Cognitive Enhancement Therapy for Early-Course Schizophrenia: Effects of a Two-Year Randomized Controlled Trial
Schizophrenia is a chronic and disabling mental disorder that is characterized by related deficits in cognition, functioning, and adjustment. The significant personal and societal costs of the disorder ( 1 ), its frequently deteriorating course ( 2 , 3 ), and the consistent negative prognosis associated with untreated illness ( 4 ) highlight the importance of early applications of evidence-based interventions to reduce long-term morbidity ( 5 ). Cognitive impairments, in particular, are promising targets for early intervention because of their early emergence ( 6 ), persistence ( 7 ), and contribution to functional outcome ( 8 ). Unfortunately, few successful efforts have been directed toward the early treatment of cognitive deficits in schizophrenia.
Pharmacological studies of antipsychotic agents (such as olanzapine and perphenazine) and newer glutamatergic agents (such as glycine and D-cycloserine) for early schizophrenia have yielded limited improvements in social and nonsocial cognitive domains that might in part reflect repeated testing ( 9 , 10 ). In addition, although several effective cognitive rehabilitation approaches exist for schizophrenia ( 11 ), the efficacy of these approaches when applied in the early course of the disorder has not been thoroughly assessed. The only two published randomized controlled trials of cognitive rehabilitation among patients with early-course schizophrenia have yielded mixed results ( 12 , 13 , 14 ). Further, these trials were conducted exclusively with patients with early- or childhood-onset illness and used relatively short-term (three-month) interventions that focused primarily on the remediation of neurocognitive deficits in attention, memory, and executive function. Long-term trials of cognitive rehabilitation approaches for patients with early-course schizophrenia are noticeably absent, and most approaches place little to no emphasis on the remediation of social cognition, which may be key to improving functional outcome ( 15 ).
Cognitive enhancement therapy (CET) ( 16 ) is an evidence-based developmental cognitive rehabilitation approach for the remediation of social and nonsocial cognitive deficits in schizophrenia that has conferred significant benefits for patients with chronic illness. In a two-year randomized controlled trial with 121 outpatients with schizophrenia who had been ill for a mean±SD of 15.70±9.30 years, those receiving CET demonstrated large and highly significant improvements in neurocognitive and social-cognitive function, as well as social adjustment ( 17 ). Further, these robust effects remained one year after treatment ended ( 18 ).
Recently, we found initial support for the efficacy of CET in improving social cognition in a preliminary sample of 38 patients with early-course schizophrenia who had completed one year of a two-year randomized trial ( 19 ). However, data for other areas of cognition and functional outcome were not yet available for analysis, leaving open questions regarding the effects of CET on broader areas of cognition and the long-term functional significance of these initial social-cognitive effects. We now report on the complete cognitive and behavioral results from all 58 individuals who entered and were treated in this two-year trial of CET for early schizophrenia. Based on our previous study of CET with outpatients with long-term schizophrenia, we hypothesized that individuals receiving CET would demonstrate significant improvements over the course of treatment in processing speed, neurocognitive and social-cognitive function, as well as social adjustment, compared with a state-of-the-art enriched supportive control condition.
Methods
Participants
Participants consisted of 58 outpatients who met diagnostic criteria of the Structured Clinical Interview for DSM-IV ( 20 ) for schizophrenia (N= 38) or schizoaffective disorder (N= 20). All were in the early course of their illness. The one-year social-cognitive effects of CET on a subset (N= 38) of these individuals have been reported previously ( 19 ). Eligible participants included those with schizophrenia, schizoaffective, or schizophreniform disorder whose illness had been stabilized on antipsychotic medication, who had experienced their first psychotic symptoms (including duration of untreated illness) within the past eight years, had an IQ ≥80, had not been abusing substances for at least two months before study enrollment, and showed significant social and cognitive disability on the Cognitive Style and Social Cognition Eligibility Interview ( 17 ).
Participants were young, with an average age of 25.92±6.31 years; over two-thirds (N=40, 69%) were male, and most were Caucasian (N=40, 69%), with 11 (19%) African American, six (10%) Asian, and one (2%) of other race-ethnicity. Although participants were eligible for this study if they had had their first psychotic symptom (including duration of untreated illness) within the previous eight years, most (N=45, 78%) had been ill for fewer than five years, with an average duration since first psychotic symptom of 3.19±2.24 years, much less than the maximal duration of illness for study eligibility. Although many had some college education (N=39, 67%), most were not employed at baseline (N=43, 74%).
Measures
A comprehensive battery of cognitive and behavioral measures was used to assess the effects of CET on cognition, adjustment, and symptomatology ( Table 1 ) ( 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ). To avoid excessive univariate inference testing that could inflate experimentwise error rates, we computed internally consistent multivariate composite indices of these domains. Individual measures were selected for these composites on the basis of literature that identifies the key domains of cognitive impairment in schizophrenia ( 39 ) and field standards for adjustment and symptom assessment ( 30 , 34 ), as well as previous CET studies ( 17 , 18 ). Measures with poor reliability (interitem r≤.10) were excluded. Four composite indices covering cognitive function were computed to represent speed of processing, neurocognition, dysfunctional cognitive style, and social cognition. Measures of neurocognitive ability and processing speed reflect the relevant domains of neurocognitive impairment identified by the NIMH-MATRICS Committee (National Institute of Mental Health-Measurement and Treatment Research to Improve Cognition in Schizophrenia) ( 40 ). Social cognition and cognitive style measures included those developed for our previous trial of CET, which have shown adequate reliability ( 17 ), and the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) ( 29 ) recommended by NIMH-MATRICS, which has demonstrated adequate psychometric properties for assessing social cognition in schizophrenia ( 41 , 42 ). A composite index was computed for social adjustment and symptomatology from multiple measures with well-documented psychometrics.
 |
Employment data were collected with the Major Role Adjustment Inventory ( 31 ), a 22-item, clinician-rated interview covering role adjustment in the domains of employment, family and household life, and social relationships. Information collected on employment with this instrument consists of vocational status, type of occupation, and number of hours a week worked at the time of the interview.
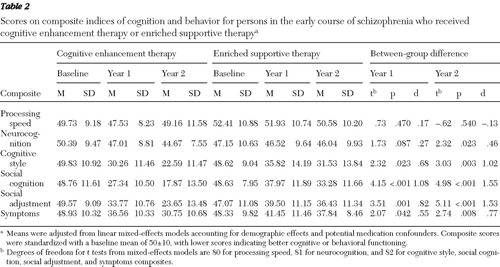
Composite indexes were scaled to a baseline mean of 50±10, with lower scores reflecting better cognitive and behavioral functioning. Social cognition, neurocognition, and processing speed composites served as primary outcome measures. Secondary outcomes included the cognitive style and social adjustment composites. Although symptomatology was assessed, differential treatment effects on symptoms were not expected.
Treatments
Medication. All participants received Food and Drug Administration-approved antipsychotic medications for the treatment of schizophrenia, schizoaffective disorder, and schizophreniform disorder as indicated by a study psychiatrist. Medication changes were allowed, although every effort was made to stabilize participants on a tolerable and efficacious antipsychotic regimen before the initiation of psychosocial treatment. All participants were seen by a clinical nurse specialist at least biweekly to monitor medication side effects and efficacy. Most participants (>98%) were given second-generation antipsychotics throughout the study, and no significant differences emerged with regard to antipsychotic dosage, type, or clinician-estimated compliance between treatment groups. [A table detailing the between-group differences in baseline demographic, clinical, and medication characteristics is available as an online supplement to this article at ps.psychiatryonline.org .]
Cognitive enhancement therapy. CET is a comprehensive, developmental approach to the remediation of social and nonsocial cognitive deficits in schizophrenia. It seeks to facilitate the development of adult social-cognitive milestones (such as perspective taking and appraisal of one's social context) by shifting thinking from reliance on effortful, serial processing to a "gistful" and spontaneous abstraction of social themes. The treatment consists of approximately 60 hours of computer-assisted neurocognitive training in attention, memory, and problem solving and 45 social-cognitive group sessions that use experiential learning opportunities to foster the development of social wisdom and success in interpersonal interactions. A broad, theoretically driven array of social-cognitive abilities are targeted in the social-cognitive groups, which range from abstracting the "gist" or main point in social interactions to perspective taking, social context appraisal, and emotion management ( 39 , 43 ). Participants engage in the social-cognitive groups by responding to unrehearsed social exchanges, presenting homework, participating in cognitive exercises that focus on experiential learning, providing feedback to peers, and chairing homework sessions. CET typically begins with approximately three months of weekly one-hour neurocognitive training in attention, after which participants begin the weekly 1.5-hour social-cognitive groups. Neurocognitive training then proceeds concurrently with social-cognitive groups throughout the remaining course of treatment. A complete description of the treatment has been provided elsewhere ( 16 ).
Enriched supportive therapy. Enriched supportive therapy (EST) is an illness management and psychoeducation approach that draws on components of the basic and intermediate phases of the demonstrably effective personal therapy ( 44 ). In this approach, outpatients are seen on an individual basis to learn and practice stress management techniques designed to forestall late postdischarge relapse and enhance adjustment. The EST treatment is divided into two phases. Phase 1 focuses on basic psychoeducation about schizophrenia, the role of stress in the disorder, and ways to avoid or minimize stress. Phase 2 involves a personalized approach to the identification and management of life stressors that pose particular challenges to adequate social and role functioning. Participants move through the two phases of EST at their own pace, although each phase is typically provided for a year. By design, phase 1 was provided on a weekly basis, and phase 2 was provided on a biweekly basis. Although no attempt was made to match CET and EST approaches with regard to hours of treatment, EST served as the active control for this trial, in part to control for the potential effects on outcome of illness management and education interventions ( 45 ), which are provided in both CET and EST. All psychosocial interventions were administered by three master's-level psychiatric nurse specialists, and clinical supervision was provided by the two treatment developers.
Procedures
Outpatients were recruited between August 2001 and January 2006 from inpatient and outpatient services at Western Psychiatric Institute and Clinic in Pittsburgh and from nearby community clinics. After recruitment, patients were screened for eligibility in consensus conferences showing videotaped interviews. Eligible persons were randomly assigned to either CET or EST by a project statistician using computer-generated random numbers. Participants were then treated for two years and assessed annually on the aforementioned measures of cognition and behavior. One-year assessments were conducted to assess intermediate improvement. Neurocognitive and some social-cognitive assessments (such as the MSCEIT) were completed via computer-based tests or administered by trained neuropsychologists, and the remaining assessments were completed by study clinicians who had been extensively trained in their use and were not blind to treatment assignment. [A figure that depicts the participant flow throughout the study is available as an online supplement to this article at ps.psychiatryonline.org .] There were no significant differences between treatment conditions with regard to demographic characteristics, attrition from the study, or symptomatology at baseline. However, as expected, individuals assigned to CET received significantly more hours of clinician contact [see the supplemental table at ps.psychiatryonline.org ].
This research was conducted between August 2001 and September 2007 and was approved annually by the University of Pittsburgh Institutional Review Board. All patients provided written informed consent before their participation began.
Data analysis
Intent-to-treat analyses were conducted with all 58 participants who were randomly assigned to either CET or EST and received any exposure, regardless of how limited, to their respective treatment conditions. Treatment effects were analyzed in a sequential fashion in order to avoid excessive inference testing that could not be realistically corrected with type I error correction algorithms. We accomplished this by using linear mixed-effects models to first examine the main effects of treatment assignment on multivariate composite indexes of cognition and behavior while adjusting for potentially confounding demographic (age, gender, illness duration, and IQ) and medication (dose) effects. Univariate main effects within composites were then examined with the same mixed-effects strategy for only the domains that had significant multivariate effects. All mixed-effects analyses used random intercept and slope models and an autoregressive error structure most suitable for longitudinal data ( 46 ). Skewed data were handled by using nonlinear or rank transformations, and neuropsychological and processing speed outliers were handled by winsorization ( 47 ).
Results
Main effects on composite indexes of cognition and behavior
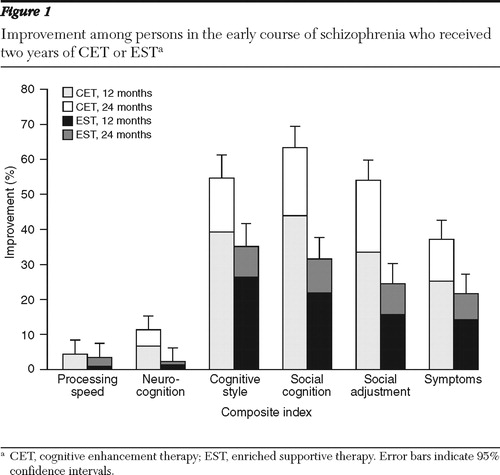
We began our analysis of the effects of CET and EST by first examining their effects on multivariate composite indexes of cognition and behavior. In the first year of treatment, persons receiving CET had significant and medium to large differential improvements in dysfunctional cognitive style, social cognition, social adjustment, and symptomatology compared with those receiving EST ( Table 2 ). After two years of treatment, highly significant and large differential effects were observed favoring CET on the composite indexes of cognitive style, social cognition, social adjustment, and symptomatology ( Figure 1 ). In addition, CET participants showed significant and medium-size improvement on the neurocognitive composite by the second year of treatment.
 |
Univariate effects on cognitive and behavioral composite indexes
Having demonstrated significant and large effects of CET in improving cognition and behavior on multivariate composite indexes by the second year of treatment, we proceeded to investigate the nature of these effects by examining differential rates of improvement for the individual components of these composites. Improvement on the neurocognitive composite was seen in select measures of verbal memory, executive functioning and planning, and neurological soft signs ( Table 3 ). Differential effects on the cognitive style composite centered on improvements with motivation problems and disorganization, whereas effects on the social cognition composite were broader and ranged from significant improvements in social and emotional information processing to improved interpersonal effectiveness and foresightfulness. These large social-cognitive effects were evident not only on clinician-rated measures of social cognition but also on the performance-based MSCEIT.
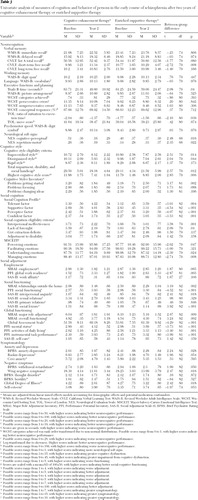 |
Improvements favoring CET on behavioral composites of social adjustment and symptomatology were also broad. Significant effects of CET were observed with regard to vocational and social functioning, global adjustment, activities of daily living, and instrumental task performance ( Table 3 ). A closer inspection of effects on employment indicated that significantly more patients receiving CET (54%) were actively engaged in paid, competitive employment (assessed through clinician interviews using the Major Role Adjustment Inventory [ 31 ]) at the end of two years of treatment, compared with recipients of EST (18%) ( χ2 =4.93, df=1, p=.026). With regard to the symptom composite, significant effects of CET were observed on multiple measures of negative symptoms, as well as on measures of anxiety and depression.
Discussion
Cognitive rehabilitation has emerged as an effective method for ameliorating the cognitive deficits associated with schizophrenia that undermine functional recovery ( 11 ). Short-term trials conducted with patients who had childhood- or early-onset schizophrenia and that focused on neurocognitive dysfunction have suggested the potential benefits of cognitive rehabilitation at the earliest stages of the illness ( 12 , 13 , 14 ). To our knowledge, this is the first study to examine the long-term effects of a comprehensive neurocognitive and social-cognitive rehabilitation program on broad domains of cognition and functioning when applied in early schizophrenia.
Results from this two-year trial broadly support our hypotheses that CET would improve cognitive and behavioral outcomes for this population. Individuals receiving CET demonstrated substantial cognitive gains during the two years of treatment, particularly in social cognition, where broad social-cognitive improvements were found on multiple performance-based and clinician-rated measures. Most important, although specific mediator analyses are needed and will be the focus of subsequent reports, these cognitive gains appear to have translated into significant reductions in disability. Compared with their counterparts assigned to EST, individuals in the CET group showed marked improvements in attaining competitive employment, social functioning, and global adjustment, and they showed reductions in negative symptoms. These effects, which could not be accounted for by group differences in antipsychotic medication use or differential rates of attrition, highlight the potential functional benefits of sufficient exposure to early cognitive rehabilitation in schizophrenia.
It is important to note that the largest cognitive effects observed during CET were in social cognition, a domain that has been linked to functional outcome ( 48 ) and remained largely unresponsive to pharmacological treatment ( 49 ). Although neurocognitive effects were moderate, it was surprising that patients with early-course schizophrenia who received CET did not show any significant improvement in processing speed, which is in contrast to our previous study with long-term patients ( 17 ). Comparison of average processing-speed scores between this early-course sample and participants in our previous study indicated that patients in the early course of their illness performed significantly better on every measure of processing speed at baseline compared with patients with chronic illness (all t values less than -2.96, all df=56, all p<.005). In fact, the pretreatment mean processing speed of individuals receiving CET in this study was comparable with that of patients with chronic illness after two years of CET treatment ( 19 ), pointing to the possibility of a ceiling effect for speed of processing. That processing speed and other aspects of attention are less impaired among patients with early-course schizophrenic illness is not novel ( 6 , 50 ), and this research suggests that more complex social-cognitive processes may be the most critical targets for early intervention programs. CET may serve as a key adjunct to pharmacotherapy in this regard.
Despite the efficacy of CET for improving cognition and behavior among patients with early-course schizophrenic illness, the results of this research need to be interpreted in the context of several limitations. Our participants were mostly male and Caucasian, and the results of this investigation may not generalize to more diverse samples. Treatment groups were also not matched for the number of hours of clinician contact; therefore, results could reflect the nonspecific effects of increased clinician contact on outcome. In addition, clinicians making the assessments were not blind to the treatments to which patients were assigned. As such, rater bias cannot be ruled out as a possible explanation for treatment effects. However, effects on performance-based measures of social cognition were as strong as clinician-rated measures; also, social adjustment effects were seen on an array of different measures, many of which leave little room for rater bias (employment, for example, although employment data did rely largely on self-report). Further, robust neurocognitive effects were also found on performance-based measures of cognition, arguing against a substantial rater bias.
Increased familiarity with computerized testing associated with CET exposure may also explain some improvements in performance on computer-based neuropsychological tests. However, CET effects on neurocognition were seen primarily on paper-and-pencil examinations that bear little resemblance to computerized training software, suggesting that although it is possible that CET influenced test-taking behavior in general, it is less likely that differential neurocognitive improvement favoring CET was the result of enhanced computer literacy or familiarity. In addition, within-composite analyses need to be interpreted with caution; although a hierarchical approach was used to avoid excessively inflating type I error, multiple univariate tests were conducted on within-composite measures. Finally, this research had a somewhat modest sample size (N= 58), which may have precluded the detection of smaller treatment effects. However, to our knowledge this is the largest and longest early-course study of cognitive rehabilitation, and our results indicate that our a priori power analyses based on previous studies ( 17 ) guided us toward a sample size that was sufficient to reliably detect the medium to large CET effects observed in this study. Consequently, it would appear that a sufficient number of individuals were included in the study to enable an adequate evaluation of the efficacy of CET in early schizophrenia. A one-year posttreatment follow-up study is being completed to ascertain the durability of these effects and to determine whether they are comparable with the sustained benefits achieved by patients with chronic schizophrenic illness ( 18 ).
Conclusions
CET is recovery-phase treatment for the remediation of social and nonsocial cognitive deficits among stable outpatients with schizophrenia. The results of this investigation suggest that the early application of CET may confer substantial benefits in cognitive functioning and broad domains of functional outcome for this population. Sufficient exposure to cognitive rehabilitation may be a vital, yet overlooked, component to early-intervention programs, ultimately providing the critical ingredients needed to help individuals recover from this disorder.
Acknowledgments and disclosures
This work was supported by grants MH 60902 (MSK) and MH 79537 (SME) from the National Institute of Mental Health. The authors are indebted to Gerard E. Hogarty, M.S.W., for his leadership and direction as co-principal investigator of this study. The authors also thank Konasale Prasad, M.D., Haranath Parepally, M.D., Diana Dworakowski, M.S., Mary Carter, Ph.D., Sara Fleet, M.S., and Michele Bauer for their help in various aspects of the study. In addition, they thank David Kupfer, M.D., for extended support throughout the project, as well as the many patients who participated in this research and the dedication they showed to their recovery, which was a constant source of inspiration.
The authors report no competing interests.
1. Rupp A, Keith SJ: The costs of schizophrenia: assessing the burden. Psychiatric Clinics of North America 16:413–423, 1993Google Scholar
2. McGlashan TH, Fenton WS: Subtype progression and pathophysiologic deterioration in early schizophrenia. Schizophrenia Bulletin 19:71–84, 1993Google Scholar
3. Lieberman JA: Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biological Psychiatry 46:729–739, 1999Google Scholar
4. Perkins DO, Gu H, Boteva K, et al: Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. American Journal of Psychiatry 162:1785–1804, 2005Google Scholar
5. McGlashan TH, Johannessen JO: Early detection and intervention with schizophrenia: rationale. Schizophrenia Bulletin 22:201–222, 1996Google Scholar
6. Saykin AJ, Shtasel DL, Gur RE, et al: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry 51:124–131, 1994Google Scholar
7. Hoff AL, Svetina C, Shields G, et al: Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophrenia Research 78:27–34, 2005Google Scholar
8. Green MF, Kern RS, Braff DL, et al: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophrenia Bulletin 26:119–136, 2000Google Scholar
9. Goldberg TE, Goldman RS, Burdick KE, et al: Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives of General Psychiatry 64:1115–1122, 2007Google Scholar
10. Buchanan RW, Javitt DC, Marder SR, et al: The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. American Journal of Psychiatry 164:1593–1602, 2007Google Scholar
11. McGurk SR, Twamley EW, Sitzer DI, et al: A meta-analysis of cognitive remediation in schizophrenia. American Journal of Psychiatry 164:1791–1802, 2007Google Scholar
12. Ueland T, Rund BR: A controlled randomized treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatrica Scandinavica 109:70–74, 2004Google Scholar
13. Ueland T, Rund BR: Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatrica Scandinavica 111:193–201, 2005Google Scholar
14. Wykes T, Newton E, Landau S, et al: Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophrenia Research 94:221–230, 2007Google Scholar
15. Couture SM, Penn DL, Roberts DL: The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin 32(suppl 1):S44–S63, 2006Google Scholar
16. Hogarty GE, Greenwald DP: Cognitive Enhancement Therapy: The Training Manual. Pittsburgh, University of Pittsburgh Medical Center, 2006. Available at www.cognitiveenhancementtherapy.com Google Scholar
17. Hogarty GE, Flesher S, Ulrich R, et al: Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry 61:866–876, 2004Google Scholar
18. Hogarty GE, Greenwald DP, Eack SM: Durability and mechanism of effects of cognitive enhancement therapy. Psychiatric Services 57:1751–1757, 2006Google Scholar
19. Eack SM, Hogarty GE, Greenwald DP, et al: Cognitive enhancement therapy improves emotional intelligence in early course schizophrenia: preliminary effects. Schizophrenia Research 89:308–311, 2007Google Scholar
20. First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
21. Ben-Yishay Y, Piasetsky EB, Rattok J: A systematic method for ameliorating disorders in basic attention; in Neuropsychological Rehabilitation. Edited by Meir MJ, Benton AL, Diller L. New York, Guilford, 1985Google Scholar
22. Bracy OL: PSSCogRehab. Indianapolis, Ind, Psychological Software Services, 1994Google Scholar
23. Wechsler D: Manual for the Wechsler Memory Scale-Revised. San Antonio, Tex, Psychological Corp, 1987Google Scholar
24. Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test Manual. San Antonio, Tex, Psychological Corp, 1987Google Scholar
25. Wechsler D: Wechsler Adult Intelligence Scale-Revised. New York, Psychological Corp, 1981Google Scholar
26. Reitan RM, Waltson, D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
27. Heaton RK, Chelune GJ, Talley JL, et al: Wisconsin Card Sorting Test Manual, rev. Odessa, Fla, Psychological Assessment Resources, 1993Google Scholar
28. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research 27:335–350, 1989Google Scholar
29. Mayer JD, Salovey P, Caruso DR, et al: Measuring emotional intelligence with the MSCEIT V2.0. Emotion 3:97–105, 2003Google Scholar
30. Schooler N, Weissman M, Hogarty GE: Social Adjustment Scale for Schizophrenics; in Resource Material for Community Mental Health Program Evaluators. DHHS pub no (ADM) 79328. Edited by Hargreaves WA, Attkisson CC, Sorenson J. Rockville, Md, National Institute of Mental Health, 1979Google Scholar
31. Hogarty GE, Goldberg SC, Schooler NR, et al: Drug and sociotherapy in the aftercare of schizophrenic patients: III. adjustment of nonrelapsed patients. Archives of General Psychiatry 31:609–618, 1974Google Scholar
32. Endicott J, Spitzer RL, Fleiss JL, et al: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 33:766–771, 1976Google Scholar
33. Disability Evaluation Under Social Security. Washington, DC, Department of Health and Human Services, 1986Google Scholar
34. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychological Reports 10:799–812, 1962Google Scholar
35. Wing JK: A simple and reliable subclassification of chronic schizophrenia. Journal of Mental Science 107:862–875, 1961Google Scholar
36. Raskin A, Schulterbrandt J, Reatig N, et al: Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. Journal of Nervous and Mental Disease 148:87–98, 1969Google Scholar
37. Lipman RS: Differentiating anxiety and depression in anxiety disorders: use of rating scales. Psychopharmacology Bulletin 18:69–77, 1982Google Scholar
38. Hogarty GE, McEvoy JP, Ulrich RF, et al: Pharmacotherapy of impaired affect in recovering schizophrenic patients. Archives of General Psychiatry 52:29–41, 1995Google Scholar
39. Hogarty GE, Flesher S: Developmental theory for a cognitive enhancement therapy of schizophrenia. Schizophrenia Bulletin 25:677–692, 1999Google Scholar
40. Green MF, Nuechterlein KH, Gold JM, et al: Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry 56:301–307, 2004Google Scholar
41. Nuechterlein KH, Green MF, Kern RS, et al: The MATRICS Consensus Cognitive Battery, Part 1: test selection, reliability, and validity. American Journal of Psychiatry 165:203–213, 2008Google Scholar
42. Eack SM, Greeno CG, Pogue-Geile MF, et al: Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophrenia Bulletin, Epub Ahead of Print, July 22, 2008, in pressGoogle Scholar
43. Selman RL, Schultz LH: Making a Friend in Youth. Chicago, University of Chicago Press, 1990Google Scholar
44. Hogarty GE: Personal Therapy for Schizophrenia and Related Disorders: A Guide to Individualized Treatment. New York, Guilford, 2002Google Scholar
45. Hogarty GE, Greenwald D, Ulrich RF, et al: Three-year trials of personal therapy among schizophrenic patients living with or independent of family: II. effects of adjustment of patients. American Journal of Psychiatry 154:1514–1524, 1997Google Scholar
46. Raudenbush DSW, Bryk DAS: Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, Calif, Sage, 2002Google Scholar
47. Dixon WJ, Tukey JW: Approximate behavior of the distribution of winsorized t (trimming/winsorization 2). Technometrics 10:83–98, 1968Google Scholar
48. Sergi MJ, Rassovsky Y, Nuechterlein KH, et al: Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. American Journal of Psychiatry 163:448–454, 2006Google Scholar
49. Sergi MJ, Green MF, Widmark C, et al: Cognition and neurocognition: effects of risperidone, olanzapine, and haloperidol. American Journal of Psychiatry 164:1585–1592, 2007Google Scholar
50. Brewer WJ, Francey SM, Wood SJ, et al: Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. American Journal of Psychiatry 162:71–78, 2005Google Scholar



