Comparison of Ketamine-Induced Thought Disorder in Healthy Volunteers and Thought Disorder in Schizophrenia
Abstract
OBJECTIVE: This study sought to determine whether thought disorder induced in healthy volunteers by the N-methyl-d-aspartic acid (NMDA) receptor antagonist ketamine resembles the thought disorder found in patients with schizophrenia. METHOD: The Scale for the Assessment of Thought, Language, and Communication was used to assess thought disorder in healthy volunteers (N=10) who received subanesthetic doses of ketamine and in a group of clinically stable inpatients with schizophrenia (N=15) who did not receive ketamine. RESULTS: Mean scores on the Scale for the Assessment of Thought, Language, and Communication for patients with schizophrenia and healthy volunteers receiving ketamine did not differ significantly. Moreover, three of the four highest rated test items in both groups were the same. CONCLUSIONS: These data suggest that ketamine-induced thought disorder in healthy volunteers is not dissimilar to the thought disorder in patients with schizophrenia and provide support for the involvement of the NMDA receptor in a cardinal symptom of schizophrenia.
Several lines of evidence suggest that the glutamatergic N-methyl-d-aspartic acid (NMDA) receptor is involved in the pathophysiology of schizophrenia. Postmortem studies have revealed a lower density of glutamatergic receptors in patients with schizophrenia, and one study of CSF reported lower levels of glutamate in patients with schizophrenia than in healthy comparison subjects (1–3). The most compelling evidence is provided by the psychotomimetic effects of the NMDA antagonists phencyclidine (PCP) and ketamine. In patients with schizophrenia, the administration of PCP and ketamine exacerbates existing psychotic symptoms and may reactivate symptoms in remittance (4–6). The administration of PCP or ketamine to healthy volunteers reproduces many of the symptoms of schizophrenia, including aspects of formal thought disorder (4, 7, 8). Because the effects of ketamine resemble many of the clinical features of schizophrenia, this ketamine-induced behavioral syndrome has been proposed as a model for schizophrenia. Nonetheless, few direct comparisons between the thought disorder of schizophrenia and the thought disorder induced by NMDA antagonists have been conducted.
Ketamine-induced thought disorder has been primarily assessed with the Brief Psychiatric Rating Scale (BPRS) (9). We have found that the BPRS conceptual disorganization item is significantly affected by the administration of ketamine (7). Krystal et al. (8) also reported a marked ketamine effect on the thought disturbance factor of the BPRS, which contains the conceptual disorganization item. Many elements of formal thought disorder, however, are poorly measured by the BPRS.
Recently, we found that the administration of ketamine to healthy volunteers significantly increases scores on the Scale for the Assessment of Thought, Language, and Communication (10). The Scale for the Assessment of Thought, Language, and Communication is a clinical, interview-based assessment of thought disorder (11) that has been used to assess thought disorder in studies of several psychiatric diagnoses and conditions (12–17). The Scale for the Assessment of Thought, Language, and Communication provides a more comprehensive evaluation of thought disorder than does the BPRS and permits an examination of subfactors (18) that address aspects of thought disorder. In this study, we used the Scale for the Assessment of Thought, Language, and Communication to compare the formal thought disorder induced by ketamine in healthy volunteers with the formal thought disorder in clinically stable inpatients with schizophrenia.
METHOD
Ten healthy volunteers (seven men and three women; mean age=35.2 years, SD=9.2) participated in this study. The subjects were in good health and underwent a brief medical evaluation. The subjects were free of psychiatric disorders on clinical examination and on the Structured Clinical Interview for DSM-III-R (19). All of these subjects had been drug- and alcohol-free for at least 6 months, and all concurrently participated in a separate study examining the effects of ketamine on healthy volunteers.
Fifteen patients (10 men and five women; mean age 33.4 years, SD=9.5) who met the DSM-IV criteria for schizophrenia (N=14) or schizoaffective disorder (N=1) were admitted to the 4th Floor East Patient Care Unit of the National Institutes of Health (NIH) Clinical Center in Bethesda, Md., or the Neurosciences Center of NIH at St. Elizabeths Hospital in Washington, D.C. Two patients were free of antipsychotic drugs, and 13 patients were undergoing pharmacotherapy during the rating period. Antipsychotic drug treatment was with olanzapine (N=6), clozapine (N=2), risperidone (N=3), and fluphenazine (N=2). All patients were clinically stable but symptomatic at the time of assessment.
The healthy volunteers and patients with schizophrenia were gender- and age-matched (age differences: t=0.47, df=23, p=0.64). After a complete description of the study to the subjects, written informed consent was obtained.
Ketamine was administered as a bolus followed by constant infusion over 1 hour to the healthy volunteers in a double-blind, placebo-controlled fashion, as previously described (6, 7, 10).
Formal thought disorder was rated using the Scale for the Assessment of Thought, Language, and Communication to assess specific aspects of thought disorder during a 15-minute interview. Healthy volunteers were rated during infusions of ketamine and placebo. Ratings were conducted by a single experienced investigator (C.M.A.).
To compare ketamine-induced thought disorder with thought disorder in patients with schizophrenia, independent t tests (two-tailed) were conducted with the scores from the Scale for the Assessment of Thought, Language, and Communication obtained from the patients with schizophrenia and the scores obtained from the healthy volunteers during ketamine infusions. Scores from the Scale for the Assessment of Thought, Language, and Communication were analyzed in total and by factors for verbal production and disconnection, factors for positive and negative symptoms, and a unidimensional severity factor (18). Individual items were examined in an exploratory manner. Effect sizes (d) were calculated for patients with schizophrenia and healthy volunteers for total and factor scores on the Scale for the Assessment of Thought, Language, and Communication.
RESULTS
There were no significant differences between the total scores for patients with schizophrenia and the total scores for healthy volunteers receiving ketamine on the Scale for the Assessment of Thought, Language, and Communication (t=0.43, df=23, p=0.67, d=0.19). There was no significant difference between the groups’ scores on any of the factors tested on the Scale for the Assessment of Thought, Language, and Communication. The scores for the unidimensional severity factor (t=–0.40, df=23, p=0.69, d=0.18), the paired factors for verbal productivity (t=0.75, df=23, p=0.46, d=0.30) and disconnection (t=0.21, df=23, p=0.84, d=0.10), and the paired factors for positive (t=0.02, df=23, p=0.98, d=0.01) and negative (t=1.10, df=23, p=0.28, d=0.45) symptoms did not differ for the patients with schizophrenic thought disorder and the patients with ketamine-induced thought disorder. Only the scores for one of the 20 individual items on the Scale for the Assessment of Thought, Language, and Communication—perseveration (t=2.13, df=23, p=0.04)—differed significantly for the two groups. This item was not found to be significantly different after use of a Bonferroni correction for the 20 comparison items (figure 1). In patients with schizophrenia and healthy volunteers receiving ketamine, scores for three of the four highest rated items on the Scale for the Assessment of Thought, Language, and Communication—poverty of speech, circumstantiality, and loss of goal—were the same.
DISCUSSION
Total scores on the Scale for the Assessment of Thought, Language, and Communication for healthy volunteers receiving ketamine did not significantly differ from total scores for a group of clinically stable inpatients with schizophrenia or from factors reflecting both positive and negative symptoms. Three of the four items most affected by ketamine administration—poverty of speech, circumstantiality, and loss of goal—were the same as three of the four highest rated items for patients with schizophrenia.
Our findings are consistent with those of Luby et al. (4), in which PCP-induced thought disorder in a group of healthy volunteers was similar to the disorganization of thought found in a group consisting for the most part of patients with schizophrenia. Similarly, these data are consistent with those of previous studies that demonstrated that ketamine induces a pattern of some schizophrenia-like symptoms in healthy volunteers (7, 8). In contrast, LSD, a serotonin agonist, and amphetamine, a dopamine agonist, induce syndromes that do not, in general, so closely resemble those of chronic schizophrenia, particularly with regard to thought disorder (20, 21). Our data, which suggest that thought disorder induced by NMDA receptor antagonists is strongly reminiscent of the thought disorder found in patients with schizophrenia, support the hypothesis that dysfunction of NMDA receptors is important in schizophrenia.
Several caveats should be considered in the interpretation of this study. Thirteen of the 15 patients with schizophrenia were undergoing treatment with antipsychotic drugs during the study. Harvey et al. (22), however, found that neuroleptics did not significantly alter the BPRS factor structure of schizophrenic symptoms and that the symptoms of patients who respond to medication do not qualitatively differ from the symptoms of patients who do not respond to medication. Moreover, our clinical experience is that while the intensity of thought disorder may decrease with medication treatment, the profile of the thought disorder is not altered.
Another important issue of this study is group size. Although the number of subjects was somewhat limited, the small effect sizes for key factors including disconnection suggested little overall difference between groups in the underlying dimension of language disorganization. Power analysis based on the effect sizes observed here revealed that a study would require more than 400 subjects to have a power of 0.80 to detect a difference in total scores at α=0.05. Therefore, only with prohibitively large study groups would a significant difference be observed. The more moderate effect size observed with the negative symptom factor of the Scale for the Assessment of Thought, Language, and Communication suggests that ketamine is a better model for the positive, rather than the negative, symptoms of schizophrenia. Given that substantially fewer items are used to calculate scores for negative factors, however, the higher effect size may be merely a function of power.
Finally, although ketamine-induced thought disorder appears to be modulated through non-NMDA mechanisms, several lines of evidence may preclude this possibility. Ketamine binds to the NMDA receptor with an affinity that is at least seven times higher than that for other sites (23–26), and behaviors induced by NMDA receptor antagonists are not blocked by opiate, cholinergic, or monoamine receptor antagonists (27). Moreover, non-NMDA, voltage-gated potassium currents are lessened only at extremely high drug levels, which suggests that low doses of ketamine are selective for the NMDA receptor (28).
In conclusion, we have demonstrated that ketamine-induced thought disorder does not differ significantly from the thought disorder of patients with schizophrenia, as measured by the Scale for the Assessment of Thought, Language, and Communication. Our findings provide additional data that show that ketamine administration in healthy volunteers produces a clinical syndrome with aspects that resemble key symptoms of schizophrenia and lend further support to the hypothesis that NMDA receptor dysfunction is involved in the pathophysiology of schizophrenia.
Received Oct. 9, 1998; revision received Feb. 9, 1999; accepted Feb. 23, 1999. From the Experimental Therapeutics Branch, NIMH, Bethesda, Md. Address reprint requests to Dr. Adler, Department of Psychiatry, University of Cincinnati College of Medicine, 231 Bethesda Ave., Cincinnati, OH 45267-0559; [email protected] (e-mail)
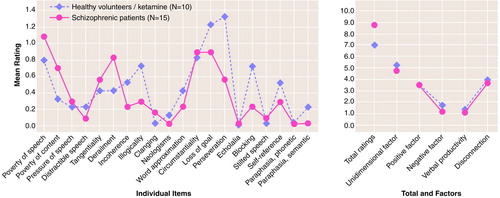
FIGURE 1. Comparison of Total, Factor, and Individual Item Scores on the Scale for the Assessment of Thought, Language, and Communication of Healthy Volunteers With Ketamine-Induced Thought Disorder and Patients With Schizophrenia
1. Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, Beckmann H: [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm 1989; 77:231–236Crossref, Medline, Google Scholar
2. Kerwin R, Patel S, Meldrum BS, Czudek C, Reynolds GP: Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet 1988; 12:25–32Google Scholar
3. Kim JS, Kornhuber HH, Holzmuller B, Schmid-Burgk W, Mergner T, Krzepinski G: Reduction of cerebrospinal fluid glutamic acid in Huntington’s chorea and in schizophrenic patients. Arch Psychiatr Nervenkr 1980; 228:7–10Crossref, Medline, Google Scholar
4. Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R: Study of a new schizophrenomimetic drug—Sernyl. Arch Neurol Psychiatry 1959; 81:363–369Crossref, Medline, Google Scholar
5. Lahti AC, Koffel B, LaPorte D, Tamminga CA: Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995; 1:9–19Crossref, Google Scholar
6. Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A: Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 1997; 17:141–150Crossref, Medline, Google Scholar
7. Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A: NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996; 14:301–307Crossref, Medline, Google Scholar
8. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
9. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
10. Adler CM, Goldberg TM, Malhotra AK, Pickar D, Breier A: Effects of ketamine on thought disorder, working memory and semantic memory in healthy volunteers. Biol Psychiatry 1998; 43:811–816Crossref, Medline, Google Scholar
11. Andreasen NC: Scale for the Assessment of Thought, Language, and Communication (TLC). Schizophr Bull 1986; 12:473–482Crossref, Medline, Google Scholar
12. Mazumdar PK, Chaturvedi SK, Sinha V, Gopinath PS: Scale for Assessment of Thought, Language and Communication in psychotic in-patients. Psychopathology 1991; 24:199–202Crossref, Medline, Google Scholar
13. Harvey PD, Earle-Boyer EA, Wielgus MS: The consistency of thought disorder in mania and schizophrenia: an assessment of acute psychotics. J Nerv Ment Dis 1984; 172:458–463Crossref, Medline, Google Scholar
14. Harvey PD, Lombardi J, Leibman M, Parrella M, White L, Powchik P, Mohs RC, Davidson M, Davis KL: Age-related differences in formal thought disorder in chronically hospitalized schizophrenic patients: a cross-sectional study across nine decades. Am J Psychiatry 1997; 154:205–210Link, Google Scholar
15. Mazumdar PK, Chaturvedi SK, Gopinath PS: A comparative study of thought disorder in acute and chronic schizophrenia. Psychopathology 1995; 28:185–189Crossref, Medline, Google Scholar
16. Cuesta MJ, Peralta V: Does formal thought disorder differ among patients with schizophrenic, schizophreniform and manic schizoaffective disorders? Schizophr Res 1993; 10:151–158Google Scholar
17. Ragin AB, Oltmanns TF: Communicability and thought disorder in schizophrenics and other diagnostic groups: a follow-up study. Br J Psychiatry 1987; 150:494–500Crossref, Medline, Google Scholar
18. Harvey PD, Lenzenweger MF, Keefe RS, Pogge DL, Serper MR, Mohs RC: Empirical assessment of the factorial structure of clinical symptoms in schizophrenic patients: formal thought disorder. Psychiatry Res 1992; 44:141–151Crossref, Medline, Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First HB: Structured Clinical Interview for DSM-III-R—Patient Version 1.0 (SCID-P). Washington, DC, American Psychiatric Press, 1990Google Scholar
20. Cohen S: LSD: the varieties of psychotic experience. J Psychoactive Drugs 1985; 17:291–296Crossref, Medline, Google Scholar
21. Tomiyama G: Chronic schizophrenia-like states in methamphetamine psychosis. Jpn J Psychiatr Neurol 1990; 44:531–539Medline, Google Scholar
22. Harvey PD, Davidson M, White L, Keefe RSE, Hirschowitz J, Mohs RC, Davis KL: Empirical evaluations of the factorial structure of clinical symptoms in schizophrenia: effects of typical neuroleptics on the Brief Psychiatric Rating Scale. Biol Psychiatry 1996; 40:755–760Crossref, Medline, Google Scholar
23. Cohen ML, Chan SL, Bhargava HN, Trevor AJ: Inhibition of mammalian brain acetylcholinesterase by ketamine. Biochem Pharmacol 1974; 23:1647–1652Google Scholar
24. Smith DJ, Pekoe GM, Martin LL, Coalgate B: The interaction of ketamine with the opiate receptor. Life Sci 1980; 26:789–795Crossref, Medline, Google Scholar
25. Smith DJ, Azzaro AJ, Zaldivar SB, Palmer S, Lee HS: Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology 1981; 20:391–396Crossref, Medline, Google Scholar
26. Oye I, Hustveit O, Moberg ER, Pausen O, Skoglund LA: The chiral forms of ketamine as probes for NMDA receptor function in humans, in NMDA Receptor Related Agents: Biochemistry, Pharmacology, and Behavior. Edited by Kameyama T, Nabeshima T, Domino EF. Ann Arbor, Mich, NPP Books, 1991, pp 381–389Google Scholar
27. Byrd LD, Standish LJ, Howell LL: Behavioral effects of phencyclidine and ketamine alone and in combination with other drugs. Eur J Pharmacol 1987; 144:331–341Crossref, Medline, Google Scholar
28. Rothman S: Noncompetitive N-methyl-d-aspartate antagonists affect multiple ionic currents. J Pharmacol Exp Ther 1988; 246:137–142Medline, Google Scholar



