Cerebral Blood Flow Changes Associated With Attribution of Emotional Valence to Pleasant, Unpleasant, and Neutral Visual Stimuli in a PET Study of Normal Subjects
Abstract
OBJECTIVE: To assist in the development of a model for the psychopathology of emotions, the present study sought to identify the neural circuits associated with the evaluation of visual stimuli for emotional valence. METHOD: Seventeen healthy individuals were shown three sets of emotionally laden pictures carrying pleasant, unpleasant, and neutral content. While subjects evaluated the picture set for emotional valence, regional cerebral blood flow was measured with the use of [15O] water positron emission tomography. Subjective ratings of the emotional valence of the picture sets were recorded. Data were analyzed by comparing the images acquired during the neutral condition with the unpleasant and pleasant image sets and the unpleasant and pleasant conditions with each other. RESULTS: Processing of pleasant stimuli was associated with increased blood flow in the dorsal-lateral, orbital, and medial frontal cortex relative to the unpleasant condition and in the cingulate, precuneus, and visual cortex relative to the neutral condition. Evaluation of unpleasant stimuli activated the amygdala, visual cortex, and cerebellum relative to the pleasant condition and the nucleus accumbens, precuneus, and visual cortex relative to the neutral condition. CONCLUSIONS: Observing and assigning emotional value to unpleasant stimuli produced activations in subcortical limbic regions, whereas evaluation of pleasant stimuli produced activations in cortical limbic areas. These findings are consistent with the notion of a subcortical and archaic danger recognition system and a system detecting pleasantness in events and situations that is phylogenetically younger, involving primarily the prefrontal cortex.
Emotion processing is composed of evaluative, experiential, and expressive components (1). The evaluation of affect may be correct or incorrect depending on an individual’s ability to identify the emotional valence carried by an event or an object and is influenced by mental illness. For example, for patients with depression, positive life events are often considered negative or harmful. Patients with schizophrenia instead seem to be unable to extract the emotional content from a situation or experience, whether the experience is pleasurable or unpleasant. Because people with psychiatric disorders lose their capacity to distinguish between pleasant and unpleasant experiences and the ability to assign the appropriate emotional valence to these experiences, developing cognitive models of emotional processing in healthy humans will assist in identifying the neural mechanisms of serious mental illnesses such as schizophrenia, depression, anxiety, and posttraumatic stress disorder (2).
Neuroimaging techniques permit identification of the in vivo neural substrates of many domains of brain function such as memory, attention, and sensation. Although several research reports have been published (3–10), the study of emotions through use of neuroimaging technology has lagged behind studies of cognition.
We report here on a study examining the functional neuroanatomy associated with the attribution of affective valence to visual stimuli carrying positive, negative, and neutral content. The study, which used the [15O] water positron emission tomography (PET) method in healthy young volunteers, focused on one component of the process of affective evaluation, i.e., the degree to which experiences or objects are judged as pleasant or unpleasant (11).
METHOD
Subjects
Subjects were 17 healthy right-handed individuals (10 women and seven men) recruited from the community. The group had a mean age of 31.2 years (SD=8.7), mean education of 14.5 years (SD=1.6, range=12–18), and no history of psychiatric/neurological disorder, alcohol/substance abuse, or current use of psychotropic medications. No gross brain abnormalities were found on magnetic resonance (MR) scans. Mean full-scale IQ (12) was 110.17 (SD=12.42, range=83–133), verbal IQ was 106.83 (SD=8.73, range=88–122), and performance IQ was 112.28 (SD=16.12, range=80–138). Subjects performed within the high end of the normative curve on the Benton Facial Recognition test, short form (mean score=23.67, SD=1.97, range=20–27) (13). All subjects gave written informed consent to protocols approved by the University of Iowa Human Subjects Institutional Review Board.
Activation Stimuli
Three sets of activation stimuli were used in the present study: pleasant images, unpleasant images, and neutral images. Each set of stimuli was shown once. In each condition subjects viewed a set of 18 complex images displayed on an 11-by-8-inch computer monitor. Images were displayed individually for 2 seconds; thus, each image set was presented for 36 seconds. The order of picture presentation was neutral-pleasant-unpleasant in order to minimize possible intensity carryover effects (14). The screen was positioned approximately 14 inches from the subject. Images were 7.75 inches wide and 7.5 inches high and subtended 29° of visual angle in height and 28° in width. Timing of the activation was such that image displays began 10 seconds before the arrival of the [15O] water bolus in the brain, assessed individually for each subject.
Images were chosen from The International Affective Picture System (15) through a multistep process that achieved a wide range of pleasant images/emotions such as happiness, appetite, satisfaction, beauty, and success. Unpleasant emotions included fear, disgust, sorrow, and disappointment. The International Affective Picture System (15) provides a large data set of standardized, emotionally evocative color pictures associated with highly reliable affective judgments and psychophysiological responses (11, 15). Pictures with high, low, and neutral normative valence ratings were first identified. Normative valence and arousal ratings were available from a large sample of healthy subjects on a scale ranging from 0 to 9 (0=very negative and 9=very positive valence or arousal) (15). Images with high arousal, sexual content, or interpersonal violence were removed. Next, the selected pictures were shown to a large group of healthy individuals and rated for valence. The pictures with the smallest standard deviations were retained in order to ensure that all subjects would have a similar emotional response during the PET experiment. The slides were rated again for valence by another group of healthy individuals. Finally, the 18 pictures with the highest and lowest ratings were selected, along with 18 neutral pictures. The three picture sets in this study were equated in terms of content (i.e., number of pictures containing people, faces, objects, and scenes). Mean normative valence scores were 2.25 (SD=0.65) for the unpleasant picture sequence, 7.68 (SD=0.36) for the pleasant picture sequence, and 5.49 (SD=0.75) for the neutral sequence. Mean normative arousal scores were 5.73 (SD=0.74) for the unpleasant sequence, 4.69 (SD=1.03) for the pleasant sequence, and 3.00 (SD=0.64) for the neutral sequence. Mean picture luminance was 12.37 feet/candles (SD=0.49) for the unpleasant picture set, 12.47 (SD=0.49) for the pleasant set, and 12.36 (SD=0.35) for the neutral set. A description of the pictures is in Appendix 1.
Before viewing each set, subjects were instructed to look at the pictures and were told that they would be rating the pictures as to how pleasant or unpleasant they were. Subjects were not told that they needed to correctly identify the emotional valence of stimuli. Immediately after acquisition of the blood flow image, subjects were asked to rate the valence of the entire image set on a verbal analog scale ranging from –7 to 7 (–7=extremely unpleasant, 0=neutral, and 7=extremely pleasant).
PET Data Acquisition
Regional cerebral blood flow (rCBF) was measured by using the bolus [15O] water method (16–18) with a GE-4096 PLUS Scanner. The subjects were oriented in the PET scanner with laser light guides aligned at the orbitomeatal line. The center of the most rostral slice was indicated by the laser guides. Fifteen slices (6.5 mm center to center), with an intrinsic in-plane resolution of 6.5 mm full width at half maximum and a 10-cm axial field-of-view, were acquired. Images were reconstructed by using a Butterworth filter (cutoff frequency=0.35 Nyquist interval). CBF was determined by using the [15O] water (50 mCi/injection) method and methods previously described (19). For each injection, arterial blood was sampled from time 0 (injection) to 100 seconds. Imaging, initiated at injection, consisted of 20 frames at 5 seconds per frame for a total of 100 seconds. The parametric image (i.e., blood flow image) was created by using a 40-second summed image (initial 40 seconds immediately after bolus transit) and the arterial input function. A preliminary injection (sham), to determine bolus arrival time, was employed to establish stimulus timing (20).
MR Image Acquisition and Processing
MR images consisted of contiguous coronal slices (1.5 mm thick) acquired on a 1.5-Tesla GE Signa scanner. Technical measures of the MR image acquisition were as follows: spoiled gradient recalled sequence, flip angle=40˚, TE=5 msec, TR=24 msec, number of excitations=2. MR images were transferred to the Image Processing Laboratory of the University of Iowa Mental Health Clinical Research Center for analysis through use of Silicon Graphics workstations and locally developed software (BRAINS) (21–27).
The initial step of postacquisition processing involved “removing” the brain from the skull by using a combination of automated edge detection techniques and manual tracing. Pixels representing surface CSF are classified through a thresholding procedure and removed from the display. All brains are realigned parallel to the anterior commissure/posterior commissure line and the interhemispheric fissure to ensure comparability of head position across subjects. Alignment also places the images in standard Talairach-Tournoux space (28). At this point, images from multiple subjects or multiple scans from a single subject can be coregistered. Finally, images are resliced in three orthogonal planes to produce a three-dimensional data set that is used for visualization and analysis.
PET Image Processing
The quantitative PET blood flow images were transferred to the Image Processing Laboratory for further analysis (21–27). The first step in image analysis involved registration of each individual’s PET and MR images. The co-registration used a two-stage process. Initially, a coarse fit based on surface matching of the MR and PET images was done (29) Then, with the surface fit data used as input, a variance minimization program was employed for the final co-registration (30). Brain landmarks identified on the MR image were used to place each co-registered image into standardized coordinate space (28). An 18-mm Hanning filter was applied to the PET images to eliminate residual anatomical variability.
Statistical Analysis
Statistical analysis of the blood flow images used a modification of the Worsley method (24, 31, 32). A within-subject subtraction of relevant conditions was performed (e.g., unpleasant pictures minus neutral pictures), followed by across-subject averaging of the subtraction images and computation of voxel-by-voxel t tests of blood flow differences.
This study examines the results from three subtractions: pleasant minus neutral pictures, unpleasant minus neutral pictures, and unpleasant minus pleasant pictures. It is not possible to determine from these subtractions whether decreased flow in a region reflects an actual decrease in blood flow in the region or relatively higher flow in that region in the comparison condition. In such analyses, conditions are measured relative to one another and are referred to as activations. Comparison of one emotional condition with both the opposite valence and neutral conditions will give insight into the neural circuitry involved.
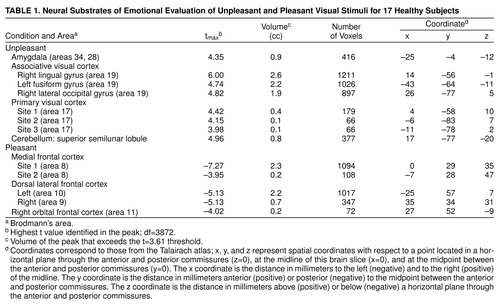
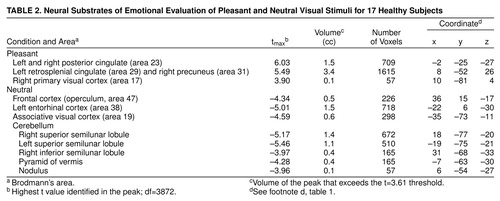
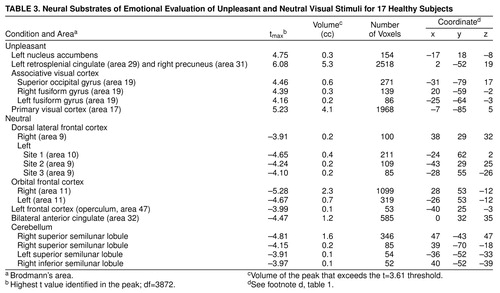
Data reported in tables 1, 2, and 3 show the location of peaks (with anatomical localization based on visual inspection of co-registered MR and PET images and Talairach-Tournoux coordinates); the x, y, z Talairach-Tournoux coordinates, the tmax (highest t value identified in the peak), and the volume of the peak (in cubic centimeters) that exceeds the t value of 3.61 (df≈3872) threshold. This threshold, which has been consistently used by our center, corresponds to an uncorrected significance level of <0.0005 per voxel. Regions of significant activation were identified on the t-map images and corrected for the large number of t tests performed, the lack of independence between voxels, and the resolution of the processed images (24, 31, 32). There were about 300,000 gray matter voxels in our images, representing approximately 242 resolution elements (31). After filtering, the three-dimensional image resolution is 2.5 cc. The degrees of freedom were extremely large for the t tests: df≈3872=number of resolution elements x (number of subjects –1). Only areas that exceeded 50 contiguous voxels were tabled, in order to omit isolated outlying values.
RESULTS
Subjects’ ratings of the unpleasant (mean score=–6.12, SD=1.4), pleasant (mean=6.10, SD=1.1), and neutral (mean=2.38, SD=1.6) picture sets were consistent with the intended valence (F=358, df=2, 15, p<0.0001). Ratings of the neutral and pleasant stimuli were statistically different (F=57, df=1, 16, p<0.0001), as were the neutral/unpleasant (F=457, df=1, 16, p<0.0001) and the unpleasant/pleasant (F=680, df=1, 16, p<0.0001) comparisons.
Evaluation of unpleasant visual stimuli relative to pleasant stimuli produced activations in primary and secondary visual cortex and in the superior semilunar lobule of the cerebellum. The left amygdala was relatively more active during evaluation of unpleasant stimuli (Table 1 and Figure 1).
Evaluation of pleasant stimuli produced activations bilaterally in the medial, orbital, and dorsal lateral frontal cortex (Table 1 and Figure 1).
Subjects showed relatively higher blood flow in the primary visual cortex, left retrosplenial cingulate, and right precuneus in the pleasant picture condition. Bilaterally, the posterior cingulate gyrus was also more active (Table 2 and Figure 2) in the pleasant than in the neutral condition.
Evaluating neutral visual stimuli produced relatively higher blood flow, compared to the evaluation of pleasant pictures, in the visual association cortex, right frontal operculum, and entorhinal cortex. Several cerebellar locations, including the superior and inferior semilunar lobules, the pyramid of the vermis, and the nodulus, were also relatively more active (Table 2 and Figure 2).
Evaluation of unpleasant stimuli produced activations in the primary and secondary visual cortex including bilateral fusiform gyri. The left retrosplenial cingulate gyrus and the right precuneus were relatively more active in a pattern very similar to that seen during evaluation of pleasant stimuli Table 3 and Figure 3). In addition, there was increased flow in the left nucleus accumbens. The neutral condition produced relative activations bilaterally in the dorsal lateral and orbital frontal cortex and in the left frontal operculum, bilaterally in the anterior cingulate, and in several locations in the cerebellum including the bilateral superior and right inferior semilunar lobules.
DISCUSSION
This study examined the functional neuroanatomy associated with the attribution of emotional valence to visual stimuli. Subcortical limbic regions were activated during attribution of valence to unpleasant stimuli, whereas cortical limbic areas were activated during attribution of valence to pleasant stimuli regardless of the comparison condition used. How may these findings be interpreted? Humans and nonhuman animals have very efficient neural mechanisms to detect danger that developed much earlier than the massive enlargement of the frontal cortex observed in primates. The danger recognition system, which is crucial for the survival of the species, evolved in order to function effectively with least cortical appraisal and thus is largely subcortical (33). Human and nonhuman animals respond to a sudden danger in a somewhat stereotypic and universal way that initially does not involve complex cognitive processing and achieves promptness to escape. The ability to appreciate the positive aspect of events and situations, however, requires more sophisticated processing of the stimuli that is individually personalized and has the characteristic of a “higher” cortical process. It is thus arguable that the detection of pleasant features relies on a rather phylogenetically newer circuit that involves largely the prefrontal cortex and the cortical executive system.
A way of isolating a differential neural response to attribution of emotional valence in pleasant and unpleasant pictorial stimuli that is least influenced by arousal is to subtract these two conditions from one another. This subtraction showed increased activity in the amygdala associated with the attribution of emotional valence to the unpleasant stimuli, whereas emotional evaluation of pleasant stimuli was associated with several areas in the orbital medial and dorsal lateral prefrontal cortex.
The role of the amygdala in emotional behavior has been recognized (34–37). Studies using affective manipulations of sensory/cognitive tasks or drugs have associated the amygdala with emotions in humans (4, 6, 8, 9, 14, 38–41). Results from this study are consistent with research showing amygdala involvement in emotion evaluation, specifically in extraction of affective content from visual stimuli (8, 9, 14, 42–46).
Whether the amygdala may specifically process only one of several possible negative emotions (9, 42) or play a very general role in all affective behavior (47–49) is one of the open questions in emotional neuroscience (14). In contrast to the view positing a role of the amygdala in the appraisal of only one negative emotion, e.g., fear (42), the present study found that increased amygdala activity was associated with evaluation of a wide range of unpleasant stimuli grouped in a pictorial sequence.
Consistent with the majority of the literature (8, 9, 42, 45), the present study found no evidence of human amygdala involvement in the attribution of positive valence to visual stimuli with pleasant content. Using picture sets derived from the same source (50) and a similar study design, Lane et al. (45) have shown left amygdala activation in response to unpleasant but not pleasant visual stimuli. The higher amygdala blood flow in response to unpleasant relative to pleasant stimuli, but not relative to neutral stimuli, is consistent with the observations of Morris et al. (9). They reported left amygdala blood flow increases with increasing fearfulness and decreases with increasing happiness in the visual stimuli (9). Rapid habituation to happy faces (14) may also account for the absence of amygdala differential activity observed in response to visually presented pleasant stimuli. Furthermore, the human amygdala may be involved in the mental processes necessary to detect affective valence for both negative and positive stimuli in other cognitive domains (47). In nonhumans, the amygdala participates in emotion processing for both negative and positive stimuli regardless of the delivery of the stimuli (33, 48, 51).
Results from the present study do suggest, however, that in humans affective evaluation of pleasant stimuli in the visual domain is carried on with a substantial contribution from the frontal lobe. This is consistent with findings from a number of research areas that suggest that the orbital frontal cortex plays an important role in representing information about reinforcing stimuli. Specifically, lesions of the orbital frontal cortex in animals interfere with short-term memory of reward information. The animal no longer can discriminate between good and bad (52). Neuronal cells in the orbital frontal cortex respond to information about reward or punishment (53–55).
The present study is also consistent with previous research in healthy humans and individuals with clinical depression. Human inferior, medial, and orbital prefrontal cortices play an important role in emotional cognitive processes (3, 4, 40, 56) including recognition of facial emotion (57). Frontal regions are also engaged in the assessment of facial attractiveness (58). Patients with full-blown depression, a condition that affects the patient’s ability to detect and take pleasure in joyful events and situations, show decreased perfusion in the left dorsal lateral and bilateral medial prefrontal regions (59, 60). In patients with brain injury, damage to the left dorsal lateral frontal cortex is associated with clinical depression (61–65). Damage to the orbital frontal cortex is also associated with diminished ability in social decision making and detection of emotional clues (66, 67), leading to changes in personality (68). Taken together, these findings support the notion that the prefrontal cortex is part of a neural system engaged in detection of positive features in objects, events, and mental states.
The relative increased blood flow in the frontal lobe during the neutral/unpleasant comparison is consistent with the above hypothesis and may be explained by the different degree of pleasant valence detected by subjects.
Increased activity of primary and associative visual cortex was found in the unpleasant/pleasant comparison, as well as in the pleasant and unpleasant versus neutral comparisons. The observation of increased blood flow in the visual cortex during processing of emotional pictorial stimuli is frequent (4, 7, 9, 45, 69) but poorly understood. Since the experimental and neutral stimuli in our and other studies (4, 9) were matched for content complexity and luminance, it is arguable that some of the visual areas in the circuit may have been activated as a result of subcortical limbic back-signaling to visual cortex for a secondary assessment of the visual stimuli (33). Consistent with previous studies (45), visual association areas were relatively more active in the unpleasant/pleasant and unpleasant/neutral comparisons. Visual association areas may be preferentially involved in the evaluation of unpleasant visual stimuli in young individuals (7, 46).
In the pleasant versus neutral subtraction, evaluation of pleasant pictures increased blood flow bilaterally in the posterior cingulate (Brodmann’s area 23). Functions attributed to the posterior cingulate include monitoring sensory events and the organism’s own behavior with respect to spatial orientation and memory (70–74). However, the role of the posterior cingulate as a component of the limbic system (75) (and therefore in emotion) should probably be reevaluated in light of recent data. The posterior cingulate cortex of the macaque is strongly connected with orbital medial prefrontal cortex area 11m (76) and participates in instrumental avoidance learning in the rabbit (77). In humans, increased blood flow in the posterior cingulate is associated with classic conditioning (78), comprehension of narratives necessitating the attribution of mental states (79) or metaphors (80), and recall of emotionally laden personal memories (81). The left posterior cingulate showed increased activity during implicit recognition of emotion (9). These and our findings suggest that the posterior cingulate gyrus may be involved in implicit and/or explicit evaluation of past and present contextual and emotional experiences.
Attribution of unpleasant valence relative to neutral showed relative increased activity in a nucleus of the basal forebrain: the nucleus accumbens. This subcortical nucleus, which is part of the limbic striatum (82), receives major input from the amygdala (83, 84) and may influence the anterior cingulate via the ventral pallidum (85). Whereas the classic view has seen the nucleus accumbens playing a role in appetitive motivation and positive reinforcement (86–90), a variety of aversive and stressful situations (e.g., active avoidance behavior) increase dopamine release within the accumbens (91–95). Recently, the function of the accumbens has been conceptualized as linking motor and motivational processes that characterize goal-directed behavior (96).
Relative to the neutral condition, evaluation of either pleasant or unpleasant stimuli activated the left retrosplenial cingulate (Brodmann’s area 29) and the right precuneus (Brodmann’s area 31). These shared neural substrates may represent memory component necessary during attribution of pleasant or unpleasant character to emotionally charged stimuli. Consistent with this hypothesis, the precuneus has been shown to be involved in episodic retrieval (97) dependent on visual imagery (98), as well as in recall, planning, and associative thinking (99–101). We posit that in order for humans to be consciously aware of a stimulus (and therefore examine its affective valence), the sensory representations of the stimulus need to be compared with past experiences of that stimulus and associated emotions (33). This evaluation and its encoding in episodic memory, which involves the retrosplenial cingulate cortex (97), are simultaneous processes.
Understanding the physiological meaning of areas of relative decreased blood flow is particularly challenging. Suspension of activity during the passive task or rest state (102) or true neural inhibitions are proposed interpretations (103). In the present study there was no “rest” state in that subjects attributed valence during all tasks. Therefore, neural inhibition or relative increased blood flow during the neutral condition (7, 24, 99) may apply. These hypotheses help in the interpretation of two main findings in the present study that were not expected: the large cerebellar activation in the neutral condition (relative to both pleasant and unpleasant conditions) and the several frontal regions activated during neutral relative to unpleasant stimuli evaluation.
No verbal response or other movements were required during the PET experiment. Eye movement-related increased cerebellar flow during the most arousing tasks might have been expected in areas such as the vermis, fastigial nuclei, and floccular lobe (104–106). Instead, relative increased neocerebellar cortex blood flow was observed during the neutral condition. Consistent with a cerebellar role in cognition (24, 107–110) including attention (111), this finding may be explained by a relatively more sustained attentional demand during the neutral condition in light of a decisional process evidently less straightforward compared to the pleasant or unpleasant condition. The increased blood flow in the anterior cingulate in the neutral/unpleasant comparison also points to a greater attentional load.
Whereas it is convenient under scholarly principles to divide emotion into evaluative, experiential, and expressive components, in real-life situations they may, albeit to a different degree, occur in some combination. Similarly to an ecological situation, the subjects in this study may have experienced “some” emotional arousal during attribution of valence. Experimental investigations using PET are currently under way in our laboratory to dissociate the functional neuroanatomy of evaluation and experience of emotion.
In conclusion, the present study has traced the functional neural anatomy associated with attribution of emotional valence in visual stimuli. Cortical limbic regions were associated with pleasant and subcortical regions with unpleasant valence regardless of the comparisons between the different experimental conditions. In the direct comparison, detection of pleasant stimuli displayed increased prefrontal activity, whereas detection of negative stimuli exhibited increased activity in the amygdala. Understanding the normal neural circuitry that supports the affective evaluation of events will help to uncover the neuroanatomical basis of psychiatric disorders with prominent emotional disturbances such as depression, anxiety disorders, posttraumatic stress disorder, and schizophrenia.
Presented in part at the Third International Conference on Functional Brain Mapping of the Human Brain, Copenhagen, Denmark, June 19–23, 1997. Received July 14, 1998; revision received March 12, 1999; accepted April 19, 1999. From the Department of Psychiatry and the Department of Radiology PET Center, Mental Health Clinical Research Center, University of Iowa College of Medicine. Address reprint requests to Dr. Paradiso, Psychiatry Research-MEB, Department of Psychiatry, University of Iowa, Iowa City, IA 52242-1000. Supported in part by grants MH-31593, MH-40856, Clinical Research Center grant MH-43271, and Research Scientist Award MH-00625 from NIMH and an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression.
 |
 |
 |
 |
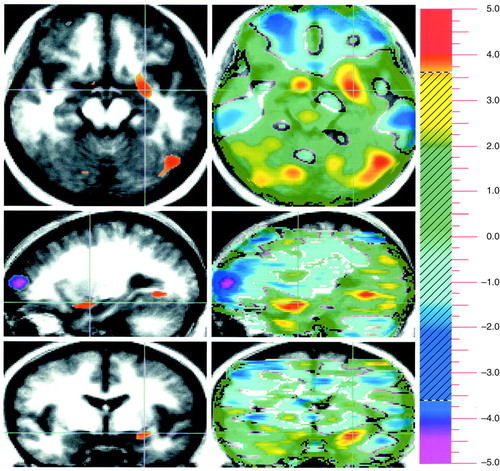
FIGURE 1. Neural Substrates of Emotion in a Study of 17 Healthy Subjects: Unpleasant Minus Pleasant Conditionsa
aThree orthogonal views are shown in each figure, with transaxial at the top, sagittal in the middle, and coronal on the bottom. Crosshairs are used to show the location of the slice. Images follow radiological convention and show location as if the viewer were standing at the foot of the bed (transaxial views) or facing the patient (coronal views). Statistical maps (t maps) of the PET data, showing regions that are significantly activated, are superimposed on a composite MR image derived by averaging the MR scans from the subjects. The value of t is shown on the color bar on the right. Two types of statistical maps are provided. The “peak map” (left side of image) shows the small areas where all contiguous voxels exceed the predefined threshold for statistical significance (t=3.61). The “t map” (right side of image) shows the value of t for all voxels in the image and provides a general overview of the landscape of increases in blood flow during the task. The planes have been chosen to illustrate the location of the relevant activity for each specific task. In this figure, activations due to attribution of pleasant valence are depicted in red/yellow tones. Blue/purple tones indicate relative increased blood flow during detection of neutral valence. Crosshairs are placed in a positive peak that is present on the left and that represents the amygdala (the limbic component); a positive peak is also seen in the left lingual gyrus. A negative peak, reflecting greater activity during the neutral condition, is present in the left frontal lobe (sagittal plane).
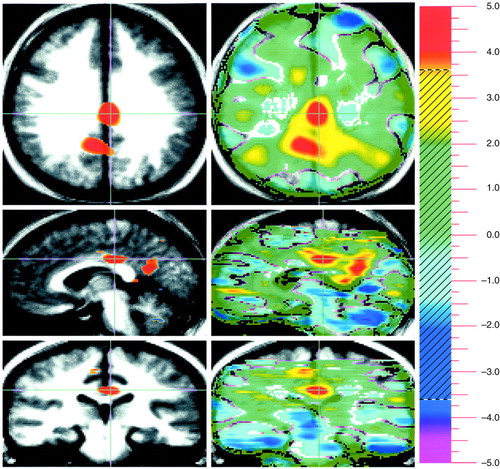
FIGURE 2. Neural Substrates of Emotion in a Study of 17 Healthy Subjects: Pleasant Minus Neutral Conditionsa
aSee figure 1 footnote for general description of images. A positive peak is present bilaterally that represents the posterior cingulate gyrus (the limbic component); positive peaks are also seen in the left retrosplenial cingulate and the right precuneus; these peaks represent the memory component of the task. A negative peak, reflecting relative greater activity during the neutral condition, is located at the level of piramis of the cerebellum.
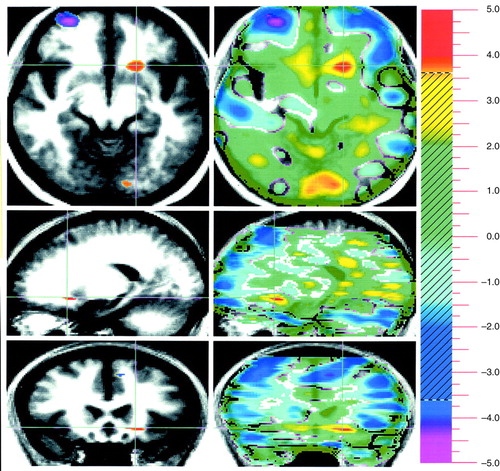
FIGURE 3. Neural Substrates of Emotion in a Study of 17 Healthy Subjects: Unpleasant Minus Neutral Conditionsa
aSee figure 1 footnote for general description of images. Crosshairs are placed in a positive peak representing the left nucleus accumbens (the limbic component). A prominent negative peak, representing relative greater blood flow during neutral, can be observed in the frontal cortex (axial view).
1. LeDoux J: Emotion, in Handbook of Physiology. Edited by Plum F. Bethesda, Md, American Psychological Society, 1987, pp 419–460Google Scholar
2. Andreasen NC: Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 1997; 275:1586–1593Google Scholar
3. Pardo JV, Pardo PJ, Raichle ME: Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150:713–719Link, Google Scholar
4. George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM: Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 1995; 152:341–351Link, Google Scholar
5. Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL: Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA 1996; 93:8016–8021Google Scholar
6. Zald DH, Pardo JV: Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 1997; 94:4119–4124Google Scholar
7. Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Watkins GL, Ponto LL, Hichwa RD: Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 1997; 154:384–389Link, Google Scholar
8. Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA: Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport 1996; 7:1765–1769Google Scholar
9. Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ: A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383:812–815Crossref, Medline, Google Scholar
10. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ: Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 1997; 154:926–933Link, Google Scholar
11. Lang PJ, Greenwald MK, Bradley MM, Hamm AO: Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 1993; 30:261–273Crossref, Medline, Google Scholar
12. Wechsler D: Wechsler Adult Intelligence Scale—Revised. New York, Psychological Corp, 1981Google Scholar
13. Benton A, Sivan A, des Hamsher K, Varney N, Spreen O: Contributions to Neuropsychological Assessment, 2nd ed. New York, Oxford University Press, 1994Google Scholar
14. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17:875–887Crossref, Medline, Google Scholar
15. Lang PJ, Ohman A, Vaitl D: The International Affective Picture System (photographic slides). Gainesville, University of Florida, Center for Research on Psychophysiology, 1988Google Scholar
16. Herscovitch P, Markham J, Raichle ME: Brain blood flow measured with intravenous [15O], I: theory and error analysis. J Nucl Med 1983; 24:782–789Medline, Google Scholar
17. Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J: Brain blood flow measured with intravenous H2(15)O, II: implementation and validation. J Nucl Med 1983; 24:790–798Medline, Google Scholar
18. Ginsberg MD, Lockwood AH, Busto R, Finn RD, Butler CM, Cendan IE, Goddard J: A simplified in vivo autoradiographic strategy for the determination of regional cerebral blood flow by positron emission tomography: theoretical considerations and validation studies in the rat. J Cereb Blood Flow Metab 1982; 2:89–98Crossref, Medline, Google Scholar
19. Hichwa RD, Ponto LLB, Watkins GL: Clinical blood flow measurements with [15O] water and positron emission tomography, in Chemists’ Views of Imaging Centers. Edited by Emran AM. New York, Plenum, 1995, pp 401–417Google Scholar
20. Hurtig RR, Hichwa RD, O’Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC: Effects of timing and duration of cognitive activation in [15O] water PET studies. J Cereb Blood Flow Metab 1994; 14:423–430Crossref, Medline, Google Scholar
21. Andreasen NC, Cizadlo T, Harris G, Swayze V II, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT: Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1993; 5:121–130Crossref, Medline, Google Scholar
22. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Crossref, Medline, Google Scholar
23. Andreasen NC, Harris G, Cizadlo T, Arndt S, O’Leary D, Swayze V, Flaum M: Techniques for measuring sulcal/gyral patterns in the brain as visualized through magnetic resonance scanning: BRAINPLOT and BRAINMAP. Proc Natl Acad Sci USA 1994; 91:93–97Crossref, Medline, Google Scholar
24. Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LL, Hichwa RD: Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA 1995; 92:5111–5115Google Scholar
25. Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW II, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WTC: Automatic atlas-based volume estimation of human brain regions from MR images. J Comp Assist Tomogr 1996; 20:98–106Crossref, Medline, Google Scholar
26. Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O’Leary DS: Tests for comparing images based on randomization and permutation methods. J Cereb Blood Flow 1996; 16:1271–1279Google Scholar
27. Cizadlo T, Andreasen NC, Zeien G, Rajaprabhakaran R, Harris G, O’Leary D, Swayze V, Arndt S, Hichwa R, Ehrhardt J, Yuh WTC: Image registration issues in the analysis of multiple-injection 15O H2O PET studies: BRAINFIT. Proceedings of the Society of Photo-Optical Instrumentation Engineers 1994; 2168:423–430Google Scholar
28. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
29. Levin DN, Pelizzari CA, Chen GT, Chen CT, Cooper MD: Retrospective geometric correlation of MR, CT, and PET images. Radiology 1988; 169:817–823Crossref, Medline, Google Scholar
30. Woods R, Mazziotta J, Cherry S: MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993; 17:536–546Crossref, Medline, Google Scholar
31. Worsley K, Evans S, Marrett S, Neelin P: A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900–918Crossref, Medline, Google Scholar
32. O’Leary DS, Andreasen NC, Hurtig RR, Hichwa RD, Watkins GL, Ponto LLB, Rogers M, Kirchner PT: A positron emission tomography study of binaurally and dichotically presented stimuli: effects of level of language and directed attention. Brain Lang 1996; 53:20–39Crossref, Medline, Google Scholar
33. LeDoux J: The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, Simon & Schuster, 1996Google Scholar
34. Aggleton JP: The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 1993; 16:328–333Crossref, Medline, Google Scholar
35. LeDoux J: Emotion: clues from the brain. Annu Rev Psychol 1995; 46:209–235Crossref, Medline, Google Scholar
36. Kluver H, Bucy PC: “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. Am J Physiol 1937; 119:352–353Google Scholar
37. Brown S, Shäfer EA: An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos Trans R Soc Lond B Biol Sci 1888; 179:303–327Crossref, Google Scholar
38. Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME: A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628–3641Google Scholar
39. Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, Willis MW, Herscovitch P, Post RM: Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 1996; 53:59–69Crossref, Medline, Google Scholar
40. Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA: A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry 1995; 52:20–28Crossref, Medline, Google Scholar
41. Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A: The neural substrate for concrete, abstract, and emotional word lexica: a positron emission tomography study. J Cogn Neurosci 1997; 9:441–461Crossref, Medline, Google Scholar
42. Adolphs R, Tranel D, Damasio H, Damasio AR: Fear and the human amygdala. J Neurosci 1995; 15:5879–5891Google Scholar
43. Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di Paola F: Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain 1996; 119(part 6):1991–2000Google Scholar
44. Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E, Roberts N, Hadley D: Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia 1998; 36:57–70Google Scholar
45. Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE: Neuroanatomical correlates of pleasant and unpleasant emotions. Neuropsychologia 1997; 35:1437–1444Google Scholar
46. Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ: A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998; 121:47–57Crossref, Medline, Google Scholar
47. LeDoux J: Emotion and the amygdala, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Edited by Aggleton J. New York, Wiley-Liss, 1992, pp 339–351Google Scholar
48. Cahill L, McGaugh JL: Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Behav Neurosci 1990; 104:532–543Crossref, Medline, Google Scholar
49. Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nägele T, Gur RC: Functional MRI reveals left amygdala activation during emotion. Psychiatry Res Neuroimaging 1997; 76:75–82Crossref, Medline, Google Scholar
50. Lang PJ, Bradley MM, Cuthbert BN: International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, University of Florida, Department of Psychology, Center of Research in Psychophysiology, 1995Google Scholar
51. Aggleton JP: The Amygdala: Neurobiological Aspects of Emotions, Memory, and Mental Dysfunction. New York, Wiley-Liss, 1992Google Scholar
52. Gaffan D, Murray EA, Fabre-Thorpe M: Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci 1993; 5:968–975Crossref, Medline, Google Scholar
53. Thorpe SJ, Rolls ET, Maddison S: The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res 1983; 49:93–115Crossref, Medline, Google Scholar
54. Ono T, Nishijo H: Neurophysiological basis of the Kluver-Bucy syndrome: responses of monkey amygdaloid neurons to biologically significant objects, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 167–190Google Scholar
55. Rolls E: Neurophysiology and functions of the primate amygdala. Ibid, pp 143–165Google Scholar
56. Partiot A, Grafman J, Sadato N, Wachs J, Hallett M: Brain activation during the generation of non-emotional and emotional plans. Neuroreport 1995; 6:1397–1400Google Scholar
57. George MS, Ketter TA, Gill DS, Haxby JV, Ungerleider LG, Herscovitch P, Post RM: Brain regions involved in recognizing facial emotion or identity: an oxygen-15 PET study. J Neuropsychiatry Clin Neurosci 1993; 5:384–394Crossref, Medline, Google Scholar
58. Nakamura K, Kawashima R, Nagumo S, Ito K, Sugiura M, Kato T, Nakamura A, Hatano K, Kubota K, Fukuda H, Kojima S: Neuroanatomical correlates of the assessment of facial attractiveness. Neuroreport 1998; 9:753–757Crossref, Medline, Google Scholar
59. Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Crossref, Medline, Google Scholar
60. Martinot J-L, Hardy P, Feline A, Huret J-D, Mazoyer B, Attar-Levy D, Pappata S, Syrota A: Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry 1990; 147:1313–1317Google Scholar
61. Robinson R, Kubos K, Starr L, Rao K, Price TR: Mood disorders in stroke patients: importance of lesion location. Brain 1984; 107:81–93Crossref, Medline, Google Scholar
62. Robinson R: Emotional and Psychiatric Disorders Associated With Brain Damage. Greenwich, Conn, JAI Press, 1996Google Scholar
63. Robinson RG, Paradiso S: Insights concerning the cerebral basis of emotion based on studies of mood disorders in patients with brain injury, in The Cerebral Basis of Emotions. Edited by Kavanaugh RD, Zimmerberg B, Fein S. Hillsdale, NJ, Lawrence Erlbaum Associates, 1995, pp 297–314Google Scholar
64. Herrmann M, Bartels C, Wallesch CW: Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry 1993; 56:672–678Crossref, Medline, Google Scholar
65. Astrom M, Adolfsson R, Asplund K: Major depression in stroke patients: a 3-year longitudinal study. Stroke 1993; 24:976–982Crossref, Medline, Google Scholar
66. Dehaene S, Changeux JP: The Wisconsin Card Sorting Test: theoretical analysis and modeling in a neuronal network. Cereb Cortex 1991; 1:62–79Crossref, Medline, Google Scholar
67. Harlow JM: Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society 1868; 2:329–347Google Scholar
68. Stuss DT, Gow CA, Hetherington CR: “No longer Gage”: frontal lobe dysfunction and emotional changes. J Consult Clin Psychol 1992; 60:349–359Crossref, Medline, Google Scholar
69. Wik G, Fredrikson M, Ericson K, Eriksson L, Stone-Elander S, Greitz T: A functional cerebral response to frightening visual stimulation. Psychiatry Res 1993; 50:15–24Crossref, Medline, Google Scholar
70. Olson CR, Musil SY: Posterior cingulate cortex: sensory and oculomotor properties of single neurons in behaving cat. Cereb Cortex 1992; 2:485–502Crossref, Medline, Google Scholar
71. Vogt BA, Finch DM, Olson CR: Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435–443Medline, Google Scholar
72. Devinsky O, Morell M, Vogt B: Contribution of anterior cingulate cortex to behavior. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
73. Swartz BE, Halgren E, Fuster J, Mandelkern M: An 18FDG-PET study of cortical activation during a short-term visual memory task in humans. Neuroreport 1994; 5:925–928Crossref, Medline, Google Scholar
74. Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG: A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA 1994; 91:8122–8126Google Scholar
75. Papez J: A proposed mechanism of emotion. Arch Neurol Psychiatry 1937; 150:713–719Google Scholar
76. Carmichael St, Price Jl: Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1995; 363:615–641Crossref, Medline, Google Scholar
77. Freeman JH Jr, Cuppernell C, Flannery K, Gabriel M: Limbic thalamic, cingulate cortical and hippocampal neuronal correlates of discriminative approach learning in rabbits. Behav Brain Res 1996; 80:123–136Crossref, Medline, Google Scholar
78. Fredrikson M, Wik G, Fischer H, Andersson J: Affective and attentive neural networks in humans: a PET study of Pavlovian conditioning. Neuroreport 1995; 7:97–101Crossref, Medline, Google Scholar
79. Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD: Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 1995; 57:109–128Crossref, Medline, Google Scholar
80. Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD: The role of the right hemisphere in the interpretation of figurative aspects of language: a positron emission tomography activation study. Brain 1994; 117(part 6):1241–1253Google Scholar
81. Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD: Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci 1996; 16:4275–4282Google Scholar
82. Joseph R: Neuropsychiatry, Neuropsychology, and Clinical Neuroscience: Emotion, Evolution, Cognition, Language, Memory, Brain Damage, and Abnormal Behavior, 2nd ed. Baltimore, Williams & Wilkins, 1996Google Scholar
83. Russchen FT, Bakst I, Amaral DG, Price JL: The amygdalostriatal projections in the monkey: an anterograde tracing study. Brain Res 1985; 329:241–257Crossref, Medline, Google Scholar
84. Amaral DG, Price JL: Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 1984; 230:465–496Crossref, Medline, Google Scholar
85. Russchen FT, Amaral DG, Price JL: The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Neurol 1987; 256:175–210Google Scholar
86. Wise RA, Bozarth MA: Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol 1981; 5:467–474Crossref, Medline, Google Scholar
87. Wise RA, Bozarth MA: Brain mechanisms of drug reward and euphoria. Psychiatry Med 1985; 3:445–460Google Scholar
88. Wise RA, Colle LM: Pimozide attenuates free feeding: best scores analysis reveals a motivational deficit. Psychopharmacology (Berl) 1984; 84:446–551Crossref, Medline, Google Scholar
89. Wise RA, Bozarth MA: Brain reward circuitry: four circuit elements “wired” in apparent series. Brain Res Bull 1984; 12:203–208Crossref, Medline, Google Scholar
90. Bozarth MA, Wise RA: Heroin reward is dependent on a dopaminergic substrate. Life Sci 1881; 29:1881–1886Google Scholar
91. Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ: Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 1989; 52:1655–1658Google Scholar
92. Bertolucci-D’Angio M, Serrano A, Scatton B: Differential effects of forced locomotion, tail-pinch, immobilization, and methyl-beta-carboline carboxylate on extracellular 3,4-dihydroxyphenylacetic acid levels in the rat striatum, nucleus accumbens, and prefrontal cortex: an in vivo voltammetric study. J Neurochem 1990; 55:1208–1215Google Scholar
93. Bertolucci-D’Angio M, Serrano A, Scatton B: Mesocorticolimbic dopaminergic systems and emotional states. J Neurosci Methods 1990; 34:135–142Crossref, Medline, Google Scholar
94. Salamone JD: The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res 1994; 61:117–133Crossref, Medline, Google Scholar
95. McCullough LD, Salamone JD: Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology (Berl) 1992; 109:379–382Crossref, Medline, Google Scholar
96. Salamone JD: The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods 1996; 64:137–149Crossref, Medline, Google Scholar
97. Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ: Brain systems for encoding and retrieval of auditory-verbal memory: an in vivo study in humans. Brain 1995; 118(part 2):401–416Google Scholar
98. Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ: Brain activity during memory retrieval: the influence of imagery and semantic cueing. Brain 1996; 119(part 5):1587–1596Google Scholar
99. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 1995; 152:1576–1585Google Scholar
100. Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Rezai K, Watkins GL, Ponto LLB, Hichwa RD: PET studies of memory, I: novel and practiced free recall of complex narratives. Neuroimage 1995; 2:284–295Crossref, Medline, Google Scholar
101. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LLB, Hichwa RD: PET studies of memory, II: novel versus practiced free recall of word lists. Neuroimage 1995; 2:296–305Crossref, Medline, Google Scholar
102. Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE: Common blood flow changes across visual tasks, II: decreases in cerebral cortex. J Cogn Neurosci 1997; 9:647–662Google Scholar
103. Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL: The functional organization of human extrastriate cortex: a PET rCBF study of selective attention to faces and locations. J Neurosci 1994; 14:6336–6353Google Scholar
104. Krauzlis RJ, Lisberger SG: Directional organization of eye movement and visual signals in the floccular lobe of the monkey cerebellum. Exp Brain Res 1996; 109:289–302Medline, Google Scholar
105. Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR: Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 1996; 75:454–468Crossref, Medline, Google Scholar
106. Luria AR: The Working Brain: An Introduction to Neuropsychology. London, Allen Lane, 1973Google Scholar
107. Schmahmann JD, Pandya DN: Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett 1995; 199:175–178Crossref, Medline, Google Scholar
108. Middleton FA, Strick PL: Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 1994; 266:458–461Crossref, Medline, Google Scholar
109. Kim S, Ugurbil K, Strick P: Activation of cerebellar output nucleus during cognitive processing. Science 1994; 265:949–951Crossref, Medline, Google Scholar
110. Paradiso S, Andreasen NC, O’Leary DS, Arndt S, Robinson RG: Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol 1997; 10:1–8Medline, Google Scholar
111. Allen G, Buxton RB, Wong EC, Courchesne E: Attentional activation of the cerebellum independent of motor involvement. Science 1997; 275:1940–1943Google Scholar



