Summer Birth and the Deficit Syndrome of Schizophrenia
Abstract
Objective: Patients with the deficit syndrome differ from other patients with schizophrenia relative to physiological correlates, course of illness, and response to treatment. Because of the abnormal seasonality of birth among persons with schizophrenia, the authors examined the relation between this risk factor and the deficit syndrome.Method: Findings in two clinical groups suggested an increase in summer births among deficit syndrome patients. The association between summer birth and the deficit syndrome was then examined in a catchment area study of first-admission patients with psychosis.Results: In the catchment area sample, summer birth was also significantly associated with the deficit syndrome; negative symptoms broadly defined were not. Conclusions: These findings add to the increasing evidence that 1) patients with the deficit syndrome have a disease with an etiopathophysiology separate from that of other patients with what is now called schizophrenia and 2) the correlates of broadly defined negative symptoms are different from those for the deficit syndrome. The previously reported association between winter birth and schizophrenia appears to apply to nondeficit schizophrenia only. Am J Psychiatry 1998; 155: 1221-1226
An amotivational, anhedonic syndrome, found in some patients with schizophrenia, has been an object of research since the time of Kraepelin (1), and descriptions of patients with both “insanity” and a “loss of will” can be found in the work of Griesinger in the middle of the nineteenth century (2). In more recent years, a number of putative subtypes, including type II schizophrenia (3), negative schizophrenia (4), and negative symptoms, have been proposed in an effort to operationalize this aspect of the psychopathology of schizophrenia.
Another approach to this aspect of schizophrenia is the concept of the deficit syndrome (5). The criteria for the deficit syndrome are unique, since they require clinical features that are enduring and not secondary to such features as suspicious withdrawal, depression, drug side effects, demoralization, a response to overwhelming stimulation, or any factor other than a loss of motivation and interests. Although there is widespread agreement that these state/trait and primary/secondary distinctions are theoretically attractive, there has been concern that they cannot be made with good reliability. However, multiple groups of investigators have developed good interrater reliability for the deficit/nondeficit categorization (6, 7, and unpublished manuscript of Amador et al.), and in large data sets, a case identification approach which does not require that the primary/secondary distinction be made explicitly has proven to delineate deficit and nondeficit groups that are quite similar to those diagnosed clinically (8-11).
Compared to other putative negative symptom subtypes, the deficit syndrome group has distinctive clinical features. Although negative symptoms tend to increase as positive symptoms increase, deficit syndrome patients do not have more severe psychotic symptoms than do nondeficit patients, and in some studies deficit patients have had a slightly lesser severity of positive symptoms (5, 8, 12. Thus, greater severity of psychotic symptoms cannot explain the lower level of functioning exhibited by deficit patients before the onset of frank psychosis (10, 12, 13), in early adulthood (9, 10), and at an average of 19 years after the deficit/nondeficit categorization was made (6, 13). This difference in functioning also cannot be attributed to two other problems frequently confronting people with schizophrenia: depression and drug abuse. Deficit patients abuse drugs less than do other patients with schizophrenia (10), and not only do they have decreased emotionality by definition (7), but deficit status predicts decreased severity of depressive mood (14) and other dysphoria (8) at follow-up. The deficit syndrome is also associated with less frequent suicidal thoughts than are found in nondeficit patients, and it may also be associated with a decreased risk of suicide (13) despite deficit patients’ greater anhedonia (15).
Aside from these differences in course of illness, other studies have suggested that the deficit syndrome delineates a group with a pathophysiology which differs from that of nondeficit schizophrenia. Compared to other people with schizophrenia, those with the deficit syndrome have differences in regional brain glucose (16) and oxygen (17) utilization as measured by positron emission tomography. Deficit and nondeficit groups also differ relative to neuropsychological functioning (18, 19), sensory integration signs on neurological examination (12), frontal lobe white matter (20), cerebellar volume (Summerfelt et al., unpublished data), oculomotor functioning (21), and risk of abnormal movements before exposure to neuroleptics (22). There are also subtle differences in the content of their delusions (11). These differences cannot be attributed to differences in duration of illness, demographic variables, or (as noted above) greater severity of psychotic symptoms in the deficit patients. This evidence suggests there are significant differences in the pathophysiology of deficit and nondeficit patients. It also raises the question of whether etiologic and risk factors also differ between the two groups.
The association of winter birth and schizophrenia, which has been extensively replicated (23-25), has had considerable theoretical influence. This risk factor appears to apply to a biological subgroup of patients. One review (25) suggested that on average, winter-born patients with schizophrenia have a relatively benign disease. It was also reported that winter birth is associated with the paranoid subtype in men(26, 27). Some studies have found an increased ventricle-brain ratio or increased sulcal prominence on computerized tomography in winter-born patients (28-30); studies that have failed to find such an association have usually had smaller sample sizes than those with a positive finding (31).
Because the deficit syndrome is a particularly severe form of illness, the findings relating the heterogeneity of schizophrenia to winter birth led us to hypothesize that winter birth would not be associated with the deficit syndrome. The evidence that some central nervous system disorders other than schizophrenia (of all types) also show seasonality of birth suggested that this was a reasonable hypothesis. One study (32) found that among patients with multiple sclerosis, there was an excess of births from March to June; a second study (33) found a similar pattern, but the sample size was one-third that of the original study, and the finding was not statistically significant. Dyslexia in boys has been found to be associated with birth in May, June, and July (34), although this finding has not, to our knowledge, been replicated. The finding that March and/or August birth is associated with autism has been replicated (35-39), and Down’s syndrome appears to show an excess of summer births (40-42).
To assess season of birth as a risk factor for the deficit syndrome, we first examined season of birth and the deficit/nondeficit categorization in a hypothesis-generating framework in two clinical study groups. We then attempted to replicate the seasonal pattern found in those groups in a catchment area sample of first-admission patients with psychosis.
METHOD
Study 1: Maryland Psychiatric Research Center
Sixty-three male and twenty-eight female patients in the outpatient program of the Maryland Psychiatric Research Center who met the DSM-III or DSM-III-R criteria for schizophrenia were studied. These patients were free of substantial drug abuse and any neurological problem, such as serious head injury, that might cause or seriously exacerbate their psychotic symptoms. Patients were categorized by two of us (B.K. and R.W.B.) into deficit (N=26) and nondeficit (N=65) groups with use of the Schedule for the Deficit Syndrome (7). These raters attained a kappa of 0.73 for the deficit/nondeficit categorization in this same clinic population (7). Previous studies of the deficit/nondeficit categorization from this clinic, and descriptions of the patients’ clinical care, have been published (7, 8, 12, 14, 15, 18, 20).
Study 2: The DSM-IV Field Trial
The relationship of month of birth to deficit/nondeficit status was also examined in another study in a hypothesis-generating framework. The DSM-IV Field Trial for Psychotic Disorders has been previously described (43, 44; see also Acknowledgments). Only subjects from the Northern Hemisphere were included in our examination of month of birth. These subjects were administered the Field Trial Instrument, which included items from the Comprehensive Assessment of Symptoms and History (45) as well as the Global Assessment of Functioning Scale (46). One hundred fifty-six patients meeting the DSM-III or DSM-III-R criteria for schizophrenia were categorized into deficit (N=55) and nondeficit (N=101) groups by one of us (B.K.) using a proxy method based on items from the Field Trial Instrument. This categorization has been described in detail (10, 11). Relative to demographic variables, this group was representative of all field trial patients meeting diagnostic criteria (data not shown).
Study 3: The Suffolk County Mental Health Project
The Suffolk County Mental Health Project is a catchment area study of patients in their first psychiatric hospital admission in Suffolk County, Long Island, N.Y. (47). Patients with psychotic symptoms were assessed at the time of hospitalization and 6 and 24 months after admission.
Subjects included in this study met the DSM-III-R criteria for definite or probable schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychosis not otherwise specified at 24-month follow-up, on the basis of structured interviews and other information reviewed in a best-estimate diagnostic conference. With use of the proxy method previously validated directly against groups defined with the Schedule for the Deficit Syndrome (8, 10, 11), 84 of these patients were categorized by one of us (B.K.) into deficit (N=32) and nondeficit (N=52) groups on the basis of their 24-month Brief Psychiatric Rating Scale (BPRS) scores, as previously described in detail (9). In this sample, this approach delineated groups that were found to be very similar to deficit and nondeficit groups diagnosed with use of the Schedule for the Deficit Syndrome (9).
Since more deficit patients in study 1 were born in June than in any other month, and in study 2 more were born in July than in any other month, in this study group we examined the association of June or July birth and the deficit syndrome. In a log-linear procedure (CATMOD, in the SAS package), we used June/July birth and gender as 0 and 1 predictor variables and deficit/nondeficit categorization as the dependent variable. Gender and an interaction term for gender and summer birth were also used as predictor variables because 1) there is evidence for a difference between men and women relative to the course of schizophrenia (48), and 2) we had previously found a greater prevalence of males in the deficit group (unpublished data) in the Treatment Strategies of Schizophrenia study (49), the largest data set (>300 subjects) in which this issue has been addressed, and in the Roscommon Family Study, a population-based study that approximated a treated-incidence sample (Kirkpatrick et al., unpublished data).
Because it has been proposed that the actual year of birth may obscure or distort the relationship to season of birth (23, 50), we examined year of birth in the deficit and nondeficit groups in this sample. We also examined month of birth for all persons born in the United States and in the state of New York in 1960 (51), which was the “average” year of birth for the Suffolk County sample. The seasonal pattern of United States births for 1960 was quite similar to the patterns for the 10 years preceding and following 1960 (51). Finally, we examined the relation between severity of negative symptoms broadly defined and month of birth for all of the patients meeting the diagnostic criteria for study 3; for this we used the mean item score on the Scale for the Assessment of Negative Symptoms (SANS) (52) obtained at the 24-month follow-up interview. Previous studies (10, 16, 42, and unpublished data of Amador et al.) have shown that results obtained when the deficit/nondeficit categorization is used may differ from those obtained when a broad definition of negative symptoms is used.
In all three of these studies, written informed consent was obtained after the procedures had been fully explained.
RESULTS
As previously reported (11), deficit and nondeficit groups in study 1 (Maryland Psychiatric Research Center) did not differ relative to demographic variables or positive psychotic symptoms. More patients with the deficit syndrome were born in June than in any other month (figure 1). Negative symptoms as measured by BPRS factor 2 were not elevated in June or in June and July combined (for the latter, t=0.41, df=73, p=0.68).
In study 2 (DSM-IV field trial), more deficit patients were born in July than in any other month (figure 2).
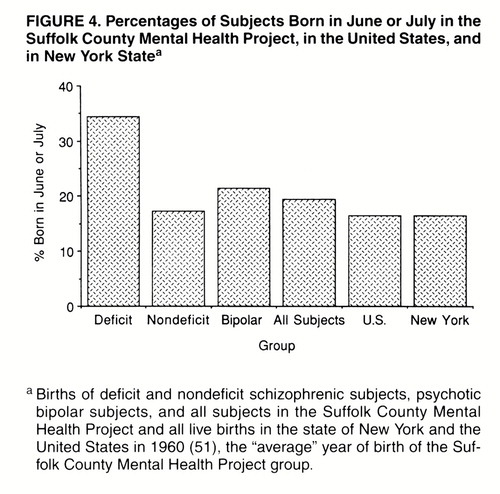
In study 3 (Suffolk County Mental Health Project), the deficit syndrome group had a significantly higher percentage of births in June or July than did the nondeficit group (34% and 17%, respectively; in the log-linear procedure, χ2=20.10, df=1, p<0.0001) (figure 3). Male gender was also a significant predictor of deficit syndrome status (χ2=6.59, df=1, p=0.01). There was no significant birth-by-gender interaction (χ2=1.56, df=1, p=0.21). To put the data in perspective, figure 4 shows the percentage of June/July births for four groups from the Suffolk County sample (patients with deficit and nondeficit schizophrenia, psychotic subjects with bipolar disorder, and all subjects) and for all persons born in the United States or in the state of New York in 1960.
Negative symptoms broadly defined were not associated with summer birth; patients born in June or July did not differ from other patients relative to SANS item scores (mean=1.72, SD=1.00, versus mean=1.70, SD=0.95; t=0.15, df=95, p=0.89) (figure 5).
In the assessment of year of birth, we found that the number of deficit patients born in a particular year was significantly correlated with the number of nondeficit patients born in the same year (Spearman rs=0.57, N=84, z=3.29, p<0.001). That is, in general, when there was an increase in births of deficit patients in a particular year, there was also an increase in the number of births of nondeficit patients.
DISCUSSION
In two clinical study groups (studies 1 and 2), we found an excess of June or July births among deficit syndrome patients. We then tested this association in a catchment area study of first-admission patients with psychosis (study 3) and were able to replicate the association. We have also replicated the association between the deficit syndrome and summer birth in another population-based sample that approximated a treated-incidence group, this one from the United Kingdom (Kirkpatrick et al., manuscript submitted for publication). Since deficit patients did not exhibit more severe positive psychotic symptoms (hallucinations, delusions, and formal thought disorder) in any of these studies, the association with summer birth reported here cannot be attributed to that aspect of the psychopathology of schizophrenia.
Our results depend on the validity of the proxy method used to categorize deficit and nondeficit patients. We originally devised this method for the purpose of making the deficit/nondeficit categorization in large studies in which it was not practical to administer the Schedule for the Deficit Syndrome and for application to existing data sets. We validated the method with respect to the Schedule for the Deficit Syndrome before applying it to other data sets (8). We have further validated it by showing that this approach delineates deficit and nondeficit groups with the combination of clinical features characteristic of groups diagnosed with the Schedule for the Deficit Syndrome (8–11 and unpublished data of Amador et al.). The replication of the season of birth effect across these studies, in two of which this approach was used,is a further validation of this method for delineating deficit and nondeficit groups.
Previous research has suggested that the risk factor of winter birth applies only to a biologically meaningful subgroup of patients with schizophrenia (25–30, 53, 54). An association between broadly defined negative symptoms and winter birth has been previously reported (55), but we did not replicate that association in the Suffolk County sample. However, other studies have found differences in the correlates of negative symptoms and those of the deficit syndrome (10, 16, 43, and unpublished data of Amador et al.). The replicated association between season of birth and other neuropsychiatric disorders (32-39) supports the biological plausibility of our finding.
One implication of our results is that there appears to be a “double dissociation” in season of birth in schizophrenia: summer birth is associated with the deficit syndrome, while winter birth is a risk factor for nondeficit schizophrenia only. Previous studies of season of birth and schizophrenia have usually required much larger study groups than ours for an effect of season of birth to be apparent, so lack of an association between winter birth and the entire schizophrenic group in these relatively small studies is not surprising.
What factor with a seasonal occurrence might be the cause of this association? Exposure to influenza during gestation has been found to increase the risk of schizophrenia in later life; the period of risk may be restricted to the second and early third trimesters (55). Influenza typically has its period of greatest incidence during winter months. Our results raise the possibility that exposure to influenza has an impact on the developing brain during midgestation and, a few months later, during the summer, there is an increase in births of children who will subsequently suffer from the deficit form of schizophrenia.
Other studies have shown biological differences between deficit and nondeficit schizophrenia (16-22), and these differences cannot be attributed to a greater severity of the same abnormalities that affect nondeficit patients. Whatever the cause of the association between the deficit syndrome and summer birth, the demonstration (and replication) of a risk factor specific to the deficit syndrome, taken with this other evidence, suggests that the patients with this syndrome have a pathophysiology that differs significantly from that of other people with schizophrenia.
ACKNOWLEDGMENTS
The authors acknowledge and thank all those involved in the data collection of the DSM-IV Field Trial for Psychotic Disorders, across the nine sites in which this study was conducted. Special thanks go to the site coordinators: Xavier Amador, Ph.D., and Jack Gorman, M.D. (New York State Psychiatric Institute, Columbia University, New York); H. Stefan Bracha, M.D. (Department of Psychiatry, University of Arkansas, Little Rock); William Edel, Ph.D., and Thomas McGlashan, M.D. (Yale Psychiatric Institute, Yale University, New Haven, Conn.); Anand Pandurangi, M.D., and Kenneth Kendler, M.D. (Department of Psychiatry, Medical College of Virginia, Richmond); Delbert Robinson, M.D., and Jeffrey Lieberman, M.D. (Department of Psychiatry, Long Island Jewish-Hillside Medical Center, Glen Oaks, N.Y.); Alfonso Ontiveros, M.D., M.Sc. (Department of Psychiatry, Autonomous University of Nuevo Leon, Monterey, Mexico); Mauricio Tohen, M.D., Dr.P.H. (Harvard University, McLean Hospital, Belmont, Mass.); and Patrick McGorry, M.D. (Department of Psychiatry, University of Melbourne, Melbourne, Australia). Finally, Michael Flaum, M.D., Nancy C. Andreasen, M.D., Ph.D., Gary Tyrrell, M.A., and Stephen Arndt, Ph.D. (University of Iowa, Iowa City) played critical roles in the design, data management, and data analysis of the overall field trial project.
Received Feb. 18, 1997; revisions received Sept. 11, 1997, and Jan. 6, 1998; accepted Jan. 30, 1998. From the Department of Psychiatry, Maryland Psychiatric Research Center, University of Maryland School of Medicine; the Department of Psychiatry, State University of New York, Buffalo; New York State Psychiatric Institute, Department of Psychiatry, Columbia University, New York; the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; McLean Hospital, Department of Psychiatry, Harvard University, Belmont, Mass.; and the Department of Psychiatry, School of Medicine, State University of New York at Stony Brook.. Address reprint requests to Dr. Kirkpatrick, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD. Supported in part by NIMH grants MH-40279, MH-44801, and MH-00925 and by a grant from the National Alliance for Research on Schizophrenia and Depression.The authors thank William T. Carpenter, Jr., M.D., for discussion of these issues, the staff of the outpatient program of the Maryland Psychiatric Research Center, and the staff of the Suffolk County Mental Health Project.




1. Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. New York, Robert E Krieger, 1971Google Scholar
2. Griesinger W: Mental Pathology and Therapeutics (1867). New York, Hafner, 1965Google Scholar
3. Crow T: The two-syndrome concept: origins and current status. Schizophr Bull 1985; 11:471–486Crossref, Medline, Google Scholar
4. Andreasen NC, Olsen S: Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789–794Crossref, Medline, Google Scholar
5. Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Link, Google Scholar
6. Fenton WS, McGlashan TH: Testing systems for assessment of negative symptoms in schizophrenia. Arch Gen Psychiatry 1992; 49:179–184Crossref, Medline, Google Scholar
7. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
8. Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr: Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Res 1993; 47:47–56Crossref, Medline, Google Scholar
9. Kirkpatrick B, Ram R, Bromet EJ: The deficit syndrome in the Suffolk County Mental Health Project. Schizophr Res 1996; 22:119–126Crossref, Medline, Google Scholar
10. Kirkpatrick B, Amador XF, Flaum M, Yale SA, Gorman JM, Carpenter WT Jr, Tohen M, McGlashan T: The deficit syndrome in the DSM-IV Field Trial, I: alcohol and other drug abuse. Schizophr Res 1996; 20:69–78Crossref, Medline, Google Scholar
11. Kirkpatrick B, Amador XF, Yale SA, Bustillo JR, Buchanan RW, Tohen M: The deficit syndrome in the DSM-IV Field Trial, II: depressive episodes and persecutory beliefs. Schizophr Res 1996; 20:79–90Crossref, Medline, Google Scholar
12. Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr: Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 1990; 147:290–294Link, Google Scholar
13. Fenton WS, McGlashan TH: Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry 1994; 151:351–356Link, Google Scholar
14. Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr: Depressive symptoms and the deficit syndrome of schizophrenia. J Nerv Ment Dis 1994; 182:452–455Crossref, Medline, Google Scholar
15. Kirkpatrick B, Buchanan RW: Anhedonia and the deficit syndrome of schizophrenia. Psychiatry Res 1990; 31:25–30Crossref, Medline, Google Scholar
16. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
17. Carpenter WT Jr, Lahti AC, Holcomb HH, Caudill PJ, Zhao M, Medoff DR, Tamminga CA: rCBF pattern differences between schizophrenic patients with and without deficit symptoms. Abstracts of the Society for Neuroscience 1995; 21:2129Google Scholar
18. Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr: Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry 1994; 51:804–811Crossref, Medline, Google Scholar
19. Wagman AM, Heinrichs DW, Carpenter WT Jr: Deficit and nondeficit forms of schizophrenia: neuropsychological evaluation. Psychiatry Res 1987; 22:319–330Crossref, Medline, Google Scholar
20. Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT Jr: Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150:59–65Link, Google Scholar
21. Thaker G, Kirkpatrick B, Buchanan RW, Ellsberry R, Lahti A, Tamminga C: Oculomotor abnormalities and their clinical correlates in schizophrenia. Psychopharmacol Bull 1989; 25:491–497Medline, Google Scholar
22. Fenton WS, Wyatt RJ, McGlashan TH: Risk factors for spontaneous dyskinesia in schizophrenia. Arch Gen Psychiatry 1994; 51:643–650Crossref, Medline, Google Scholar
23. Bradbury TN, Miller GA: Season of birth in schizophrenia: a review of evidence, methodology, and etiology. Psychol Bull 1985; 98:569–594Crossref, Medline, Google Scholar
24. Eaton WW: Update on the epidemiology of schizophrenia. Epidemiol Rev 1991; 13:320–328Crossref, Medline, Google Scholar
25. Boyd JH, Pulver AE, Stewart W: Season of birth: schizophrenia and bipolar disorder. Schizophr Bull 1986; 12:173–186Crossref, Medline, Google Scholar
26. Hsieh HH, Khan MH, Atwal SS, Cheng SC: Seasons of birth and subtypes of schizophrenia. Acta Psychiatr Scand 1987; 75:373–376Crossref, Medline, Google Scholar
27. Nasrallah HA, McCalley-Whitters M: Seasonality of birth in subtypes of chronic schizophrenia. Acta Psychiatr Scand 1984; 69:292–295Crossref, Medline, Google Scholar
28. Zipursky RB, Schulz SC: Seasonality of birth and CT findings in schizophrenia. Biol Psychiatry 1987; 22:1288–1292Crossref, Medline, Google Scholar
29. Sacchetti E, Calzeroni A, Vita A, Terzi A, Pollastro F, Cazzullo CL: The brain damage hypothesis of the seasonality of births in schizophrenia and major affective disorders: evidence from computerised tomography. Br J Psychiatry 1992; 160:390–397Crossref, Medline, Google Scholar
30. Degreef G, Mukherjee S, Bilder R, Schnur D: Season of birth and CT scan findings in schizophrenic patients. Biol Psychiatry 1988; 24:461–464Crossref, Medline, Google Scholar
31. DeQuardo JR, Goldman M, Tandon R: VBR in schizophrenia: relationship to family history of psychosis and season of birth. Schizophr Res 1996; 20:275–285Crossref, Medline, Google Scholar
32. Templer DI, Trent NH, Spencer DA, Trent A, Corgiat MD, Mortensen PB, Gorton M: Season of birth in multiple sclerosis. Acta Neurol Scand 1992; 85:107–109Crossref, Medline, Google Scholar
33. Sadovnick AD, IM Yee: Season of birth in multiple sclerosis. Acta Neurol Scand 1994; 89:190–191Crossref, Medline, Google Scholar
34. Livingston R, Adam BS, Bracha HS: Season of birth and neurodevelopmental disorders: summer birth is associated with dyslexia. J Am Acad Child Adolesc Psychiatry 1993; 32:612–616Crossref, Medline, Google Scholar
35. Mouridsen SE, Nielsen S, Rich B, Isager T: Season of birth in infantile autism and other types of childhood psychoses. Child Psychiatry Hum Dev 1994; 25:31–43Crossref, Medline, Google Scholar
36. Konstantareas MM, Hauser P, Lennox C, Homatidis S: Season of birth in infantile autism. Child Psychiatry Hum Dev 1986; 17:53–63Crossref, Medline, Google Scholar
37. Gilberg C: Do children with autism have March birthdays? Acta Psychiatr Scand 1990; 82:152–156Google Scholar
38. Barak Y, Ring A, Sulkes J, Gabbay U, Elizur A: Season of birth and autistic disorder in Israel. Am J Psychiatry 1995; 152:798–800Link, Google Scholar
39. Bartlik BD: Monthly variation in births of autistic children in North Carolina. J Am Med Wom Assoc 1981; 36:363–368Medline, Google Scholar
40. Puri BK, Singh I: Season of birth in Down’s syndrome. Br J Clin Pract 1995; 49:129–130Medline, Google Scholar
41. Seifert C, Sommer A: A summertime peak of Down’s syndrome in Franklin County, Ohio. Am J Dis Child 1986; 140:822–824Medline, Google Scholar
42. Rothman KJ, Fabia JJ: Place and time aspects of the occurrence of Down’s syndrome. Am J Epidemiol 1976; 103:560–564Crossref, Medline, Google Scholar
43. Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, Gorman JM: Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry 1994; 51:826–836Crossref, Medline, Google Scholar
44. Strakowski SM, Tohen M, Flaum M, Amador X, DSM-IV Field Trial Work Group: Substance abuse in psychotic disorders: associations with affective syndromes. Schizophr Res 1994; 14:73–81Crossref, Medline, Google Scholar
45. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
46. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
47. Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovaszny B, Lavelle J, Miller A, Pato C, Ram R, Rich C: The epidemiology of psychosis: the Suffolk County Mental Health Project. Schizophr Bull 1992; 18:243–255Crossref, Medline, Google Scholar
48. Castle DJ, Murray RM: The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med 1991; 21:565–575Crossref, Medline, Google Scholar
49. Schooler NR, Keith SJ, Severe JB, Matthews SM, Bellak AS, Glick ID, Hargreaves WA, Kane JM, Ninan PT, Frances A, Jacobs M, Lieberman JA, Mance R, Simpson GM, Woerner MG: Relapse and rehospitalization during maintenance treatment of schizophrenia: the effects of dose reduction and family treatment. Arch Gen Psychiatry 1997; 54:453–463Crossref, Medline, Google Scholar
50. Lewis MS: Age incidence and schizophrenia, part I: the season of birth controversy. Schizophr Bull 1989; 15:59–73Crossref, Medline, Google Scholar
51. Vital Statistics of the United States, 1960, vol 1: Natality. Washington, DC, US Department of Health, Education, and Welfare, 1960Google Scholar
52. Andreasen NC: Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry 1982; 39:784–788Crossref, Medline, Google Scholar
53. Franzek E, Beckmann H: Season-of-birth effect reveals the existence of etiologically different groups of schizophrenia. Biol Psychiatry 1992; 32:375–378Crossref, Medline, Google Scholar
54. Ohlund LS, Ohman A, Ost LG, Lindstrom LH, Wieselgren IM: Electrodermal orienting response, maternal age, and season of birth in schizophrenia. Psychiatry Res 1991; 36:223–232Crossref, Medline, Google Scholar
55. McGrath J, Castle D: Does influenza cause schizophrenia? a five-year review. Aust NZ J Psychiatry 1995; 29:23–31Crossref, Medline, Google Scholar



