Anterior Cingulate Gyrus Dysfunction and Selective Attention Deficits in Schizophrenia: [15O]H2O PET Study During Single-Trial Stroop Task Performance
Abstract
OBJECTIVE: Attentional deficits are a prominent aspect of cognitive dysfunction in schizophrenia. The anterior cingulate gyrus is proposed to be an important component of frontal attentional control systems. Structural and functional abnormalities have been reported in this region in schizophrenia, but their relationship to attentional deficits is unknown. The authors investigated the function of the anterior cingulate gyrus and the related neural systems that are associated with selective attention in patients with schizophrenia. METHOD: While subjects performed multiple blocks of a single-trial Stroop task, [15O]H2O positron emission tomography scans were obtained. Fourteen patients with schizophrenia were compared with 15 normal subjects matched for age, gender, and parental education. RESULTS: The patients with schizophrenia responded at the same rate but made more errors in color naming during the color-incongruent condition. Consistent with the authors' hypothesis, patients with schizophrenia showed significantly less anterior cingulate gyrus activation while naming the color of color-incongruent stimuli. CONCLUSIONS: Patients with schizophrenia fail to activate the anterior cingulate gyrus during selective attention performance. This finding adds to the understanding of the functional significance of the structural and metabolic abnormalities in schizophrenia that have been previously reported in this region of the brain. (Am J Psychiatry 1997; 154:1670–1675)
Selective attention, the ability to enhance the processing of information relevant to our goals and limit the processing of that which is irrelevant, is a fundamental cognitive capability that is essential for everyday functioning. Disturbances of selective attention are among the earliest described and most clinically apparent cognitive deficits that are present in schizophrenia (1), and these disturbances appear to be related to behavioral disorganization in this illness (2).
With the advent of functional brain imaging it has become possible to investigate the neural substrates of selective attention deficits. Positron emission tomography (PET) studies that employ continuous performance tasks have reported decreased metabolism in lateral and medial prefrontal cortex in patients with schizophrenia (3, 4). These studies used continuous performance tasks to stabilize the subjects' psychological state and place demands upon systems involved in vigilance and attention. These studies did not include control tasks or seek to establish specific relationships between abnormal attentional processes and the metabolic response of neural systems.
With the development of the [15O]H2O PET brain mapping technique, in which repeated scans are performed during different psychological states, it has become possible to design experiments that associate discrete neural system responses with specific cognitive processes. This involves constructing control tasks that match activation tasks on sensory, motor, and cognitive processes that are not specifically of interest. Comparisons of regional cerebral blood flow (CBF) between activation and control states isolate the regional CBF responses that are related to the specific processes of interest. A recent application of this technique used a dichotic listening task to show impaired task-appropriate modulation of blood flow in the superior temporal gyrus in patients with schizophrenia (5).
In studies of attentional processes analyzed by human brain mapping, considerable emphasis has been placed upon the role of the anterior cingulate gyrus (6). The anterior cingulate gyrus is activated when subjects divide their attention across more than one feature of a stimulus (7) but not when they attend to a single feature. During the Stroop task (8), a paradigmatic measure of selective attention in which subjects must avoid reading a word (a pre-potent response) while naming its color, anterior cingulate gyrus activation has been reported in three independent studies (9–11); the precise rostro-caudal location of activation varied with the particular stimulus parameters employed (11). In contrast, the dorsolateral prefrontal cortex and parietal cortex, but not the anterior cingulate gyrus, were activated when subjects maintained attention for the occurrence of visual and somatosensory stimuli (12). It has been proposed that anterior cingulate gyrus activation is associated with the selection of one of several competing responses (6, 13) and that it has an executive role in the control of selective attention (6, 10).
Anterior cingulate gyrus abnormalities have been reported in morphometric and histopathological studies of schizophrenia (14, 15). Medial frontal hypometabolism was found in patients at rest (16) and during performance of a continuous performance task (17, 18). Decreased medial frontal activation, probably in the anterior cingulate gyrus, was seen in patients during performance of the Tower of London task (19), and impaired activation was seen in the anterior cingulate gyrus during paced verbal fluency, compared to word repetition, tasks in patients with schizophrenia (20). We recently reported decreased activation in the anterior cingulate gyrus, dorsolateral prefrontal cortex, and superior temporal gyrus during supraspan memory performance (21). Liddle and colleagues reported significant correlations between anterior cingulate gyrus regional CBF and disorganization in chronically symptomatic subjects (22). Together these studies suggest that there are behaviorally relevant structural and functional anterior cingulate gyrus abnormalities in patients with schizophrenia. However the relationship between these abnormalities and specific functional deficits, such as impaired selective attention, remains unknown. The present study sought to address this question by using [15O]H2O PET and a single-trial Stroop task.
METHOD
Subjects
The study was approved by the University of Pittsburgh biomedical institutional review board. All subjects gave written informed consent after the procedure was fully explained to them. Fourteen patients with schizophrenia and 15 healthy comparison subjects participated. All were right-handed (determined by their response to the first three questions of the Edinburgh Inventory [23]), had normal or corrected-to-normal vision that could discern color, and were native English speakers. The comparison subjects were matched as a group with the patients with schizophrenia on age (patients with schizophrenia: mean=35.7 years, SD=4.4; comparison subjects: mean=34.3, SD=9.1), gender (patients with schizophrenia: six women, eight men; comparison subjects: eight women, seven men), and years of parental education (patients with schizophrenia: mean=12.0, SD=1.6; comparison subjects: mean=12.5, SD=1.8). Regional CBF data from the normal comparison group, analyzed by using an earlier version of Statistical Parametric Mapping, have been reported elsewhere (11). All patients met DSM-IV criteria for chronic schizophrenia (N=12) or schizoaffective disorder, bipolar type (N=2), and had been clinically stable on fixed-dose antipsychotic regimens for a minimum of 3 months. Two patients were being treated with clozapine, and two were receiving risperidone. The rest were being treated with a variety of conventional antipsychotics. Schizophrenic symptoms were rated on the day of study by using the Positive and Negative Symptom Scale (24). We examined correlations among symptoms, performance, and regional CBF responses by combining items from the Positive and Negative Symptom Scale to produce three syndromes. The positive syndrome consisted of the symptoms of hallucinations and delusions. Blunted affect, emotional withdrawal, passive social avoidance, motor retardation, and lack of spontaneity were combined to form the negative syndrome. Finally, the symptoms of conceptual disorganization, difficulty abstracting, and poor attention comprised the disorganization syndrome. Alphas for these syndromes were 0.85, 0.87, and 0.78, respectively.
Procedures
PET images were obtained with a Seimens ECAT951r/31 camera. Subjects' heads were immobilized by using an individually molded thermoplastic mask. The PET gantry was rotated and tilted so that the lowest imaging plane was parallel to, and approximately 1 cm above, the canthomeatal line. A 10-minute transmission scan that used three rotating “pin” sources of 68Ge/68Ga for the purpose of calculating attenuation factors preceded the blood flow studies.
Brain activity was measured after an intravenous injection of 50 mCi of [15O]H2O in 5–7 ml of saline. Twenty seconds after injection, a 60-second emission scan was acquired and reconstructed to approximately 10 mm full width at half maximum to create a map that is highly proportional to CBF (25). In subsequent sections we will follow the convention of referring to these data as regional CBF.
The Stroop task was programmed by using PsyScope (26) on a Macintosh IIci computer. Subjects began the task 40 seconds before injection and continued for 3 minutes. Each trial began with a central fixation cross for 500 msec that was followed by the stimulus (a colored word) for 1250 msec. Subjects' responses were registered by using a voice-activated relay and timing device with millisecond accuracy. Responses were also tape recorded to measure accuracy. In the two previous PET studies of single-trial Stroop performance that included color-congruent stimuli, these stimuli comprised an entire block of trials (9, 10). In discussing these studies, Taylor et al. (13) pointed out that when subjects are presented with a block of color-congruent trials they may change strategy and read the word rather than name the color. To control for this, we mixed neutral stimuli in with the color-congruent and color-incongruent stimuli. Facilitation blocks consisted of trials in which 50% of the stimuli were color-congruent (e.g., the word RED printed in red), and 50% were neutral (e.g., the word DOG printed in red). Interference blocks consisted of trials in which 50% of the stimuli were color-incongruent (e.g., the word RED printed in green), and 50% were neutral. Neutral blocks consisted of animal name words that were printed in color. Stimuli in all blocks were presented in random order. Subjects were instructed to fixate on the center of the monitor and ignore the words while naming their colors as quickly and accurately as possible. Subjects performed each of the three experimental conditions three times. The order was counterbalanced across subjects. While in the PET scanner, but before the first regional CBF scan, subjects received one practice block of the condition that they had been randomized to receive first during the PET study.
Data Analysis
Individual PET images were registered by using an automated algorithm to correct for small head movements (27), then registered to the individual subject's MRI scan (28). Spatial normalization was accomplished by registering these data to a standard MRI that had been previously transformed to the coordinates of the atlas of Talairach and Tournoux (29). PET images were then analyzed by using Statistical Parametric Mapping (30) software. Data were normalized to an average value of 50 ml/100 ml/minute by using analysis of covariance (ANCOVA). A gaussian filter (12 mm full width at half maximum) was applied to the data to reduce the effects of high frequency noise and individual differences in anatomy. The mean difference between the color-incongruent and the neutral conditions was compared between the patients with schizophrenia and the healthy subjects by using a split plot ANCOVA. The resulting image of t statistics was transformed to z scores to allow the associated display to be independent of the degrees of freedom. The comparison of patients with schizophrenia and normal subjects was undertaken in two steps. The first analysis was hypothesis driven and focused on the anterior cingulate gyrus. The critical threshold for this comparison was a z score of 2.32, which corresponded to a p value of 0.01. The second comparison was exploratory and sought to identify other regions throughout the brain in which activation in patients with schizophrenia was less than that of comparison subjects during performance of the Stroop task. The critical z score for this comparison was 3.09, which corresponded to a p value of 0.001.
RESULTS
Task Performance
Because of initial technical difficulties in measuring verbal responses in the scanner, reliable reaction time data were available for only nine comparison subjects and 10 patients with schizophrenia. Accuracy ratings, scored from taped responses, were obtained for all but one subject in each group. Both groups were able to maintain the pace of responding to a stimulus every 1750 msec without omissions. Hence, differences between the groups' regional CBF response cannot be due to reduced rates of responding, which is a frequent confound in mapping studies of cognitively impaired subjects.
As in previous studies of single-trial Stroop task performance (31), the patients with schizophrenia did not show greater interference, as measured by the difference in reaction times for color-incongruent stimuli (patients with schizophrenia: mean=958.6 msec [SD=186.4], comparison subjects: mean=902.3 msec [SD=229.4]) and neutral stimuli (patients with schizophrenia: mean=855.9 msec [SD=171.0], comparison subjects: mean=805.3 msec [SD=192.3]) (F=0.03, df=1,17, p<0.87). In the error analysis, overall error rates were low, which confirms that subjects understood the task and were performing as instructed. The patients with schizophrenia showed a larger increase in errors with color-incongruent stimuli (mean=11.1%, SD=9.2%) than with neutral stimuli (mean=2.2%, SD=1.9%) than did the comparison subjects (mean=1.9% [SD=3.4%] and mean=0.5% [SD=0.9%], respectively) (F=7.9, df=1,25, p<0.01). These errors invariably consisted of reading the word rather than naming its color. This greater error interference remained significant whether the comparison was made for all subjects or just those with reaction time data. Pearson's correlation coefficients between reaction times and error rates for color-incongruent stimuli for the whole group (r=0.10, df=18, p<0.34), the comparison subjects (r=0.36, df=8, p<0.34), and the patients with schizophrenia (r=–0.02, df=9, p<0.34) were not significant. The nonparametric Spearman's rank order correlation showed a similar result (whole group: rs=0.14, N=19, p<0.26; comparison subjects: rs=0.42, N=9, p<0.26; patients with schizophrenia: rs=–0.12, N=10, p<0.26). Hence, the higher frequency of errors was not simply due to a speed-accuracy tradeoff by the patients with schizophrenia. Rather, the greater number of errors in the color-incongruent condition indicates attentional dysfunction in the patients with schizophrenia, which reflects a greater influence of the irrelevant dimension of the stimulus (the word) over naming the color (32).
Performance during color-congruent blocks did not reveal differences between the patients with schizophrenia and the comparison subjects in either reaction times (color-congruent mean=723.7 msec [SD=209.7] and 665.7 msec [SD=160.0], respectively; neutral mean=790.8 msec [SD=218.5] and 751.2 msec [SD=176.2], respectively) or errors (color-congruent mean=0.1% [SD=0.3%] and 0.0% [SD=0.0%], respectively; neutral mean=1.4% [SD=1.2%] and 0.6% [SD=1.1%], respectively). Comparison subjects showed more reaction time facilitation than patients, but this was not significant. This would be expected if patients with schizophrenia have a deficit in the control of selective attention, since in the current design the color-congruent condition was a mix of neutral and color-congruent stimuli. Comparison subjects would be better able to strategically allow more reading, thus taking advantage of the presence of color-congruent color names to improve performance.
Regional CBF
The regional CBF analysis focuses on the comparison of the two groups' responses to color-incongruent versus neutral conditions, since it is in this comparison that patients showed significant evidence of impaired selective attention.
Consistent with our hypothesis, patients with schizophrenia showed significantly less activation than comparison subjects in the right anterior cingulate gyrus (pixel maxima at Talairach coordinates 12, 46, 4) (z=2.88, N=29, p<0.002). The location and extent of this group difference is shown in figure 1.
Figure 2 shows other regions of the brain that increased less in patients with schizophrenia than in normal comparison subjects in response to naming the color of color-incongruent stimuli. Differences are seen in the left precentral gyrus (Talairach coordinates –26, –14, 52) (z=3.24, N=29, p<0.001), and right hippocampal gyrus (Talairach coordinates 12, –38, 4) (z=3.45, N=29, p<0.001). Orthogonal projection views of these group differences in activation are shown in figure 2.
Group differences in the magnitude of regional CBF responses seen in the anterior cingulate gyrus, left precentral gyrus, and right hippocampal region were not due to greater variability in these regions. The patients with schizophrenia showed smaller and generally less variable responses than comparison subjects in the anterior cingulate gyrus (patients with schizophrenia: mean change=1.2% [SD=2.4%], comparison subjects: 2.4% [SD=3.4%]) and the precentral gyrus (patients with schizophrenia: mean=–0.2% [SD=2.2%], comparison subjects: mean=3.3% [SD=3.4%]). The hippocampal gyrus difference reflected a region that decreased more in the patients with schizophrenia (mean=–2.4%, SD=3.7%) than in the normal comparison subjects (mean=0.3%, SD=4.3%).
Correlations Between Regional CBF Response and Behavior
Pearson's product moment correlation coefficients were calculated between errors in the color-incongruent condition and regional CBF response in the anterior cingulate gyrus. This correlation was positive and approached significance at a trend level in both groups (comparison subjects: r=0.48, df=13, p<0.09; patients with schizophrenia: r=0.48, df=12, p<0.10). To evaluate the specificity of this finding, correlations were computed between errors and the regional CBF response at the two other regions that distinguished patients with schizophrenia from comparison subjects: the left precentral gyrus (patients with schizophrenia: r=–0.52, df=12, p<0.07; comparison subjects: r=–0.23, df=13, p<0.16) and the right hippocampal region (patients with schizophrenia: r=0.44, df=12, p<0.14; comparison subjects: r=–0.40, df=13, p<0.16).
Within the schizophrenia group correlation coefficients were computed for anterior cingulate gyrus activation and positive, negative, and disorganization syndrome scores. None of these correlations approached significance. The correlation between errors in the color-incongruent condition and disorganization, which we have found in studies of larger groups of patients (33), was also not significant.
DISCUSSION
Consistent with our hypothesis, patients with schizophrenia showed significantly less anterior cingulate gyrus activation than normal comparison subjects during the color-incongruent condition of the Stroop task. This is consistent with previous studies that suggested abnormal medial frontal physiology in schizophrenia (16–22). Because of our experimental design we are able to attribute functional relevance to this finding. We compared regional CBF under attentionally demanding conditions (response competition) to a state in which sensory, motor, and cognitive components unrelated to resolving this competition were identical. Hence, we conclude that failure to activate the anterior cingulate gyrus in the color-incongruent condition is related to the selective attention demands of the task.
The primary purpose of this study was to test a focal hypothesis: patients with schizophrenia would fail to activate the anterior cingulate gyrus during selective attention performance. Our results confirm this hypothesis while also showing that other regions respond abnormally to task performance in patients with schizophrenia. This result is consistent with recent PET findings that suggested that patients with schizophrenia fail to modulate cortical activity in distributed networks in a task-appropriate manner (5, 34). One hypothesis that has been invoked to account for these findings is that corticocortical or corticostriatal-thalamic connectivity is disturbed in schizophrenia (5, 35). The caveat to this interpretation is that a failure to activate a region involved in executive control would also have widely distributed ramifications within a neural network engaged by the task. The design of previous studies as well as the current one do not allow us to draw firm conclusions regarding which of these two interpretations is more accurate. Further studies with novel designs and quantitative methods for evaluating connectivity are needed to resolve these two competing views regarding the underpinnings of abnormal patterns of cortical activation during cognitive performance in schizophrenia.
In both groups, anterior cingulate gyrus activation correlated with errors in the color-incongruent condition. Similar correlations between poor performance and anterior cingulate gyrus activation have been previously reported during a Stroop-like task (13) and during a response inhibition task (36). A baseline level of task-related activation, with incremental increases with increasing error rates, is consistent with the hypothesized role of the anterior cingulate gyrus in monitoring for or detecting errors and in strategically reactivating selected circuits to compensate for their occurrence (37, 38). Such a function would reflect the connectivity between the anterior cingulate gyrus and both the cortical systems involved in cognition and the limbic regions involved in emotion and motivation. Physiological dysfunction in this region could result in the lower regional CBF response and greater number of color-naming errors seen in patients in the current study, with preservation of the correlational relationship between these two measures.
The relationship between impaired selective attention performance and disorganization reported in other studies (2, 31) was not found. There were no significant relationships between symptoms and cingulate regional CBF response, which may reflect a restricted range of patient symptoms. All patients were mildly ill and clinically stable outpatients.
All patients in the study were treated with neuroleptics. It is possible that the failure of the anterior cingulate gyrus to activate during Stroop performance in the patients with schizophrenia reflects the effects of antipsychotic medications rather than processes related to the pathophysiology of schizophrenia. Decreases in resting anterior cingulate gyrus regional CBF and metabolism have been reported 3–4 weeks after neuroleptic withdrawal in patients with schizophrenia (39, 40), which confirms that increasing dopamine tone increases resting cingulate blood flow and metabolism. However, since previous studies that reported reduced anterior cingulate gyrus activation during Tower of London performance (19) and verbal fluency (34) found this result in unmedicated patients, we believe that this account of our results is unlikely. A definitive conclusion on this point must await a replication in an unmedicated group of patients.
The locus of the reduced regional CBF response associated with naming the color of color-incongruent stimuli is within the rostral anterior cingulate gyrus. Similar loci of activation have previously been reported during Stroop performance (10). On the basis of animal studies and a limited review of human PET studies (41), it has been proposed that within the anterior cingulate gyrus the more caudal region is involved with cognition and the more rostral region with emotions. The results of this and other Stroop studies suggest that a role in attention extends to the pregenual portion of the cingulate. Reports of caudal anterior cingulate gyrus activation in response to pain (42) are also inconsistent with a simplified functional division of ventral and dorsal regions along cognitive versus emotional lines. Of course, one can always argue that activation associated with pain reflects attention to this sensation and that activation during a cognitive task reflects an emotional response. However, the association between performance and anterior cingulate gyrus activation in the present study, as well as those of Taylor et al. (13) and Casey et al. (36), argues against a nonspecific emotional response driving anterior cingulate gyrus activation. More functional imaging studies that focus on this region are needed before we will have a clear understanding of the nature of functional specialization within the human anterior cingulate gyrus.
While the precise pathophysiological mechanisms that underlie a failure to increase regional CBF in the anterior cingulate gyrus during selective attention in schizophrenia are unknown, recent histopathological studies have reported findings that may begin to illuminate this issue. Benes (15) reported greater numbers of vertical glutamatergic fibers in layer II of the anterior cingulate gyrus in postmortem schizophrenic brains along with a reduction of GABAergic interneurones and up-regulation of postsynaptic GABA receptors. Benes proposed a model of a disturbed intrinsic circuitry of the anterior cingulate gyrus in which an increase in excitatory input and a reduction in GABAergic inhibition within layer II cause unmodulated pyramidal cell activity in this region and its distant projection sites. A similar model that emphasized disturbances in glutamatergic, dopaminergic, and GABAergic neurotransmission in the cingulate in schizophrenia has been also proposed by Olney and Farber (43).
In the present study we found that patients with schizophrenia showed significantly less activation in the anterior cingulate gyrus, which was associated with impaired selective attention. This suggests that the functional significance associated with histopathological and physiological abnormalities that have been previously reported in this region of the brain in schizophrenia includes a relationship with deficits in selective attention. These results demonstrate the utility of the Stroop task as an activating procedure in functional imaging studies and invite further studies that evaluate the relationship of anterior cingulate gyrus function, attentional deficits, and associated clinical manifestations in schizophrenia.
Presented at the Second International Conference on Functional Mapping of the Human Brain, Boston, June 17–21, 1996. Received Dec. 6, 1996; revisions received May 7 and June 13, 1997; accepted July 3, 1997. From the Department of Psychiatry, University of Pittsburgh, Western Psychiatric Institute and Clinic. Address reprint requests to Dr. Carter, Department of Psychiatry, University of Pittsburgh, Western Psychiatric Institute and Clinic, 3811 O'Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and NIMH Mentored Research Scientist Development Award for Clinicians MH-01306 to Dr. Carter. The authors thank David Kupfer, M.D., James McClelland, Ph.D., Rohan Ganguli, M.D., and Nina Schooler, Ph.D., for advice and support and MaryBeth Wiseman, B.S., Amy Herbster, B.S., and Renee Ralke, B.A., for technical assistance.
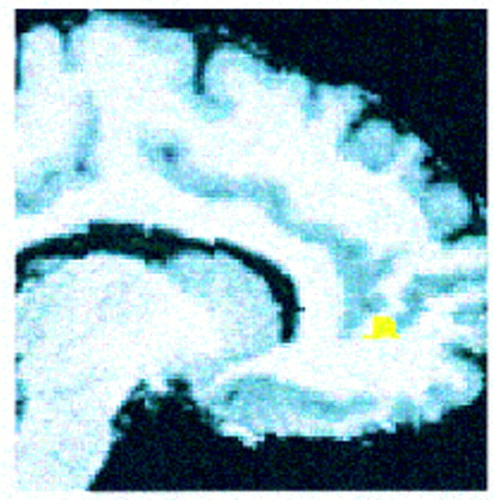
FIGURE 1. Extent and Location of the Difference Between Normal Comparison Subjects and Patients With Schizophrenia in Anterior Cingulate Gyrus Regional CBF Response to Naming the Color of Color-Incongruent Stimulia
aResults are rendered onto a T1-weighted structural MRI scan that has been spatially transformed to the coordinates of the atlas of Talairach and Tournoux (29).
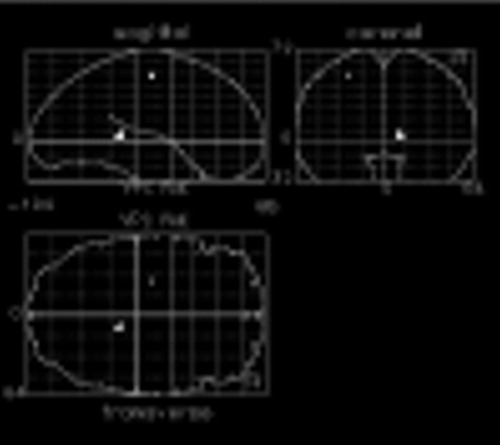
FIGURE 2. Additional Regional Differences Between Normal Comparison Subjects and Patients With Schizophrenia in Regional CBF Activation Response to Naming the Color of Color-Incongruent Stimuli Versus Naming the Color of Noncolor Words
1. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
2. Liddle PF, Morris DL: Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry 1991; 158:340–345Crossref, Medline, Google Scholar
3. Cohen RM, Semple WE, Gross M, Nordahl TE, DeLisi L, Holcomb H, King C, Marhisa JM, Pickar D: Dysfunction in a prefrontal substrate of sustained attention in schizophrenia. Life Sci 1987; 40:2031–2039Google Scholar
4. Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, Lohr J, Wu J, Lottenberg S, Jerabek PA, Trenary M, Tafalla R, Reynolds C, Bunney W: Frontostriatal disorder of cerebral metabolism in never medicated schizophrenics. Arch Gen Psychiatry 1992; 49:935–942Crossref, Medline, Google Scholar
5. O'Leary DS, Andreasen NC, Hurtig RR, Kesler ML, Rogers M, Arndt S, Cizadlo T, Watkins GL, Ponto LLB, Kirchner PT, Hichwa RD: Auditory attentional deficits in patients with schizophrenia: a positron emission tomography study. Arch Gen Psychiatry 1996; 53:633–641Crossref, Medline, Google Scholar
6. Posner MI, Dehaene S: Attentional networks. Trends Neurosci 1994; 17:75–79Crossref, Medline, Google Scholar
7. Corbetta M, Miezin FM, Dobmeyer S, Shulman G, Petersen SE: Selective and divided attention during visual discrimination of shape, color and speed. J Neurosci 1991; 11:2383–2402Google Scholar
8. Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
9. Pardo JV, Pardo J, Janer W, Raichle ME: The ACG cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 1990; 87:256–259Crossref, Medline, Google Scholar
10. Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ: Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 1993; 31:907–922Crossref, Medline, Google Scholar
11. Carter CS, Mintun M, Cohen JD: Interference and facilitation effects during selective attention: an H2O15O PET study of Stroop task performance. Neuroimage 1995; 2:264–272Crossref, Medline, Google Scholar
12. Pardo JV, Fox P, Raichle ME: Localization of a system for sustained attention by positron emission tomography. Nature 1991; 349:61–64Crossref, Medline, Google Scholar
13. Taylor SF, Kornblum S, Minoshima S, Oliver LM, Koeppe RA: Changes in medial cortical bloodflow with a stimulus-response compatibility task. Neuropsychologia 1994; 32:249–255Crossref, Medline, Google Scholar
14. Albanese AM, Merlo A, Mascitti TA, Tornee EB, Gomez EE, Konopka V, Albanese EF: Inversion of the hemispheric laterality of the ACG gyrus in schizophrenics. Biol Psychiatry 1995; 38:13–21Crossref, Medline, Google Scholar
15. Benes FM: Is there a neuroanatomic basis for schizophrenia? Neuroscientist 1995; 1:112–120Google Scholar
16. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia with fluorodeoxyglucose and neocortical alterations with the deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
17. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
18. Siegel BV Jr, Buchsbaum MS, Bunney WE Jr, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG, Wu JC: Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am J Psychiatry 1993; 150:1325–1336Google Scholar
19. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O'Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
20. Dolan R, Frith CD, Friston KJ, Frackowiak RSJ, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the ACG in schizophrenia. Nature 1995; 378:180–183Crossref, Medline, Google Scholar
21. Ganguli R, Carter CS, Mintun M, Brar JS, Becker JT, Sarma TN, Bennington E: PET brain mapping study of auditory verbal supraspan memory versus visual fixation in schizophrenia. Biol Psychiatry 1997; 41:33–42Crossref, Medline, Google Scholar
22. Liddle P, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RSJ: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
23. Oldfield RC: The assessment and analysis of handedness; the Edinburgh inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
24. Kay S: Pyramidical model of schizophrenia, in Positive and Negative Symptoms in Schizophrenia: Assessment and Research. Edited by Kay S. New York, Brunner/Mazel, 1991, pp 230–231Google Scholar
25. Raichle ME, Martin WRW, Herscovitch P, Mintun MA, Markham J: Brain blood flow measured with intravenous H215O, II: implementation and validation. J Nucl Med 1983; 24:790–798Medline, Google Scholar
26. Cohen JD, Macwhinney B, Flatt MR, Provost J: PsyScope: a new graphic interactive environment for designing psychology experiments. Behavior Res Methods, Instruments, and Computers 1993; 25:101–113Crossref, Google Scholar
27. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620–633Crossref, Medline, Google Scholar
28. Woods RP, Mazziotta JC, Cherry SR: MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993; 17:536–546Crossref, Medline, Google Scholar
29. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
30. Friston FJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ: Statistical parametric mapping in functional imaging: a general approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
31. Carter CS, Robertson LC, Nordahl TE: Abnormal processing of irrelevant information in chronic schizophrenia: selective enhancement of Stroop facilitation. Psychiatry Res 1992; 41:137–146Crossref, Medline, Google Scholar
32. Cohen JD, Servan-Schreiber D: Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Crossref, Medline, Google Scholar
33. Barch DM, Carter CS, Hachten PC: Mechanism of selective attention in schizophrenia (abstract). Biol Psychiatry 1996; 39(274A):579Google Scholar
34. Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ: Local and distributed effects of apomorphine on fronto-temporal function in acute unmedicated schizophrenia. J Neurosci 1996; 16:7055–7062Google Scholar
35. Friston KJ, Frith CD: Schizophrenia: a disconnection syndrome? Clin Neuroscience 1995; 3:89–97Google Scholar
36. Casey BJ, Orendi JL, Trainor RJ, Shubert AB, Noll DC: A functional MRI study of sex differences in prefrontal activation during performance of a go-no-go task (abstract). Human Brain Mapping 1996 3:S174Google Scholar
37. Ghering WJ, Goss B, Coles MGH, Meyer DE, Donchin E: A neural system for error detection and compensation. Psychol Sci 1993; 4:385–390Crossref, Google Scholar
38. Dehaene S, Posner MI, Tucker DM: Localization of a neural system for error detection and compensation. Psychol Sci 1994; 5:303–305Crossref, Google Scholar
39. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA: Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry 1996; 153:41–49Link, Google Scholar
40. Miller DD, Andreasen NC, O'Leary DS, Rezai K, Watkins L, Boles Ponto LL, Hichwa RD: Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography (PET) (abstract). Neuroimage 1996; 3:S499Google Scholar
41. Devinsky O, Morrel MJ, Vogt B: Contributions of anterior cingulate cortex to behavior. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
42. Jones AKP, Brown WD, Friston KJ, Qi LY, Frackowiak RSJ: Cortical and subcortical localization of pain in man using positron emission tomography. Proc R Soc Lond 1991; 244:39–44Crossref, Medline, Google Scholar
43. Olney JW, Farber NB: Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52:998–1007Google Scholar



