Attention Bias Modification Treatment Versus a Selective Serotonin Reuptake Inhibitor Or Waiting List Control for Social Anxiety Disorder: A Randomized Clinical Trial
Abstract
Objective:
Social anxiety disorder is common and impairing. The efficacy of pharmacotherapy is moderate, highlighting the need for alternative therapies. This study compared the efficacy of gaze-contingent music reward therapy (GC-MRT), an eye-tracking-based attention bias modification treatment, with a selective serotonin reuptake inhibitor (SSRI) treatment or a waiting list control condition in reducing social anxiety disorder symptoms. Superior clinical effects of similar magnitude were expected for the active treatments relative to the control condition.
Methods:
Participants were 105 treatment-seeking adults with social anxiety disorder, randomly allocated to 12 weeks of GC-MRT, SSRI, or waiting list control. Mean changes in clinician-rated and self-reported social anxiety symptoms from baseline to mid- and posttreatment assessments were compared between groups using generalized estimating equations. Changes in attentional dwell time on threat were also examined.
Results:
Analysis indicated a significant differential reduction in symptoms between groups. Patients in the GC-MRT and SSRI groups had lower social anxiety scores at the mid- and posttreatment assessments compared with patients in the waiting list group. The efficacy of the active treatments did not differ. Only patients in the GC-MRT group showed reduction in dwell time on threat from baseline to posttreatment assessment.
Conclusions:
Eye-tracking-based attention bias modification is an acceptable and effective treatment option for social anxiety disorder.
Social anxiety disorder involves chronic fear and avoidance of scrutiny (1). Lifetime prevalence is 4%–12.1% (2–4), and impact on functioning is marked (2, 5). Selective serotonin reuptake inhibitors (SSRIs) and cognitive-behavioral therapy (CBT) are first-line treatments, both with medium clinical effect sizes (6), and approximately 50% of patients remain symptomatic following treatment (7–9). Moreover, less than 15% of patients receive minimally adequate treatment (10). This has led to a call for technology-driven interventions to increase access and reduce costs (11). In this study, we tested the efficacy of one such treatment for social anxiety disorder, an eye-tracking-based attention bias modification called gaze-contingent music reward therapy (GC-MRT) (12), relative to standard SSRI treatment and a waiting list control condition.
Patients with social anxiety, as compared to healthy peers, dwell longer on scowling facial expressions (13–16), a tendency implicated in the maintenance of the disorder (17). GC-MRT reduces this tendency through feedback. An initial randomized controlled trial of GC-MRT (12) and two open trials (18, 19) indicated reliable target engagement and considerable symptom reduction. The present study extends this work by comparing GC-MRT, SSRI treatment, and a waiting list control condition over a 12-week period. We expected both GC-MRT and SSRI to induce greater symptom reductions than waiting list control at the midtreatment (6 weeks) and posttreatment (13 weeks) assessments. Based on previous studies, we expected similar symptomatic changes in the two active treatments (6, 12). To explore cognitive target engagement, we recorded threat-related gaze at the baseline, midtreatment, and posttreatment assessments. Reduction in dwell time on threat was expected only in the GC-MRT group. Finally, given the high comorbidity between social anxiety disorder and depression (2, 3), depression was assessed at the pre- and posttreatment assessments. Previous studies indicate effectiveness of SSRIs (20) and limited effectiveness of GC-MRT for depression (21). Therefore, we expected depressive symptoms to improve only in the SSRI group.
Methods
Participants
Participants were 105 treatment-seeking patients with social anxiety disorder; 62 were male, and the mean age was 30.71 years (SD=8.25). Inclusion criteria were social anxiety disorder as the main source of distress and impairment, age 18–65 years, a Liebowitz Social Anxiety Scale score ≥50, and a Clinical Global Impressions severity score ≥4. Exclusion criteria were posttraumatic stress, psychotic, or bipolar disorders; epilepsy or brain injury; suicidal ideation or risk; drug abuse; and concurrent pharmacological or psychological treatment. All participants provided written informed consent prior to clinical assessment. The study was approved by the Tel Aviv University, Tel Aviv Sourasky Medical Center, and Sheba Medical Center Institutional Review Boards and was conducted at the university’s anxiety clinic. The study protocol and relevant data sets are available in the Open Science Framework (OSF) repository (https://osf.io/mya75/?view_only=6c948605d4e24b479b8de856ba5fb8db, DOI 10.17605/OSF.IO/MYA75).
Sample Size Calculation
Power analyses were conducted using G*Power, version 3.1.9.7 (22). We estimated the necessary sample size for a repeated-measures, within-between interaction F test, with three groups and three repeated measurements to allow detection of a significant small effect size (Cohen’s f=0.15) (23) at 0.80 power and an alpha of 0.05, with an expected correlation among repeated measures set at 0.5, to require 93 participants. We preregistered and enrolled 105 participants (35 per group).
Randomization and Blinding
Participants were randomly allocated in a 1:1:1 ratio to GC-MRT, SSRI, or waiting list control using randomly permuted blocks stratified by age (under or over 40 years) and gender. The allocation list was created using a computer-generated random number sequence prior to recruitment. Enrolled patients were assigned to treatment by a coordinator with no other involvement in the study. The independent evaluators assessing patients’ clinical status were blind to study design, treatments, and patients’ group assignment. Patients and independent evaluators were instructed not to discuss treatment aspects during the clinical interviews.
Interventions
GC-MRT.
This 12-week protocol consisted of 11 sessions. After the initial week-1 session dedicated to establishing rapport, providing psychoeducation, and explaining procedures (45 minutes), 10 sessions of GC-MRT designed to reduce dwell time on threat faces (12) were delivered (∼20 minutes per session). Training was delivered twice weekly in weeks 2, 3, 4, and 5 and once a week in weeks 8 and 11. For each session, patients selected a 12-minute music track. Thirty face matrices were presented (24 seconds each), with gaze tracking throughout. Patients heard the music play when they fixated on the neutral faces, and the music stopped when they fixated on threat faces. This operant conditioning setup induces attentional preference for neutral over threat stimuli. For an illustration of stimuli and setup, see Figure 3A.
SSRI.
Standard 12-week flexible-dose escitalopram treatment (24, 25) was provided, starting at 5 mg/day, which was increased to 10 mg/day on day 5 (barring side effects), and further increased to 20 mg/day on day 21 according to treatment response. The mean maximum dosage for this sample was 11.6 mg/day. Four visits with a psychiatrist (at weeks 1, 3, 6, and 13) were used for intake and prescription, progress monitoring, dosage modification, and treatment conclusion.
Waiting list control.
Patients in this group were told that they would receive GC-MRT following a 12-week waiting period. Clinical assessments and self-reported symptoms were collected during the waiting period.
Study Outcomes
The primary outcome was clinician-rated total severity score on the Liebowitz Social Anxiety Scale (LSAS) (26). Cronbach’s alpha values were 0.86, 0.92, and 0.92 at the pre-, mid-, and posttreatment assessments, respectively. Total score on the self-reported Social Phobia Inventory (SPIN) (27) served as a secondary outcome. Cronbach’s alpha values were 0.81, 0.84, 0.89, 0.91, 0.91, 0.93, and 0.92 at the pretreatment, weeks 2, 4, 6, 8, 10, and posttreatment assessments, respectively. The LSAS rather than the SPIN was designated the primary outcome for two reasons. First, many previous trials have utilized clinician-rated measures as primary outcomes, as these are scored by a small number of highly trained professionals, whereas self-reported measures are individually scored by each patient. And second, the LSAS was administered in the clinic under controlled conditions, whereas the SPIN was completed both in the clinic and at home. This may reduce variability in LSAS versus SPIN rating procedures, making the former more reliable.
Additional outcomes were the Clinical Global Impressions improvement score (CGI-I) at the mid- and posttreatment assessments and the Patient Health Questionnaire–9 (PHQ-9) depression scale at the baseline and posttreatment assessments (28, 29). The Credibility/Expectancy Questionnaire (CEQ) was used to explore expectancies and treatment credibility (30). The Client Satisfaction Questionnaire (CSQ-8) was used to measure satisfaction with treatment (31).
Threat-related attention was assessed at the pre-, mid-, and posttreatment assessments using 30 trials of the free-viewing eye-tracking task (13). Each trial began with a fixation cross (until 1,000 ms fixation). Then a matrix of 16 faces appeared (6,000 ms), followed by a 2,000-ms intertrial interval. Faces were of different actors than those displayed in treatment and had an equal frequency of male/female and scowling/neutral expressions (“threat” area of interest/“neutral” area of interest), respectively. Participants were instructed to look at each matrix in any way they chose until it disappeared. Percent dwell time on threat was calculated as the proportion of time fixating on the threat area of interest relative to the total time fixating on faces. Cronbach’s alphas representing internal consistency between the 30 matrices were 0.89, 0.93, and 0.92 at the pre-, mid-, and posttreatment assessments, respectively.
Trial Procedures
The trial was advertised on social media, and respondents were screened by telephone. A score ≥30 on the SPIN warranted clinical interview. After enrollment in the study, interviews were conducted at weeks 1 (baseline), 6 (midtreatment/wait), and 13 (posttreatment/wait) by two clinical psychologists trained to 85% reliability with a senior clinician. Social anxiety disorder diagnosis was established using the LSAS interview. Primary and comorbid diagnoses were further validated using the Mini-International Neuropsychiatric Interview (MINI) (32). Eligible participants were invited for a baseline assessment of threat-related gaze patterns using the free-viewing eye-tracking task and then randomized to study conditions.
Self-reported SPIN scores were collected every 2 weeks throughout the trial. Two additional clinical assessments (MINI, LSAS) and gaze-tracking assessments were conducted at midtreatment and 1 week posttreatment. Pre- and posttreatment MRI scans were performed (results to be reported elsewhere). The study was conducted from July 2018 to December 2021, and data were analyzed from January 2022 to February 2022.
Statistical Analysis
Analyses were performed using SPSS, version 28 (IBM, Armonk, N.Y.). Treatment effects and cognitive target engagement were evaluated using generalized estimating equations (33). Generalized estimating equations apply an intention-to-treat approach, accommodating missing data and correlations among repeated measures by estimating marginal means, including data from all randomized participants based on the missing-at-random principle (for tests confirming the validity of the missing-at-random principle, see the online supplement). To represent within-subject dependencies, an unstructured correlation matrix was used. For each outcome, we first applied a full factorial model containing the effects of time (treated as a categorical variable), group, and their interaction. Wald chi-square tests were used to test effects for the continuous outcomes. The time-by-group interaction term represented differential effects among study conditions. Significant interactions were decomposed by comparing each group with the others at each time point. For the primary outcome (LSAS) and for attentional dwell time on threat, the pre-, mid-, and posttreatment assessments were considered; for the secondary outcome (SPIN), the pretreatment, weeks 2, 4, 6, 8, 10, and posttreatment assessments were considered; for depression (PHQ-9), the pre- and posttreatment assessments were considered.
Clinically significant change and reliable change (34) cutoffs were determined based on the test-retest reliability data from Baker et al. (35) and pretreatment LSAS scores from the authors’ previous trials data (N=169). The cutoff for clinically significant change on the LSAS was set at 46.29, and the cutoff for reliable change was set at 1.96. Pearson chi-square tests were used to compare the number of patients displaying clinically significant change and reliable change at the mid- and posttreatment assessments in the different groups. To handle missing data in categorical outcomes, patients who dropped out were first considered as not displaying reliable change or clinically significant change (according to the last observation carried forward principle). For a completers-only analysis, see the online supplement.
A Pearson chi-square test was used to assess group differences in clinical improvement (CGI-I). The number of patients who were rated as “much” or “very much” improved at the posttreatment assessment was compared with the number of patients with lower scores across groups. Dropouts were considered as not improved (for completers-only analyses, see the online supplement).
To examine differences in pretreatment expectancy and credibility (CEQ) and posttreatment satisfaction with treatment (CSQ), generalized estimating equation analyses were conducted contrasting the two active treatment groups. A Pearson chi-square test was used to assess group differences in completion rates.
Within the GC-MRT group, session-to-session changes in dwell time on threat were estimated using generalized estimating equations. To examine generalization of training to a new set of faces and in the absence of music reinforcement, Pearson correlation between reduction in dwell time on threat from training session 1 to 10 and from baseline to posttreatment assessment was used. To assess the relations between changes in threat-related attention and symptom reduction in the GC-MRT group, Pearson correlations were computed between reduction in dwell time on threat and reductions in LSAS and SPIN scores from the pretreatment to the midtreatment and posttreatment assessments.
All statistical tests were two-sided, using an alpha of 0.05 to control for type I error in the primary analyses. In the remaining analyses, multiple comparisons were corrected using the Benjamini-Hochberg procedure for controlling the false discovery rate (36). Effect sizes are reported when appropriate using Cohen’s d.
Results
See Figure 1 for the progress of participants through the study and Table 1 for demographic and clinical characteristics.
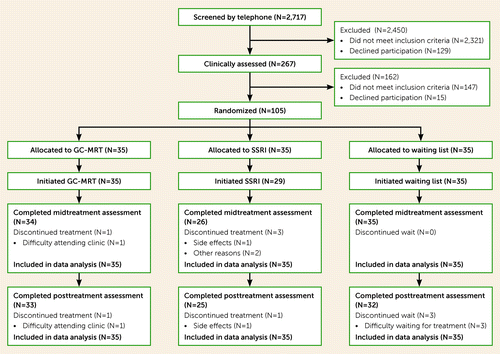
FIGURE 1. CONSORT flow diagram of participants in a trial of gaze-contingent music reward therapy in social anxiety disordera
aThe midtreatment assessment occurred at week 6 of the 12-week intervention or wait, and the posttreatment assessment at week 13. GC-MRT=gaze-contingent music reward therapy; SSRI=selective serotonin reuptake inhibitor.
| Variable and Assessment | GC-MRT Group (N=35) | SSRI Group (N=35) | Control Group (N=35) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 30.80 | 8.06 | 30.83 | 9.71 | 30.51 | 6.98 |
| LSAS score | ||||||
| Baseline | 76.83 | 15.51 | 80.03 | 14.28 | 81.49 | 16.50 |
| Midtreatment | 59.00 | 18.96 | 60.86 | 19.24 | 75.97 | 17.52 |
| Posttreatment | 56.78 | 19.71 | 54.70 | 20.04 | 74.03 | 18.16 |
| SPIN score | ||||||
| Baseline | 47.80 | 9.74 | 48.06 | 7.37 | 49.60 | 7.94 |
| Midtreatment | 34.46 | 12.07 | 32.87 | 12.92 | 44.61 | 10.06 |
| Posttreatment | 35.12 | 13.89 | 30.85 | 12.92 | 44.14 | 10.18 |
| PHQ-9 score | ||||||
| Baseline | 13.26 | 5.01 | 13.94 | 5.85 | 14.74 | 5.35 |
| Posttreatment | 9.73 | 5.53 | 7.83 | 4.97 | 11.52 | 7.30 |
| % Dwell time on threat | ||||||
| Baseline | 45.29 | 10.48 | 47.34 | 8.19 | 46.93 | 6.92 |
| Midtreatment | 37.40 | 14.31 | 45.00 | 9.90 | 46.14 | 8.44 |
| Posttreatment | 35.45 | 15.01 | 44.37 | 8.77 | 46.21 | 8.02 |
| N | % | N | % | N | % | |
| Maleb | 22 | 62.86 | 19 | 54.29 | 21 | 60.00 |
| Comorbidities | ||||||
| Mild depressive episode | 10 | 28.57 | 14 | 40.00 | 16 | 45.71 |
| Dysthymia | 4 | 11.43 | 4 | 11.43 | 3 | 8.57 |
| Generalized anxiety disorder | 3 | 8.57 | 6 | 17.14 | 4 | 11.43 |
| Panic disorder | 2 | 5.71 | 2 | 5.71 | 2 | 5.71 |
| Agoraphobia | 2 | 5.71 | 1 | 2.86 | 1 | 2.86 |
TABLE 1. Clinical and demographic characteristics of participants in a trial of gaze-contingent music reward therapy in social anxiety disordera
Symptom Severity
Primary outcome.
Change over time in clinician-rated social anxiety symptom severity is summarized in Figure 2A. Significant effects of time (Wald χ2=112.16, df=2, p<0.001), group (Wald χ2=14.48, df=2, p<0.001), and a time-by-group interaction (Wald χ2=26.87, df=4, p<0.001) were noted. The groups did not differ significantly at baseline (p values >0.2), but relative to patients in the control group, those in the GC-MRT and SSRI groups showed lower symptom severity at the midtreatment assessment (p values <0.001; d=0.93, 95% CI=0.43, 1.41, and d=−0.82, 95% CI=0.32, 1.30, respectively) and at the posttreatment assessment (p values <0.001; d=0.91, 95% CI=0.41, 1.39, and d=1.01, 95% CI=0.5, 1.5, respectively). No difference was noted between the two active treatment groups (p values, 0.68 and 0.66; d=0.10, 95% CI=−0.57, 0.37, and d=0.10, 95% CI=−0.36, 0.057, for the midtreatment and posttreatment assessments, respectively).
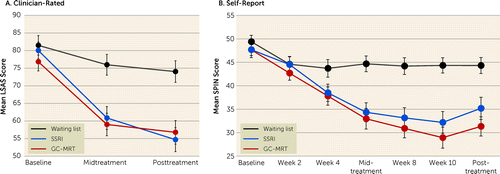
FIGURE 2. Clinician-rated and self-reported social anxiety symptom severity, by group and timea
a Higher values indicate more severe social anxiety. Values reflect estimated generalized estimating equation means, and error bars indicate standard error of the estimated mean. GC-MRT=gaze-contingent music reward therapy; LSAS=Leibowitz Social Anxiety Scale; SPIN=Social Phobia Inventory; SSRI=selective serotonin reuptake inhibitor.
Of the patients in the GC-MRT, SSRI, and control groups, 37.1%, 28.6%, and 5.71%, respectively, showed clinically significant change following treatment (χ2=10.18, df=2, p=0.006). Follow-up analyses indicate higher frequencies of patients with clinically significant change in the GC-MRT and SSRI groups relative to the control group (χ2=10.27, df=1, p=0.001, and χ2=6.44, df=1, p=0.011, respectively), with no significant difference between the two active treatment groups (χ2=0.58, df=1, p=0.45). Reliable change was noted in 48.6% of the patients in both the GC-MRT and the SSRI groups and in 14.3% in the control group (χ2=11.75, df=2, p=0.003). Significantly higher frequencies of reliable change were noted in the GC-MRT and SSRI groups relative to the control group separately (in both cases, χ2=9.55, df=1, p=0.002). The findings were replicated when only treatment completers were analyzed (see the online supplement).
Secondary outcome.
Changes over time in self-reported social anxiety symptom severity are summarized in Figure 2B. Effects of time (Wald χ2=173.24, df=6, p<0.001), group (Wald χ2=19.21, df=2, p<0.001), and time-by-group interaction (Wald χ2=74.41, df=12, p<0.001) were noted. The groups did not differ significantly in self-reported social anxiety severity at baseline and after 2 weeks of treatment (p values >0.35). Thereafter both active treatment groups (GC-MRT and SSRI, respectively) showed significantly lower symptom severity than the control group (week 4: p values, 0.03 and 0.047; d=0.52, 95% CI=0.04, 0.099, and d=0.48, 95% CI=−0.005, 0.94; week 6: p values <0.001; d=0.94, 95% CI=0.43, 1.42, and d=1.01, 95% CI=0.51, 1.5; week 8: p values <0.001; d=0.94, 95% CI=0.44, 1.42, and d=1.18, 95% CI=0.66, 1.68; week 10: p values <0.001; d=1.03, 95% CI=0.52, 1.51, and d=1.33, 95% CI=0.8, 1.83; and week 13: p values <0.002 and 0.001; d=0.75, 95% CI=0.26, 1.23, and d=1.17, 95% CI=0.65, 1.66). No significant difference was noted between the GC-MRT and SSRI groups at any of the time points.
Additional Clinical Outcomes
Improvement was rated by clinicians as “much” or “very much” improved at posttreatment assessment in 40% of patients in both the GC-MRT and SSRI groups, and 8.5% in the control group (CGI-I, χ2=11.08, df=2, p=0.004), with more improved patients in each of the active treatment groups relative to the control group (in both cases, χ2=9.40, df=1, p=0.002). Similar results were obtained when only treatment completers were analyzed (see the online supplement). Analysis of depression scores revealed a main effect of time (Wald χ2=79.32, df=1, p<0.001), no effect of group (Wald χ2=3.13, df=2, p=0.21), and a time-by-group interaction (Wald χ2=6.88, df=2, p=0.03). The groups did not differ significantly in depression at baseline (p values >0.2), but lower depression was noted at the posttreatment assessment in the SSRI group relative to the control group (p=0.01; d=0.59, 95% CI=0.11, 1.06), with no difference relative to the GC-MRT group (p=0.13; d=0.36, 95% CI=−0.11, 0.83) or between the GC-MRT and control groups (p=0.25; d=0.28, 95% CI=−0.2, 0.74).
Cognitive Target Engagement
Change in percent dwell time on threat by group and time is summarized in Figure 3B. Main effects of time (Wald χ2=19.33, df=2, p<0.001), group (Wald χ2=9.86, df=2, p=0.007), and time-by-group interaction (Wald χ2=14.40, df=4, p=0.006) were noted. The groups did not differ significantly in dwell time on threat at baseline (p values >0.35). Lower dwell time on threat was noted for the GC-MRT group relative to the SSRI and control groups at the midtreatment assessment (p values, 0.01 and 0.002; d=0.62, 95% CI=0.13, 1.09, and d=0.74, 95% CI=0.25, 1.22, respectively) and at the posttreatment assessment (p values <0.002 and 0.001; d=0.73, 95% CI=0.23, 1.20, and d=0.89, 95% CI=0.39, 1.38, respectively). No differences were noted between the SSRI and control groups (p values, 0.61 and 0.36; d=0.12, 95% CI=−0.35, 0.59, and d=0.22, 95% CI=−0.25, 0.69, for the mid- and posttreatment assessments, respectively). A significant reduction of 9.84% on average in dwell time on threat over time was noted for the GC-MRT group (Wald χ2=21.45, df=2, p<0.001). No changes were observed in the SSRI and control groups (Wald χ2=3.13, df=2, p=0.21, and Wald χ2=0.63, df=2, p=0.73, respectively).
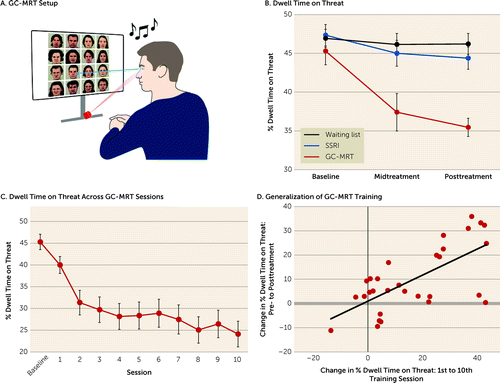
FIGURE 3. Gaze-contingent music reward therapy setup and analysis of dwell time on threata
a Panel A shows the setup for gaze-contingent music reward therapy (GC-MRT). Music is played when patients fixate on a neutral face and stops when they fixate on a scowling face. Panel B indicates a progressive decline in mean percent dwell time on threat by group and time. Error bars indicate standard error of the estimated mean. Panel C shows declines in threat-related attention in the GC-MRT group throughout training sessions. Panel D is a scatterplot of the correlation between reduction in dwell time on threat from pre- to posttreatment assessment and reduction in dwell time on threat from treatment session 1 to treatment session 10 in the GC-MRT group. During pre- and posttreatment measurements, different face stimuli were used than those used in treatment and no music reinforcement was played. The positive correlation indicates a near-transfer generalization of training.
Changes in dwell time on threat across training sessions in the GC-MRT group are summarized in Figure 3C. A significant reduction across sessions was noted (Wald χ2=122.88, df=9, p<0.001), with significant reductions between consecutive sessions 1 and 2 (p<0.001), 7 and 8 (p<0.001), and 9 and 10 (p=0.015). Change in dwell time on threat from session 1 to session 10 was correlated with change in dwell time on threat from pre- to posttreatment assessment (r=0.68, N=30, p<0.001) (Figure 3D), indicating generalization of attentional rule learning to different faces and without music reinforcement. Reductions in symptom severity were positively associated with reductions in dwell time on threat in the GC-MRT group. However, these associations were modest and nonsignificant (self-reported social anxiety, assessed by the SPIN: midtreatment assessment, r=0.35, N=33, p=0.048; posttreatment assessment, r=0.23, N=33, p=0.208; clinician-rated social anxiety, assessed by the LSAS: midtreatment assessment, r=0.19, N=33, p=0.294; posttreatment assessment, r=0.07, N=33, p=0.691) (for scatterplots, see Figure S1 in the online supplement).
Adherence, Credibility, Expectation, and Treatment Satisfaction
Of the 33 GC-MRT completers, 29 attended all treatment sessions and four missed one session. No training sessions were cut short. Of the 25 SSRI completers, 24 attended all sessions with the psychiatrist and one attended three sessions. Ninety-six percent of the patients reported taking the medication as instructed. All patients reported refraining from parallel treatments throughout the study. Patients were similarly satisfied with GC-MRT and SSRI (assessed by the CSQ: Wald χ2=0.35, df=1, p=0.56; mean=3.08, SD=0.50, and mean=3.19, SD=0.85, respectively) and found both treatments highly credible (assessed by the CEQ; Wald χ2=2.60, df=1, p=0.11; mean=8.00, SD=0.95, and mean=7.74, SD=0.94, respectively). Patients expected symptoms to improve by 55% and 58%, on average, in the GC-MRT and SSRI groups, respectively (CEQ; Wald χ2=0.48, df=1, p=0.49). Completion rates differed between groups (χ2=8.87, df=2, p=0.012), with higher attrition in the SSRI group (28.6%) relative to the waiting list (8.6%) and GC-MRT (5.7%) groups (χ2=4.63, df=1, p=0.031, and χ2=6.44, df=1, p=0.011, respectively).
Discussion
This study generated two key findings. First, comparably large clinical effects were noted for GC-MRT and SSRI treatments relative to a waiting list control group. Second, a significant reduction in attentional threat processing occurred only in the GC-MRT group, providing evidence of cognitive target engagement.
GC-MRT-related symptom reductions replicate previous results (12, 18). This study extends previous results by also showing acceptable treatment credibility and satisfaction, comparable to that reported for SSRI treatment. Thus, GC-MRT may offer an effective, low-cost treatment alternative.
The reduction in dwell time on threat in the GC-MRT group reflects effective target engagement. This generalized to a novel face set, viewed without music reinforcement. These findings are consistent with previous GC-MRT studies reporting effective target engagement (12, 19). Future studies could evaluate the presence of such effects during real-life social interactions. The present study was not powered to detect group differences in mediation. One study reported a small partial mediation effect of symptom reduction by reduction in dwell time on threat following GC-MRT training (12). This outcome did not replicate in our study. A modest correlation was only noted between dwell time on threat and self-reported clinical change at the midtreatment assessment. Larger studies are needed to evaluate potential mediation of clinical outcomes by reduced threat-related attention. Analyses also indicate that SSRI treatment was associated with lower depression relative to waiting list control at the posttreatment assessment, a difference not observed for GC-MRT. This may suggest an advantage of SSRI treatment over GC-MRT in patients with comorbid depression. This result corresponds with reported SSRI efficacy in depression (20) and with findings suggesting that GC-MRT might not be effective for depression (21).
Adherence to, credibility of, and satisfaction with both treatments was high and comparable, indicating that GC-MRT was viewed as a viable treatment. The dropout rate in the SSRI group (28%) is consistent with previous studies of social anxiety disorder finding rates in the range of 16%–37% (8, 25, 37). Dropout was lower in the GC-MRT group (6%) and was similar to the rate reported in a previous trial of GC-MRT for social anxiety disorder (12). These results suggest that for patients with social anxiety disorder, GC-MRT may be better tolerated than SSRI treatment.
Limitations of this study should be noted. First, waiting list may be considered a weak control for the two active treatments. An alternative design could use two control groups, each linked to one of the active treatments (e.g., placebo pills for SSRI and noncontingent music for GC-MRT). Without such a design, the comparable improvement in the two active treatments could be viewed as reflecting a low-level, nonspecific effect associated with many forms of treatment. However, the relatively high response rates, comparable to rates in previous controlled studies, is inconsistent with such a nonspecific effect (6, 12, 25, 38). An additional alternative approach could utilize a noninferiority design comparing GC-MRT to either CBT or an SSRI. Because previous trials had shown efficacy using tight controls separately for SSRIs (6, 25, 38) and GC-MRT (12), we focused this study on comparing the two treatments with a waiting list control condition. Second, because it did not use a noninferiority design, the present study possesses insufficient statistical power to rule out differences between GC-MRT and SSRI on symptom reductions. However, the selected sample size provided sufficient power to detect a small to medium effect size (f=0.19) between the two treatments, had such differences existed. Future studies could determine whether a smaller but still significant difference might exist between SSRI and GC-MRT. Third, this study did not include a follow-up on treatment outcomes. This is an important limitation given that SSRI continuation is recommended for at least a year. Future studies could determine the relative longer-term effects of these treatments. Finally, both active treatments were limited in their overall clinical effectiveness. Mean LSAS scores remained above the suggested clinical cutoff at the posttreatment assessment, and less than 50% of patients showed reliable change. This outcome is consistent with the literature on SSRI treatments for social anxiety disorder (7) but is lower than that previously reported for GC-MRT (12). The higher GC-MRT efficacy noted previously (12) could be attributed to differences in the enrolled populations. Although baseline social anxiety scores were similar in this and previous GC-MRT trials, it is possible that because of potential randomization into pharmacological treatment in this trial, a different patient profile was attracted.
In summary, this randomized controlled trial is the first to compare GC-MRT with SSRI treatment for social anxiety disorder. The results indicate that the clinical efficacy of GC-MRT is comparable to this first-line treatment. GC-MRT presents low demands on patients, is short-term, and requires minimally trained professionals, making it a viable treatment option. SSRIs and GC-MRT may engage different mechanisms to reduce social anxiety symptoms, consistent with differential effects on comorbid depression. Hence, the two therapies may possess additive therapeutic value for social anxiety. GC-MRT and SSRIs could be combined or sequenced, consistent with recommendations to initiate treatment for social anxiety with a psychosocial intervention (39). GC-MRT could also serve as an alternative treatment for patients unwilling or unable to receive pharmacological treatment (e.g., pregnant or breastfeeding women, patients concerned about medication side effects) or CBT. Currently, GC-MRT requires access to eye-tracking equipment operated by trained staff. However, advances in telemedicine following the COVID-19 pandemic are mirrored by advances in remote eye-tracking applications (40), suggesting that GC-MRT may become available even from home in the near future.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013 Crossref, Google Scholar
2 : The cross-national epidemiology of social anxiety disorder: data from the World Mental Health Survey Initiative. BMC Med 2017; 15:143Crossref, Medline, Google Scholar
3 : Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med 2008; 38:15–28Crossref, Medline, Google Scholar
4 : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
5 : Functional impairment in social anxiety disorder. J Anxiety Disord 2012; 26:393–400Crossref, Medline, Google Scholar
6 : Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2014; 1:368–376Crossref, Medline, Google Scholar
7 : Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry 2004; 61:1005–1013Crossref, Medline, Google Scholar
8 : Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry 2001; 158:275–281Link, Google Scholar
9 : Remission in CBT for adult anxiety disorders: a meta-analysis. Clin Psychol Rev 2018; 61:1–8Crossref, Medline, Google Scholar
10 : Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:629–640Crossref, Medline, Google Scholar
11 : Behavioral intervention technologies: evidence review and recommendations for future research in mental health. Gen Hosp Psychiatry 2013; 35:332–338Crossref, Medline, Google Scholar
12 : Gaze-contingent music reward therapy for social anxiety disorder: a randomized controlled trial. Am J Psychiatry 2017; 174:649–656Link, Google Scholar
13 : Social anxiety is related to increased dwell time on socially threatening faces. J Affect Disord 2016; 193:282–288Crossref, Medline, Google Scholar
14 : Increased attention allocation to socially threatening faces in social anxiety disorder: a replication study. J Affect Disord 2021; 290:169–177Crossref, Medline, Google Scholar
15 : Social anxiety and difficulty disengaging threat: evidence from eye-tracking. Cogn Emot 2012; 26:300–311Crossref, Medline, Google Scholar
16 : Difficulty disengaging attention from social threat in social anxiety. Cognit Ther Res 2010; 34:99–105Crossref, Medline, Google Scholar
17 : Social anxiety and social anxiety disorder. Annu Rev Clin Psychol 2013; 9:249–274Crossref, Medline, Google Scholar
18 : Fast evidence accumulation in social anxiety disorder enhances decision making in a probabilistic reward task. Emotion 2022; 22:1–18Crossref, Medline, Google Scholar
19 : Gaze-contingent music reward therapy for clinically anxious 7- to 10-year-olds: an open multiple baseline feasibility study. J Clin Child Adolesc Psychol 2020; 49:618–625Crossref, Medline, Google Scholar
20 : Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs 2010; 24:769–796Crossref, Medline, Google Scholar
21 : A randomized controlled trial of gaze-contingent music reward therapy for major depressive disorder. Depress Anxiety 2021; 38:134–145Crossref, Medline, Google Scholar
22 : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–191Crossref, Medline, Google Scholar
23 : Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Erlbaum, 1988Google Scholar
24 : Update on the efficacy of pharmacotherapy for social anxiety disorder: a meta-analysis. Expert Opin Pharmacother 2014; 15:2281–2291Crossref, Medline, Google Scholar
25 : Escitalopram in the treatment of social anxiety disorder: randomised, placebo-controlled, flexible-dosage study. Br J Psychiatry 2005; 186:222–226Crossref, Medline, Google Scholar
26 : Social phobia, in Modern Trends in Pharmacopsychiatry, vol 22. Basel, Switzerland, Karger, 1987 Google Scholar
27 : Psychometric properties of the Social Phobia Inventory (SPIN): new self-rating scale. Br J Psychiatry 2000; 176:379–386Crossref, Medline, Google Scholar
28 : Evaluation of the Clinical Global Impression Scale among individuals with social anxiety disorder. Psychol Med 2003; 33:611–622Crossref, Medline, Google Scholar
29 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
30 : Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry 2000; 31:73–86Crossref, Medline, Google Scholar
31 : Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979; 2:197–207Crossref, Medline, Google Scholar
32 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
33 : Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44:1049–1060Crossref, Medline, Google Scholar
34 : Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991; 59:12–19Crossref, Medline, Google Scholar
35 : The Liebowitz Social Anxiety Scale as a self-report instrument: a preliminary psychometric analysis. Behav Res Ther 2002; 40:701–715Crossref, Medline, Google Scholar
36 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57:289–300 Google Scholar
37 : A randomized, controlled trial of the effectiveness of cognitive-behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry 2006; 14:255–263Crossref, Medline, Google Scholar
38 : Efficacy of escitalopram in the treatment of social anxiety disorder: a meta-analysis versus placebo. Eur Neuropsychopharmacol 2016; 26:1062–1069Crossref, Medline, Google Scholar
39 : Recognition, assessment, and treatment of social anxiety disorder: summary of NICE guidance. BMJ 2013; 346:f2541Crossref, Medline, Google Scholar
40 : Gaze and eye-tracking solutions for psychological research. Cogn Process 2012; 13(suppl 1):S261–S265Crossref, Medline, Google Scholar



