Caffeine Intake, Tolerance, and Withdrawal in Women: A Population-Based Twin Study
Abstract
OBJECTIVE: Caffeine is by far the most commonly consumed psychoactive substance. The use and abuse of most other licit and illicit psychoactive drugs have been shown to be substantially heritable. However, the impact of genetic factors on caffeine consumption, heavy use, intoxication, tolerance, and withdrawal is largely unknown. METHOD: Caffeine consumption, in the form of brewed coffee, instant coffee, tea, and caffeinated soft drinks, as well as caffeine intoxication, tolerance, and withdrawal, were assessed by personal interviews of 1,934 individual twins from female-female pairs ascertained from the population-based Virginia Twin Registry. The sample included both members of 486 monozygotic and 335 dizygotic pairs. Twin resemblance was assessed by probandwise concordance, odds ratios, and tetrachoric correlations. Biometrical model fitting was also performed. RESULTS: The resemblance in twin pairs for total caffeine consumption, heavy caffeine use, caffeine intoxication, caffeine tolerance, and caffeine withdrawal was substantially greater in monozygotic than in dizygotic twin pairs. Model fitting suggested that twin resemblance for these measures could be ascribed solely to genetic factors, with estimated broad heritabilities of between 35% and 77%. CONCLUSIONS: Caffeine is an addictive psychoactive substance. Similar to previous findings with other licit and illicit psychoactive drugs, individual differences in caffeine use, intoxication, tolerance, and withdrawal are substantially influenced by genetic factors.
“How sweet the coffee tastes, more delicious than a thousand kisses . . . . Coffee, I must have coffee . . . . If I don’t get my three cups of coffee each day, I’ll shrivel up like a piece of roast goat.”
— Lieschen in J.S. Bach’s Coffee Cantata, written in 1732 (1)
Caffeine is by far the most commonly used psychoactive substance, consumed daily by approximately 80% of the world’s population (2). While the adverse health consequences of caffeine consumption may be small, given widespread use, even minimal risk could produce substantial public health effects (2). While caffeine currently has a benign public image, as appreciated by the fictional young lady Lieschen in the early 1700s, caffeine use is influenced by the reinforcing properties of taste, hedonic psychoactive effects, and the desire to avoid withdrawal (2–5). There are wide interindividual variations in preference for caffeine and in sensitivity to withdrawal (6, 7). What is responsible for these differences?
Given the importance of genetic factors in use and dependence of other psychoactive drugs (8–13), genetic variation might also influence individual differences in caffeine use, tolerance, and dependence. While several twin studies have found heritable influences on coffee consumption (e.g., references 14–18), we are unaware of studies in genetically informative populations that have assessed total caffeine consumption or symptoms of caffeine toxicity, tolerance, or withdrawal.
METHOD
Sample and Interviewers
Subjects in this study were from Caucasian, female, same-sex twin pairs from the Virginia Twin Registry (19)—a population-based register formed from a systematic review of birth certificates in the Commonwealth of Virginia. Twins were initially ascertained through mailed surveys to female twin pairs in the registry, the response to which was approximately 64%. The true cooperation rate was higher because such large-scale mailings contain a significant percentage of questionnaires that do not reach the intended recipient. Twins were then interviewed face to face, at which time our refusal rate was approximately 8%. In the current phase of the project, 2,288 members of female-female pairs from the Virginia Twin Registry were eligible, on the basis of prior cooperation with at least one interview, to participate in a structured telephone interview. Of these twins, 1,937 were successfully interviewed in 1995–1997, three had died, 33 were lost to follow-up, one was too medically ill to be interviewed, three had incomplete interviews, 58 neither refused participation nor completed an interview by the end of the study, and 253 refused. Thus, we succeeded in interviewing 84.7% of the entire sample and 86.2% of the eligible sample. Zygosity was determined blindly by standard questions (20), photographs, and, when necessary, DNA (19, 21). All interviews were conducted blind to information about the co-twin. The mean age at interview was slightly greater in monozygotic (36.1, SD=8.0) than in dizygotic (35.4, SD=8.6) twins (t=1.89, df=1,931, p=0.06).
This project was approved by the Committee for the Conduct of Human Research at Virginia Commonwealth University. Written informed consent was obtained before face-to-face interviews and verbal assent before phone interviews. Clinically experienced interviewers were initially trained for 40 hours and received regularly scheduled review sessions over the course of the study.
Caffeine use was assessed for the year before interview and for the time of maximum caffeine consumption. For each time period, we assessed the frequency of caffeine consumption. For days in which the twins consumed caffeine, we asked separately for the average daily consumption of cups of caffeinated coffee, cups of caffeinated tea, and servings of caffeinated soda. If they reported drinking coffee, we inquired whether the coffee was usually brewed or instant. Our calculation of caffeine consumption used the following estimates of caffeine content: brewed coffee, 125 mg/cup, instant coffee, 90 mg/cup, tea, 60 mg/cup, and caffeinated soft drinks, 40 mg/can (2). Caffeine consumption per month was estimated by multiplying the average consumption per day by the average numbers of days per month in which caffeine was consumed. We report last-year rather than maximum lifetime caffeine use because of the higher reliability of the former measure.
Caffeine toxicity was assessed by the item: “During the time when you consumed caffeinated beverages the most, did you ever feel ill or shaky or jittery after drinking caffeinated beverages?” Caffeine tolerance was assessed by the response to the following two questions adapted from the Psychoactive Substance Abuse Section of the Structured Clinical Interview for DSM-III-R—Patient Version (22): “During the time when you were consuming caffeinated beverages the most, did you find that you needed to drink a lot more to get the desired effect than you did when you first drank them?” and “What about finding out that when you drank the same amount, it had much less effect than before?” A positive response to either question was assumed to reflect tolerance.
All individuals who responded positively to the question, “…did you ever stop or try to cut down on your consumption of caffeinated beverages?” were asked about the four DSM-IV symptoms of caffeine withdrawal: headaches, marked fatigue or drowsiness, marked anxiety or depression, and nausea or vomiting. We defined caffeine withdrawal following DSM-IV criteria as the presence of headache and at least one of the other three additional symptoms.
We defined “heavy use” as daily or near-daily consumption of 625 mg or more of caffeine. The distribution of milligrams of caffeine per month was substantially skewed and was, therefore, log-transformed before analysis.
To test the validity of the equal environment assumption—that monozygotic and dizygotic twins are equally correlated for exposure to relevant environmental risk factors—we assessed twin similarity for three kinds of environments: childhood (how often the twins shared the same room at home and class at school and were dressed alike), adolescence (how often they had the same friends, were in the same social group, and went together to movies and dances), and adulthood (how often, in the last year, they were in contact with one another).
Twin studies provide a method for detecting cooperation bias. If heavy caffeine use both is correlated in twin pairs and predicts noncooperation, then the rates of heavy caffeine use in a twin could be predicted from the interview status (cooperated versus refused) of the co-twin.
Presentation of Results From Twin Studies
We report twin resemblance in three different ways, which we will illustrate for heavy caffeine use. Probandwise concordance is defined as the proportion of co-twins of heavy-consuming proband twins who themselves are heavy users. The odds ratio reflects the increase in risk for heavy caffeine use in co-twins of twins with heavy use relative to that found in co-twins of twins without heavy use. Odds ratios and their 95% confidence intervals were obtained from the logistic regression procedure in SAS (23). The significance of the difference in odds ratios was determined by the Breslow-Day test (24). The tetrachoric correlation, or “correlation of liability” (25, 26), assumes that underlying the observed division of twins into those with and without heavy caffeine use, there exists a latent distribution that reflects their underlying “liability.” We assume a threshold exists on this liability distribution such that individuals with a liability above the threshold develop heavy caffeine use, while those with a liability below the threshold do not. The tetrachoric correlation represents the correlation in twins for this underlying liability. This model further assumes that caffeine use, heavy use, intoxication, tolerance, and dependence have a multifactorial etiology involving a number of genetic and environment risk factors of small to moderate effect (27).
On the basis of this liability-threshold model, we performed biometrical modeling fitting (for further details, see references 28 and 29). We assumed that twin resemblance resulted from three possible sets of latent factors: 1) additive genes (A), which reflect the additive effect of alleles at susceptibility loci and contribute twice as much to the correlation in monozygotic as in dizygotic twins (because monozygotic twins share all their genes identical by descent, while dizygotic twins, like nontwin siblings, share on average half of their genes); 2) dominance genes (D), which reflect the nonadditive interaction between alleles at the same locus and contribute four times as much to the correlation in monozygotic as in dizygotic twins (because, at any given locus, monozygotic twins always share both alleles identical by descent, while dizygotic twins share them both one time in four); and 3) family or “common” environment (C), which contributes equally to the correlation in monozygotic and dizygotic twins. In addition to “common” environment (those environmental factors that make members of a twin pair similar for liability to heavy caffeine use), the model also contains individual-specific environment (E), which, in addition to measurement error, is a measure of the impact of those environmental experiences that make members of a twin pair different for liability to heavy caffeine use.
The formal analysis of our twin data began with fitting an ACE model. This model, and all other models here reported, were fitted directly to contingency tables through use of the program Mx (30) by weighted least squares. (The exception to this was for caffeine consumption—where models were fitted directly to variance-covariance matrices.) The ACE model includes additive genes (A), common environment (C), and individual-specific environment (E). We then fit three additional models. The ADE model contains dominance genetic variance (D) instead of common environment. This model assumes that all twin resemblance results from genetic factors, some of which are additive in effect and others of which are interactive. Because the estimates of A and D in the ADE model are highly negatively correlated in twin studies (28), we focus on broadly defined heritability that equals a2 + d2. The AE model contains only additive genes (A) and individual-specific environment (E) and assumes that all familial aggregation results from additive genetic effects. The CE model, which contains only common environment (C) and individual-specific environment (E), assumes that all familial aggregation is due to the effects of family or “common” environment. For a familial trait, the presence of genetic risk factors is directly tested by the attempt to reject the CE model, which assumes that all observed familial aggregation is the result of shared environmental influences.
The goal in model fitting is to explain the observed data as well with as few parameters as possible. We operationalize this goal with the use of Akaike’s information criterion (31, 32), which equals the chi-square value of the model minus twice the degrees of freedom. The model with the lowest value of Akaike’s information criterion reflects the best balance of goodness of fit and parsimony. In addition, the fit of the CE or AE model can be directly compared with that of the ACE model by a chi-square difference test (df=1).
The final step in twin analysis is to estimate, on the basis of the best-fitting model, the proportion of variance in liability to caffeine use or misuse due to individual-specific environment (e2) and, depending upon the results of model fitting, additive gene action (a2), dominance gene action (d2), or common environment (c2). The proportion of variance in liability due to additive or dominance genetic effects or both is often termed “heritability.”
RESULTS
Complete data for caffeine were available for 1,934 individual twins, including both members of 486 monozygotic and 335 dizygotic pairs.
Reliability
A total of 192 twins were interviewed twice, by different interviewers, with a mean interval of 4.3 weeks (SD=1.5). The intraclass correlation for last-year monthly caffeine consumption was r=0.84. For the remaining dichotomous measures, reliabilities, evaluated by chance-corrected agreement (kappa) (33) and tetrachoric correlation (r), were as follows: heavy use, kappa=0.88, r=0.99; toxicity, kappa=0.60, r=0.82; tolerance, kappa=0.41, r=0.66; and withdrawal, kappa=0.65, r=0.85.
Tests for Potential Biases
The level of past-year caffeine consumption did not differ significantly in monozygotic and dizygotic twins (t=1.41, df=1, 640, p=0.16). While the frequency of heavy caffeine use was significantly greater in dizygotic than in monozygotic twins (χ2=4.2, df=1, p=0.04), no significant differences were seen in the rates of caffeine toxicity (χ2=1.5, df=1, p=0.22), tolerance (χ2=0.6, df=1, p=0.43), or withdrawal (χ2=0.0, df=1, p=0.96).
To test the equal environment assumption, we examined whether—when we controlled for zygosity—similarity of childhood, adolescent, and adult environments could predict twin resemblance for caffeine consumption, heavy caffeine use, and caffeine toxicity, tolerance, and dependence. Of these 15 analyses, one was statistically significant: increased current contact predicted greater twin resemblance for current caffeine consumption. However, this effect was inconsistent across zygosity groups. Within dizygotic twins, high-contact pairs were more highly correlated for caffeine consumption than were low-contact pairs (0.21 versus 0.07). By contrast, high-contact monozygotic pairs were less correlated in their level of caffeine use than were low-contact monozygotic pairs (0.41 versus 0.48).
No difference was seen in the level of caffeine use or in the frequency of heavy use, toxicity, tolerance, or withdrawal between twins whose co-twins were or were not interviewed in this study.
Caffeine Consumption
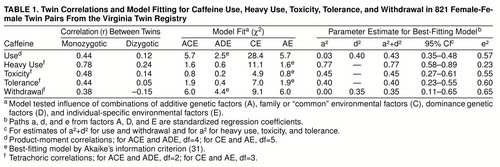
Pearson product-moment correlations for the log-transformed last-year daily caffeine consumption were 0.44 in monozygotic and 0.12 in dizygotic twin pairs. The best-fitting twin model was the ADE model (table 1). The CE model could be confidently rejected (versus the ACE model, χ2=22.7, df=1, p<0.0001). Broad heritability of caffeine consumption from the ADE model was estimated at 43%.
Heavy Caffeine Use and Caffeine Toxicity, Tolerance, and Withdrawal
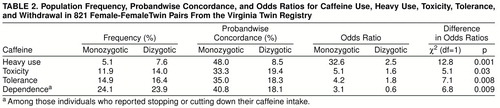
Table 2 depicts the frequency rates, probandwise concordance rates, and odds ratios for heavy caffeine use and caffeine toxicity, tolerance, and dependence in monozygotic and dizygotic twins. For all four traits, both concordance rates and odds ratios in monozygotic twins were substantially greater than those found in dizygotic twins.
For heavy caffeine use and caffeine toxicity, tolerance, and withdrawal, table 1 depicts both the estimated tetrachoric correlations in monozygotic and dizygotic twins and the results of model fitting. For three of the four traits, the CE model could be rejected by the chi-square difference test (versus the ACE model, all df=1): heavy use, χ2=9.5, p=0.002; toxicity, χ2=4.1, p=0.04; and tolerance, χ2=5.1, p=0.02. For withdrawal, the CE model fit considerably worse than the ACE model, but this difference fell short of significance (χ2=3.1, p=0.08). For heavy caffeine use, caffeine toxicity, and caffeine tolerance, the best balance of fit and parsimony (as indicated by the lowest Akaike information criterion score) was obtained by the AE model. For caffeine withdrawal, by contrast, the best Akaike information criterion score was obtained with the ADE model. While the broad heritabilities of caffeine toxicity, tolerance, and withdrawal were all similar, ranging from 35% to 45%, the heritability of heavy caffeine use, estimated at 77%, was substantially greater.
DISCUSSION
Genetic factors appear to substantially influence a woman’s vulnerability to caffeine use, heavy use, intoxication, tolerance, and withdrawal. With respect to the etiologic role of genetic factors, caffeine appears to be qualitatively similar to the two other common licit psychoactive drugs—nicotine (8, 34) and ethanol (11, 12)—as well as to the illicit psychoactive drugs such as cocaine and cannabis (13, 35). Our results are consistent with rodent studies demonstrating strain differences in behavioral responses to caffeine (36–38).
Our findings on caffeine consumption are consistent with previous twin studies that found the heritability of coffee consumption to range from 36% to 51% (15–18). In accord with the largest of these studies (17), we found no evidence that family environment contributes to twin resemblance for caffeine intake.
Since human twin studies are quasi-experimental designs of nature, the possibility of biases should always be considered. In particular, excess monozygotic over dizygotic similarity for caffeine use might result from closer environmental contact between monozygotic twins. We examined this hypothesis and found it supported by only one of 15 tests—a finding easily explained by chance (39). Furthermore, the observed effect—that similarity for current caffeine intake was related to current frequency of contact—was not consistent across zygosity groups. We also fitted a twin model that estimates heritability, correcting for the effect of environmental similarity (40). This model indicated no decline in the heritability of current caffeine use when the effect of current contact was incorporated. These results argue against a strong bias in our findings due to violations of the equal environment assumption. We also found no evidence that refusal to participate in our study was systematically related to caffeine intake or the history of caffeine toxicity, tolerance, or withdrawal.
These results should be interpreted in the context of eight potential methodologic limitations. First, the sample was entirely female, and our findings may not extrapolate to male subjects. Second, we did not assess all dietary sources of caffeine—omitting those from chocolate and over-the-counter medications. However, these sources account for less than 10% of caffeine consumption by U.S. adults (6). Third, the traits examined in this study had variable reliability. In standard twin studies, errors of measurement result in an overestimation of the individual-specific environment (e2) and an underestimation of heritability (a2) (29). For measures such as caffeine intake, these biases are likely to be modest. For others, such as caffeine tolerance, the moderate reliability may result in a substantial underestimation of heritability. Fourth, our data on caffeine intake and sequelae were collected by self-report. Prior research has found that self-reported caffeine intake correlates substantially with the level of caffeine and its metabolites in plasma (41) and saliva (42). In addition, consistent with prior investigators (43, 44), we found good test-retest reliability on our measures of caffeine intake and at least moderate reliability for our assessments of toxicity, tolerance, and withdrawal. Fifth, the actual amount of caffeine in tea, coffee, and soft drinks is variable and depends on many factors including brand and, for tea and coffee, method of brewing (45). Since it was not feasible to measure the caffeine content of beverages consumed in this population, our approximations undoubtedly introduced some error. Sixth, in our sample, significantly more dizygotic than monozygotic twins were heavy caffeine users. However, this difference was modest, and none of the other four caffeine-related phenotypes differed in frequency in the two zygosity groups. Seventh, since our analyses of caffeine intake ignored the impact of age and body weight on caffeine consumption, we repeated the analyses, correcting for these variables. The broad heritability of caffeine consumption declined from 43% to 40%. Eighth, the level of caffeine consumption was obtained by multiplying two measures reflecting quantity (milligrams per day) and frequency (days per month) of caffeine consumption. To test whether this composite measure obscured important differences in heritability, we performed twin analyses separately for quantity and frequency. For both measures, ADE was the best-fitting model, with broad heritability estimates that were a bit higher than those found for the composite measure: 50% (95% confidence interval=43%–55%) for quantity and 61% (95% confidence interval=56%–64%) for frequency. A bivariate twin analysis, which informs us about the causes of correlation between these measures, indicated that the same genetic factors influenced the quantity and frequency of caffeine consumption. These results suggest that it was not misleading to combine these two measures into a single index of overall caffeine use.
CONCLUSIONS
Genetic differences between individuals contribute substantially to differences in liability to heavy caffeine intake and to caffeine-related sequelae including intoxication, tolerance, and dependence. Genetic risk factors could be operating at many levels including personality (2), vulnerability to psychopathology (46), caffeine metabolism (47), and variation in the adenosine receptors that may mediate the psychoactive effects of caffeine (48, 49).
Our results have implications for the debate over the status of caffeine as a drug of abuse (2, 4). By demonstrating genetic influences on caffeine tolerance and withdrawal in man, our findings provide evidence in support of the validity of these phenomena.
Received Jan. 14, 1998; revisions received May 21 and July 17, 1998; accepted Aug. 18, 1998. From the Departments of Psychiatry and Human Genetics, Medical College of Virginia/Virginia Commonwealth University, and the Virginia Institute for Psychiatric and Behavioral Genetics, Richmond. Address reprint requests to Dr. Kendler, Box 980126, 800 East Leigh St., Suite 1-101, Rm. 122, Richmond, VA 23298-0126; [email protected] (e-mail). Supported by grants MH-40828 from NIMH and AA/DA-09095 from the National Institute on Alcohol Abuse and Alcoholism, with a supplement from the National Institute on Drug Abuse, and by Research Scientist Award MH-01277 to Dr. Kendler. The Virginia Twin Registry, established by W. Nance, M.D., Ph.D., and maintained by L. Corey, Ph.D., is supported by grant HD-26746 from the National Institute of Child Health and Human Development and by grant NS-31564 from the National Institute of Neurological and Communicative Disorders and Stroke. Debra Foley, Ph.D., aided in the development of the assessment instrument, and Charles Gardner, Ph.D., assisted in data analysis. The data were collected under the direction of L. Halberstadt, M.A., and B. Brooke, M.S.W.
 |
 |
1. Picander CFH: Schweigt stille, plaudert nicht (“Be silent, not a word”), Coffee Cantata, BWV 211; translated by Greenberg RM, in Bach and The High Baroque, Part III. Springfield, Va, Teaching Co, 1995, p 68Google Scholar
2. James JE: Understanding Caffeine: A Biobehavioral Analysis: Thousand Oaks, Calif, Sage Publications, 1997Google Scholar
3. Griffiths RR, Mumford GK: Caffeine—a drug of abuse? in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom FE, Kupfer DJ. New York, Raven Press, 1995, pp 1699–1713Google Scholar
4. Hughes JR, Oliveto AH, Helzer JE, Higgins ST, Bickel WK: Should caffeine abuse, dependence, or withdrawal be added to DSM-IV and ICD-10? Am J Psychiatry 1992; 149:33–40Google Scholar
5. Strain EC, Mumford GK, Silverman K, Griffiths RR: Caffeine dependence syndrome: evidence from case histories and experimental evaluations. JAMA 1994; 272:1043–1048Crossref, Medline, Google Scholar
6. Gilbert RM: Caffeine Consumption. New York, Alan R Liss, 1984Google Scholar
7. Hughes JR, Oliveto AH, Bickel WK, Higgins ST, Badger GJ: Caffeine self-administration and withdrawal: incidence, individual differences, and interrelationships. Drug Alcohol Depend 1993; 32:239–246Crossref, Medline, Google Scholar
8. Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC, Eaves LJ: Genetic contribution to risk of smoking initiation: comparisons across birth cohorts and across cultures. J Subst Abuse 1993; 5:221–246Crossref, Medline, Google Scholar
9. Jardine R, Martin NG: Causes of variation in drinking habits in a large twin sample. Acta Genet Med Gemellol 1984; 33:435–450Crossref, Medline, Google Scholar
10. Kaprio J, Koskenvuo M, Langinvainio H: Finnish twins reared apart, IV: smoking and drinking habits: a preliminary analysis of the effect of heredity and environment. Acta Genet Med Gemellol 1984; 33:425–433Crossref, Medline, Google Scholar
11. Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ: A population-based twin study of alcoholism in women. JAMA 1992; 268:1877–1882Crossref, Medline, Google Scholar
12. McGue M: Genes, environment and the etiology of alcoholism, in The Development of Alcohol Problems: Exploring the Biopsychosocial Matrix of Risk. Edited by Costello CG. Washington, DC, National Institute on Alcohol Abuse and Alcoholism, 1994, pp 1–40Google Scholar
13. Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Meyer JM, Eaves LJ: Genetic influences on abuse of illicit drugs: a study of 3,297 twin pairs. Am J Med Genet 1996; 67:473–477Crossref, Medline, Google Scholar
14. Conterio F, Chiarelli B: Study of the inheritance of some daily life habits. Heredity 1962; 17:347–359Crossref, Medline, Google Scholar
15. Kaprio J, Sarna S, Koskenvuo M, Rantasalo I: The Finnish Twin Registry: Baseline Characteristics, Section II. Helsinki, University of Helsinki Press, 1978Google Scholar
16. Partanen J, Bruun K, Markkanen T: Inheritance of Drinking Behavior. Helsinki, Finnish Foundation for Alcohol Studies, 1966Google Scholar
17. Carmelli D, Swan GE, Robinette D, Fabsitz RR: Heritability of substance use in the NAS-NRC twin registry. Acta Genet Med Gemellol 1990; 39:91–98Crossref, Medline, Google Scholar
18. Pedersen NL: Twin similarity for usage of common drugs, in Twin Research 3: Epidemiological and Clinical Studies, vol 69C. Edited by Gedda L, Parisi P, Nance WE. New York, Alan R Liss, 1981, pp 53–59Google Scholar
19. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A population-based twin study of major depression in women: the impact of varying definitions of illness. Arch Gen Psychiatry 1992; 49:257–266Crossref, Medline, Google Scholar
20. Eaves LJ, Eysenck HJ, Martin NG, Jardine R, Heath AC, Feingold L, Young PA, Kendler KS: Genes, Culture and Personality: An Empirical Approach. London, Oxford University Press, 1989Google Scholar
21. Spence JE, Corey LA, Nance WE, Marazita ML, Kendler KS, Schieken RM: Molecular analysis of twin zygosity using VNTR DNA probes (abstract). Am J Hum Genet 1988; 43:A159Google Scholar
22. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1987Google Scholar
23. SAS Language: Reference, Version 6, 1st ed. Cary, NC, SAS Institute, 1990Google Scholar
24. Breslow NE, Day NE: Statistical Methods in Cancer Research, vol 1. Lyon, France, International Agency for Research on Cancer, 1980Google Scholar
25. Pearson K: Mathematical contributions to the theory of evolution, VIII: on the correlation of characters not quantitatively measurable. Proc R Soc 1901; 66:241–244Crossref, Google Scholar
26. Falconer DS: The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet 1965; 29:51–76Crossref, Google Scholar
27. Kendler KS, Kidd KK: Recurrence risks in an oligogenic threshold model: the effect of alterations in allele frequency. Ann Hum Genet 1986; 50:83–91Crossref, Medline, Google Scholar
28. Neale MC, Cardon LR: Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands, Kluwer Academic, 1992Google Scholar
29. Kendler KS: Twin studies of psychiatric illness: current status and future directions. Arch Gen Psychiatry 1993; 50:905–915Crossref, Medline, Google Scholar
30. Neale MC: Mx: Statistical Modeling, 2nd ed. Richmond, Va, Commonwealth University of Virginia, Medical College of Virginia, Department of Psychiatry, 1994Google Scholar
31. Akaike H: Factor analysis and AIC. Psychometrika 1987; 52:317–332Crossref, Google Scholar
32. Williams LJ, Holahan PJ: Parsimony-based fit indices for multiple-indicator models: do they work? Structural Equation Modeling 1994; 1:161–189Google Scholar
33. Cohen J: A coefficient of agreement for nominal scales. Educational and Psychol Measurement 1960; 20:37–46Crossref, Google Scholar
34. Boomsma DI, Koopmans JR, Van Doornen LJP, Orlebeke JF: Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction 1994; 89:219–226Crossref, Medline, Google Scholar
35. Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA: Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry 1995; 52:42–52Crossref, Medline, Google Scholar
36. Castellano C: Effects of pre- and post-trial caffeine administrations on simultaneous visual discrimination in three inbred strains of mice. Psychopharmacology (Berl) 1977; 51:255–258Crossref, Medline, Google Scholar
37. Satinder KP: Genotype-dependent effects of d-amphetamine sulphate and caffeine on escape-avoidance behavior of rats. J Comp Physiol Psychol 1971; 76:359–364Crossref, Medline, Google Scholar
38. Seale TW, Roderick TH, Johnson P, Logan L: Complex genetic determinants of susceptibility to methylxantine-induced locomotor activity changes. Pharmacol Biochem Behav 1986; 24:1333–1341Crossref, Medline, Google Scholar
39. Feild HS, Armenakis AA: On use of multiple tests of significance in psychological research. Psychol Rep 1974; 35:427–431Crossref, Google Scholar
40. Hettema JM, Neale MC, Kendler KS: Physical similarity and the equal-environment assumption in twin studies of psychiatric disorders. Behav Genet 1995; 25:327–335Crossref, Medline, Google Scholar
41. James JE, Paull I, Cameron-Traub E, Miners JO, Lelo A, Birkett DJ: Biochemical validation of self-reported caffeine consumption during caffeine fading. J Behav Med 1988; 10:15–30Crossref, Google Scholar
42. James JE, Bruce MS, Lader MH, Scott NR: Self-report reliability and symptomatology of habitual caffeine consumption. Br J Clin Pharmacol 1989; 27:507–514Crossref, Medline, Google Scholar
43. Methodological Issues in Assessment of Caffeine Intake: A Method for Quantifying Consumption and a Test-Retest Reliability Study. New York, Raven Press, 1981Google Scholar
44. Schreiber GB, Maffeo CE, Robins M, Masters MN, Bond AP: Measurement of coffee and caffeine intake: implications for epidemiologic research. Prev Med 1988; 17:280–294Crossref, Medline, Google Scholar
45. Stavric B, Klassen R, Watkinson B, Karpinski K, Stapley R: Variability in caffeine consumption from coffee and tea: possible significance for epidemiological studies. Food Chem Toxicol 1988; 26:111–118Crossref, Medline, Google Scholar
46. Leibenluft E, Fiero PL, Bartko JJ, Moul DE, Rosenthal NE: Depressive symptoms and the self-reported use of alcohol, caffeine, and carbohydrates in normal volunteers and four groups of psychiatric outpatients. Am J Psychiatry 1993; 150:294–301Link, Google Scholar
47. Cascorbi I, Drakoulis N, Brockmoller J, Maurer A, Sperling K, Roots I: Arylamine N-acetyltransferase (NAT2) mutations and their allelic linkage in unrelated Caucasian individuals: correlation with phenotypic activity. Am J Hum Genet 1995; 57:581–592Crossref, Medline, Google Scholar
48. Porkka-Heiskanen T, Strecker RE, Thakkar M, Bj�rkum AA, Greene RW, McCarley RW: Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997; 276:1265–1268Crossref, Medline, Google Scholar
49. Ledent C, Vaugeois J-M, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen J-J, Costenin J, Heath JK, Vassart G, Parmentier M: Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997; 388:674–678Crossref, Medline, Google Scholar



