Rostral and Orbital Prefrontal Cortex Dysfunction in the Manic State of Bipolar Disorder
Abstract
OBJECTIVE: This study investigated prefrontal cortex function in the manic state of bipolar disorder. METHOD: High-sensitivity [15O]H2O positron emission tomography and a word generation activation paradigm were used to study regional cerebral blood flow in five manic and six euthymic individuals with bipolar disorder and in five healthy individuals. RESULTS: Decreased right rostral and orbital prefrontal cortex activation during word generation and decreased orbitofrontal activity during rest were associated with mania. CONCLUSIONS: The data support the presence of rostral and orbital prefrontal dysfunction in primary mania. These findings, when seen in the context of the human brain lesion and the behavioral neuroanatomic literatures, may help to explain some of the neurobehavioral abnormalities characteristic of the manic state.
Behaviors similar to those in bipolar disorder have been associated with prefrontal cortex lesions. Depressive symptoms are more often associated with left prefrontal lesions, and manic symptoms more frequently with right prefrontal lesions (1). Previous functional neuroimaging studies of mania, performed while subjects were in resting states, reported decreased anterior-posterior cortical gradients, decreased prefrontal and basotemporal activity, and increased ventral anterior cingulate activity (1–8). The current study is, to our knowledge, the first to use a high-sensitivity [15O]H2O positron emission tomography (PET) technique (9), in combination with a reliable neuropsychological probe of prefrontal function (10), to measure regional cerebral blood flow (rCBF) as an index of local neuronal activity (11) in mania.
METHOD
We studied 11 subjects with bipolar I disorder, without comorbid axis I or II disorders (except for substance abuse in remission for at least 5 years): five manic subjects (four women, one man; mean age=34.2 years, SD=12.2) and six euthymic subjects (four women, two men; mean age=32.5 years, SD=11.0). Five nonpatient subjects (four women, one man; mean age=30.0 years, SD=6.7) were also studied. Diagnoses were established by the Structured Clinical Interview for DSM-IV Axis I and II Disorders (12). Subjects were right-handed, spoke English as a first language, and were without neurologic or medical illness. Subjects denied family history of psychotic or anxiety disorder, and nonpatients also denied family history of mood disorder. Patient medication regimens included mood stabilizers, benzodiazepines, typical and atypical antipsychotics, antidepressants, thyroid hormone, and antihistamine. A full spectrum of manic symptoms were represented in the manic population. Mean mania scores (13) were as follows: for the manic group, 21.8 (SD=6.7); the euthymic group, 2.5 (SD=4.5); and the nonpatient group, 0.2 (SD=0.4). After complete description of the study to the subjects, written informed consent was obtained.
Four scans were performed in each of three conditions (order counterbalanced): activation—the generation of single words beginning with letters presented binaurally at a rate of one every 5 seconds, control—repetition of letters presented at the same rate, and rest. All scans were performed with the subjects’ eyes closed, lights dimmed, and low noise levels. “Word generation” connotes the subtraction of the activation and control conditions.
A General Electric ADVANCE positron emission tomograph was operated in three-dimensional mode (14). [15O]H2O delivery and data acquisition were performed according to a previously published technique (9). Image processing included image realignment, transformation into standard stereotactic space (15), and smoothing with a 15-mm Gaussian filter. Global flow was normalized to a mean of 50 ml/100 g per minute by analysis of covariance. Multiple linear regression analyses between groups (within and across conditions) were performed at every voxel with Statistical Parametric Mapping 96 according to the general linear model, and probability estimates were performed according to the theory of random fields (16). All Statistical Parametric Mapping maxima above a significance level of p<0.001 (one-tailed) for relative between-group decreases in mania are reported.
RESULTS
There was decreased activation during word generation in the right middle frontal gyrus (Brodmann’s area 10) in the manic group compared to both the euthymic group (Talairach coordinates x=30, y=56, z=8; and t statistic transformed to the unit normal distribution z=3.21) and the healthy group (coordinates x=30, y=54, z=20; and z=4.13). An additional area of decreased activation was detected in the comparison of the manic and euthymic bipolar groups in right orbitofrontal cortex (Brodmann’s area 11) (coordinates x=18, y=18, z=–26; and z=3.01) (p=0.0013). Figure 1 shows the comparison of the manic and euthymic groups. There was no significant difference between groups in response time or accuracy during word generation (p<0.05).
Adjusted rCBF measured while subjects were at rest was 21.8% lower in orbitofrontal cortex in the manic group (mean=54.76, SD=3.36) than in the healthy group (mean=70.01, SD=3.02) in an area that extended bilaterally with a maxima at x=–2, y=46, z=–28; and z=3.18.
DISCUSSION
This study of primary mania provides evidence of decreased right rostral and orbital prefrontal cortex activation during a probe of prefrontal function and decreased bilateral orbitofrontal cortex activity while subjects were at rest.
Decreased prefrontal executive control may underlie the cognitive and emotional symptoms of mania. Dysfunction of rostral prefrontal cortex may lead to impaired planning, judgment, and insight (17). Impairment of orbitofrontal cortex may result in inappropriate behavioral responses to changing inner drive and external environmental contexts (18). Disrupted orbitofrontal cortex modulation of amygdala and hypothalamus (18) could contribute to the manic dysregulation of more primitive motivational, chronobiologic, and endocrinologic processes. Right hemispheric dysfunction during activation in mania is consistent with reports of secondary mania in right prefrontal brain lesions (1).
These observations are limited by the small group size and by patient medication due to issues associated with studying acutely manic patients in this setting. Generalization will require replication and minimization of these limitations. However, this preliminary study is the first to employ high-sensitivity PET paired with a prefrontal probe to examine regional brain activity in mania. The findings, when seen in the context of the human lesion and behavioral neuroanatomic literatures, may help to explain some of the neurobehavioral abnormalities characteristic of the manic state.
Presented in part at the 36th annual meeting of the American College of Neuropsychopharmacology, Waikiloa, Hawaii, Dec. 8–12, 1997; and the Fourth International Conference on the Functional Mapping of the Human Brain, Montreal, Canada, June 7–12, 1998. Received Nov. 16, 1998; revision received May 12, 1999; accepted May 13, 1999From the Functional Neuroimaging Laboratory, Department of Psychiatry, New York Hospital-Cornell Medical Center, New York; and the Department of Neurology, North Shore University Hospital-New York University School of Medicine, Manhassett, N.Y. Address reprint requests to Dr. Blumberg, Department of Psychiatry, VA Connecticut Healthcare System, 116A, 950 Campbell Ave., West Haven, CT 06516.Supported by the DeWitt-Wallace Fund in the New York Community Trust. The authors thank the volunteers for their participation and Claude Marguleff, M.S., Abdelfatihe Belakhlef, Ph.D., and Robert Dahl, Ph.D., for their technical expertise.
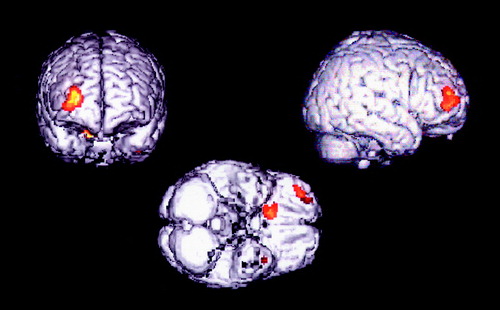
Figure 1. Brain Areas of Decreased Activation in the Manic Group (N=5) Compared to the Euthymic Group (N=6) of Patients With Bipolar Disordera
aFrontal (left), right lateral (right), and ventral (bottom) brain surface renderings demonstrate areas of decreased rCBF (p<0005) superimposed on a T1-weighted magnetic resonance imaging template.
1. Starkstein SE, Mayberg HS, Berthier ML, Fedoroff P, Price TR, Dannals RF, Wagner HN, Leiguarda R, Robinson RG: Mania after brain injury: neuroradiologic and metabolic findings. Ann Neurol 1990; 27:652–659Crossref, Medline, Google Scholar
2. Migliorelli R, Starkstein SE, Teson A, de Quiros G, Vazquez S, Leiguarda R, Robinson RG: SPECT findings in patients with primary mania. J Neuropsychiatry Clin Neurosci 1993; 5:379–383Crossref, Medline, Google Scholar
3. Baxter LR, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, Sumida RM: Cerebral metabolic rates for glucose in mood disorders: studies with positron emission tomography and fluorodeoxyglucose F18. Arch Gen Psychiatry 1985; 42:441–447Crossref, Medline, Google Scholar
4. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824–827Crossref, Medline, Google Scholar
5. O’Connell RA, Van Heertum RL, Luck D, Yudd AP, Cueva JE, Billick SB, Cordon DJ, Gersh RJ, Masdeu JC: Single-photon emission computed tomography of the brain in acute mania and schizophrenia. J Neuroimaging 1995; 5:101–104Crossref, Medline, Google Scholar
6. Rubin E, Sackeim HA, Prohovnik I, Moeller JR, Schnur DB, Mukherjee S: Regional cerebral blood flow in mood disorders, IV: comparison of mania and depression. Psychiatry Res 1995; 61:1–10Crossref, Medline, Google Scholar
7. Al-Mousawi AH, Evans N, Ebmeier KP, Roeda D, Chaloner F, Ashcroft GW: Limbic dysfunction in schizophrenia and mania: a study using 18F-labeled fluorodeoxyglucose and positron emission tomography. Br J Psychiatry 1996; 169:509–516Crossref, Medline, Google Scholar
8. Gyulai L, Alavi A, Brioch K, Reilley J, Ball WB, Whybrow PC: I-123 Iofetamine single-photon computed emission tomography in rapid cycling bipolar disorder: a clinical study. Biol Psychiatry 1997; 41:152–161Crossref, Medline, Google Scholar
9. Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S, Spinks T, Clark J, Frackowiak R, Jones T: Detection of thirty-second cognitive activations in single subjects with positron emission tomography: a new low-dose H215O regional cerebral blood flow three-dimensional imaging technique. J Cereb Blood Flow Metab 1993; 13:617–629Crossref, Medline, Google Scholar
10. Frith CD, Friston K, Liddle PF, Frackowiak RSJ: Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci 1991; 244:241–246Crossref, Medline, Google Scholar
11. Raichle ME: The nervous system, in Handbook of Physiology, section 1, vol V. Edited by Mountcastle VB. Bethesda, Md, American Physiological Society, 1987, pp 643–674Google Scholar
12. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I and II Disorders, version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
13. Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM: The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability and validity. Biol Psychiatry 1994; 36:124–134Crossref, Medline, Google Scholar
14. Lewellen TK, Kohlmyer RS, Miyaoka RS, Kaplan MS, Stearns CW, Schubert SF: Investigation of the performance of the General Electric ADVANCE positron emission tomograph in 3D mode. IEEE Transactions on Nuclear Sci 1996; 43:2199–2207Google Scholar
15. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
16. Friston KJ, Holmes A, Worsley K, Poline J, Frith C, Heather J, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
17. Mesulam M-M (ed): Principles of Behavioral Neurology. Philadelphia, FA Davis, 1985Google Scholar
18. Rolls ET: The orbitofrontal cortex. Philos Trans R Soc Lond B Biol Sci 1996; 351:1433–1444Google Scholar



