) that neuronal activation in response to patterns of flashing lights in mildly demented subjects is abnormal only at frequencies that elicit the greatest functional demand in healthy comparison subjects, but not with lower frequencies or at rest.
In summary, this study shows that the development of the Alzheimer’s disease neuropathology results in a limitation of the ability of neurons to respond to stimulation and that the degree of functional failure is correlated with the severity of the cognitive impairment. A PET-FDG method with sensory or cognitive stimulation can be used for the in vivo assessment of synaptic integrity and for detecting functional impairment in the preclinical stages of Alzheimer’s disease, aiding early diagnosis of at-risk subjects (6, 7). Moreover, given that most drugs currently used with Alzheimer’s disease patients act by enhancing cholinergic transmission and thus require viable synapses (4), this information may be useful in determining which patients are most likely to respond to treatment.
Presented in part at the 26th Annual Meeting of the Society for Neuroscience, Washington, D.C., Nov. 16–21, 1996. Received April 14, 1998; revision received Aug. 11, 1998; accepted Aug. 19, 1998. From the Laboratory of Neurosciences, National Institute on Aging; and the Department of Human and Environmental Sciences and the Department of Psychiatry, Neurobiology, Pharmacology, and Biotechnologies, University of Pisa, Pisa, Italy. Address reprint requests to Dr. Pietrini, Laboratory of Neurosciences, National Institute on Aging, NIH, Rm. 6C414, Bldg. 10, 9000 Rockville Pike, Bethesda, MD 20892; [email protected] (e-mail). Supported by the National Institute on Aging/NIH Intramural Program. Dr. Dani was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression. Dr. Guazzelli was partially supported by the Italian Minister of University and Scientific and Technological Research and the National Research Council of Italy. The authors thank Dr. P. Herscovitch for organization of the NIH PET program, P. Baldwin and the other PET technologists, and Prof. P. Nichelli for reviewing this manuscript.
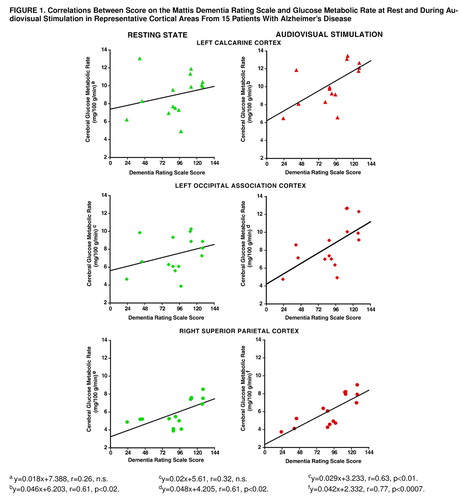
FIGURE 1. Correlations Between Score on the Mattis Dementia Rating Scale and Glucose Metabolic Rate at Rest and During Audiovisual Stimulation in Representative Cortical Areas From 15 Patients With Alzheimer’s Disease
y=0.018x+7.388, r=0.26, n.s.
y=0.02x+5.61, r=0.32, n.s.
y=0.029x+3.233, r=0.63, p<0.01.
y=0.046x+6.203, r=0.61, p<0.02.
y=0.048x+4.205, r=0.61, p<0.02.
y=0.042x+2.332, r=0.77, p<0.0007.
1. Jeuptner M, Weiller C: Does measurement of regional cerebral blood flow reflect synaptic activity? implications for PET and fMRI. Neuroimage 1995; 2:148–156Crossref, Medline, Google Scholar
2. Sokoloff L: Relationships among local functional activity, energy metabolism and blood flow in the central nervous system. Fed Proc 1981; 40:2311–2316Medline, Google Scholar
3. Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, Moore A, Schlageter N, Larson S, Rapoport SI: Positron emission tomography in Alzheimer’s disease. Neurology 1986; 36:879–887Crossref, Medline, Google Scholar
4. DeKosky ST, Scheff SW: Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990; 27:457–464Crossref, Medline, Google Scholar
5. Dani A, Pietrini P, Furey M, McIntosh AR, Grady CL, Horwitz B, Freo U, Alexander GE, Schapiro MB: Brain cognition and metabolism in Down syndrome adults in association with development of dementia. Neuroreport 1996; 7:2933–2936Crossref, Medline, Google Scholar
6. Mentis MJ, Alexander GE, Krasuski J, Pietrini P, Furey ML, Schapiro MB, Rapoport SI: Increasing required neural response to expose abnormal brain function in mild versus moderate or severe Alzheimer’s disease: PET study using parametric visual stimulation. Am J Psychiatry 1998; 155:785–794Abstract, Google Scholar
7. Pietrini P, Dani A, Furey ML, Alexander GE, Freo U, Grady CL, Mentis MJ, Mangot D, Simon EW, Horwitz B, Haxby JV, Schapiro MB: Low glucose metabolism during brain stimulation in older Down’s syndrome subjects at risk for Alzheimer’s disease prior to dementia. Am J Psychiatry 1997; 154:1063–1069Link, Google Scholar
8. Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW: The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients in Alzheimer’s disease. Cereb Cortex 1991; 1:103–116Crossref, Medline, Google Scholar
9. Pietrini P, Horwitz B, Grady CL, Maisog J, Gonzales-Aviles A, De Micheli E, Rapoport SI, Schapiro MB: Regional cerebral blood flow and glucose metabolism at rest and during activation in healthy human aging assessed by positron emission tomography. Society for Neuroscience Abstracts 1992; 18:1266Google Scholar
10. Mattis S: Mental status examination for organic mental syndrome in the elderly patient, in Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. Edited by Belack L, Karasu TB. New York, Grune & Stratton, 1976, pp 77–121Google Scholar
11. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
12. Cohen J, Cohen P: Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1983, pp 56–57Google Scholar
13. Mata M, Fink DJ, Gainer H, Smith CB, Davidsen L, Savaki H, Schwartz WJ, Sokoloff L: Activity-dependent energy metabolism in rat posterior pituitary reflects sodium pump activity. J Neurochem 1980; 34:213–215Crossref, Medline, Google Scholar
14. Duara R, Gross-Gleen K, Barker WW, Chang JY, Apicella A, Loewenstein D, Boothe T: Behavioral activation and the variability of cerebral glucose metabolic measurements. J Cereb Blood Flow Metab 1987; 7:266–271Crossref, Medline, Google Scholar
15. Rapoport SI, Hatanp�� K, Brady DR, Chandrasekaran K: Brain energy metabolism, cognitive function and down-regulated oxidative phosphorylation in Alzheimer disease. Neurodegeneration 1996; 5:473–476Crossref, Medline, Google Scholar



