Racial Disparities in Adversity During Childhood and the False Appearance of Race-Related Differences in Brain Structure
Abstract
Objective:
Black Americans in the United States are disproportionately exposed to childhood adversity compared with White Americans. Such disparities may contribute to race-related differences in brain structures involved in regulating the emotional response to stress, such as the amygdala, hippocampus, and prefrontal cortex (PFC). The authors investigated neuroanatomical consequences of racial disparities in adversity.
Methods:
The sample included 7,350 White American and 1,786 Black American children (ages 9–10) from the Adolescent Brain Cognitive Development Study (public data release 2.0). Structural MRI data, parent and child self-reports of adversity-related measures, and U.S. Census neighborhood data were used to investigate the relationship between racial disparities in adversity exposure and race-related differences in brain structure.
Results:
Black children experienced more traumatic events, family conflict, and material hardship on average compared with White children, and their parents or caregivers had lower educational attainment, lower income, and more unemployment compared with those of White children. Black children showed lower amygdala, hippocampus, and PFC gray matter volumes compared with White children. The volumes of the PFC and amygdala, but not the hippocampus, also varied with metrics of childhood adversity, with income being the most common predictor of brain volume differences. Accounting for differences in childhood adversity attenuated the magnitude of some race-related differences in gray matter volume.
Conclusions:
The results suggest that disparities in childhood adversity contribute to race-related differences in gray matter volume in key brain regions associated with threat-related processes. Structural alterations of these regions are linked to cognitive-affective dysfunction observed in disorders such as posttraumatic stress disorder. More granular assessments of structural inequities across racial/ethnic identities are needed for a thorough understanding of their impact on the brain. Together, the present findings may provide insight into potential systemic contributors to disparate rates of psychiatric disease among Black and White individuals in the United States.
Children across the United States grow up in vastly different environments that shape their responses to stress and ability to function later in life. Uncontrollable factors such as the neighborhood children are born into can contribute to significant early-life adversity, such as enduring socioeconomic disadvantage or increased risk of violence exposure. In the United States, Black children are disproportionately burdened with these adverse life experiences compared with White children (1). Current U.S. Census data show that Black households, on average, have a lower median income, lower educational attainment, and higher rates of unemployment and poverty compared with White households (2). Moreover, research suggests that Black children are more likely to be exposed to trauma and domestic violence and are more likely to have a parent who died, an incarcerated parent, or divorced or separated parents compared with White children (3–5). Additionally, research has shown that Black children live in disproportionately disadvantaged neighborhoods and are more likely than White children to be exposed to neighborhood violence (6, 7). These racial disparities are not random. Rather, they are deep-rooted structural inequalities that result from a history of disenfranchisement of racially minoritized groups (e.g., slavery, segregation) that reinforce themselves through societal norms and practices (i.e., systemic racism) (8).
Early-life adversity can have lasting negative consequences on mental health in adulthood. Several studies have found positive associations between childhood adversity (e.g., witnessing violence and low socioeconomic status) and prevalence of poor psychosocial and behavioral outcomes later in life, including posttraumatic stress disorder (PTSD), anxiety, and depression, problematic drug and alcohol use, low life satisfaction, suicide attempts and ideation, and perpetration of violence (9–15). Thus, the literature demonstrates a strong relationship between adverse life experiences and outcomes such that more adversity experienced in childhood is tied to a greater risk of deleterious mental health outcomes later in life. Further, recent research has emphasized that different types of adversity are associated with distinct outcomes. Specifically, “threat” type adversity (e.g., physical or sexual abuse, witnessing violence) is more often associated with dysregulated emotional responses, whereas “deprivation” type adversity (e.g., poverty, neglect) is more typically associated with language and cognitive deficits (16–18).
Previous work has shown that early exposure to adversity (i.e., either threat or deprivation) is associated with structural alterations of brain regions, such as the prefrontal cortex (PFC), amygdala, and hippocampus, which support healthy emotional functioning in response to threat and stress (19–21). Therefore, racial disparities in childhood adversity may contribute to race-related differences in the structure of the PFC, hippocampus, and amygdala. The Adolescent Brain and Cognitive Development (ABCD) Study, a large MRI study of childhood development in the United States, may be well-suited to investigate the impacts of racial disparities in adversity on the brain. Previous ABCD Study analyses have found that socioeconomic status (22) and trauma exposure (23) are associated with differences in thickness and volume of threat-related brain regions, and that greater neighborhood disadvantage is associated with greater amygdala reactivity in response to faces (24). Further, socioeconomic status partially mediates the association between race and some aspects of gray matter morphology (25, 26). Relatedly, previous work outside the ABCD Study found lower neural reactivity to threat within the PFC, hippocampus, and amygdala in Black participants compared with White participants, and these differences were partially attributable to racial disparities in negative life experiences (27). The literature thus suggests that adversity is associated with differential structure and functional responses within threat-related neural circuitry, although no work that we are aware of has investigated the relationship between racial disparities in adversity and the structure of this circuitry as a whole during childhood. While emergent research has investigated the impacts of racial discrimination on the brain, it is also important to understand how contextual factors (e.g., systemic racism) may also impact threat neurocircuitry (28–31). Understanding the potential effects of such disparities on these brain structures is critical for a fuller understanding of the impacts of stress on the developing brain and creating generalizable neurobiological models of disease.
In this study, we investigated the relationship between racial disparities in adversity exposure and race-related differences in brain structure among participants in the ABCD Study. We hypothesized that Black American children would have experienced more adversity than White American children in the sample. We further hypothesized that greater exposure to adverse life experiences would be related to lower gray matter volume in the amygdala, the hippocampus, and several subregions of the PFC. Finally, we anticipated that Black and White children would show differences in gray matter volume of these regions and that these differences would be partially explained by racial differences in exposure to adversity.
Methods
Participants
We used data from the ABCD Study’s annual curated NIH public release 2.0 (released in March 2019; accessed in July 2019 from the NIMH Data Archive [NDA]) (32). Participants (N=11,878) ages 9–10 were recruited from 21 research sites across the United States. The present analyses included 9,382 participants (White, N=7,516; Black, N=1,866; male, N=4,921; female, N=4,461) (descriptive statistics are provided in Table 1). Children were primarily contacted and recruited through U.S. public and private schools within the 21 catchment areas. Less than 10% of the sample was recruited through other methods, which included mailing lists, affiliates and referrals, summer programs, and twin registries. The methods for sampling and recruiting have been described in detail elsewhere (33).
| Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total N | White American | Black American | Statistic | df | p | ||
| Mean | SD | Mean | SD | |||||
| Age (months) | 9,382 | 119.03 | 7.50 | 118.82 | 7.26 | t=1.09 | 9380 | 0.28 |
| N | % | N | % | |||||
| Gender | 9,382 | χ2=5.86 | 0.02 | |||||
| Male | 3,989 | 53.1 | 934 | 50.1 | ||||
| Female | 3,527 | 46.9 | 932 | 49.9 | ||||
| Parental education | 9,373 | t=33.15a | 2802 | <0.001 | ||||
| Grade school | 288 | 3.8 | 221 | 11.9 | ||||
| High school diploma or equivalent | 520 | 6.9 | 449 | 24.1 | ||||
| Some college | 1,054 | 14.0 | 436 | 23.4 | ||||
| Associate’s degree | 907 | 12.1 | 314 | 16.9 | ||||
| Bachelor’s degree | 2,490 | 33.1 | 237 | 12.7 | ||||
| Master’s degree | 1,719 | 22.9 | 179 | 9.6 | ||||
| Doctoral or professional degree | 534 | 7.1 | 25 | 1.3 | ||||
| Parental employment | 9,121 | χ2=344.90 | <0.001 | |||||
| Not currently employed | 409 | 5.6 | 342 | 19.0 | ||||
| Currently employed | 6,914 | 94.4 | 1456 | 81.0 | ||||
| Annual family income | 8,654 | t=40.30a | 1985 | <0.001 | ||||
| <$5,000 | 88 | 1.2 | 225 | 14.2 | ||||
| $5,000–$11,999 | 128 | 1.8 | 178 | 11.2 | ||||
| $12,000–$15,999 | 97 | 1.4 | 93 | 5.9 | ||||
| $16,000–$24,999 | 226 | 3.2 | 155 | 9.8 | ||||
| $25,000–$34,999 | 301 | 4.3 | 194 | 12.2 | ||||
| $35,000–$49,999 | 463 | 6.5 | 211 | 13.3 | ||||
| $50,000–$74,999 | 987 | 14.0 | 221 | 13.9 | ||||
| $75,000–$99,999 | 1,164 | 16.5 | 122 | 7.7 | ||||
| $100,000–$199,999 | 2,611 | 36.9 | 153 | 9.7 | ||||
| >$200,000 | 1,004 | 14.2 | 33 | 2.1 | ||||
| Mean | SD | Mean | SD | |||||
| Neighborhood disadvantageb | 8,840 | 90.30 | 23.91 | 105.94 | 22.25 | t=−25.66a | 2706 | <0.001 |
| Family conflictb | 9,363 | 1.96 | 1.94 | 2.43 | 2.01 | t=−9.17a | 2786 | <0.001 |
| Material hardshipb | 9,296 | 0.30 | 0.89 | 1.01 | 1.49 | t=−19.63a | 2166 | <0.001 |
| Trauma historyb | 9,043 | 0.48 | 1.10 | 0.67 | 1.02 | t=−7.26a | 2965 | <0.001 |
TABLE 1. Demographic characteristics of participants in a study of childhood adversity and brain structure
Measures
Demographic history.
Family demographic data were acquired using a standardized survey, completed by participants’ parents (NDA: pdem02), that assessed both parent and child race/ethnicity, parental education and employment, and family income, among other variables. Parents identified their children as a member of one or more racial identities from 16 categories (e.g., White, Black/African American, Alaska Native, Samoan, Vietnamese). The present analyses focused on environmental and brain structure relationships specifically in White and Black children. Children who were identified by their parents as both Black and White were excluded from our analysis.
Parents and caregivers self-reported their current employment status, their highest educational attainment, and their total family annual income at the time of the interview. Parent educational attainment was self-reported for 22 levels, from “never attended/kindergarten only” through “doctoral degree,” and was recoded into seven ordinal groups (see Table 1) for the present analyses. Employment status was recategorized from 11 possible categories into two groups of “currently employed” or “not currently employed.” The “currently employed” group consisted of parents/caregivers who endorsed “working now,” “stay-at-home parent,” “student,” “maternity leave,” or “sick leave” as their employment status. The “not currently employed” group consisted of those who endorsed “temporarily laid off,” “looking for work,” “disabled,” or “unemployed, not looking for work” as their employment status. Retired individuals and those who did not provide employment information were excluded from the analyses. Family income was self-reported for 10 levels, <$5,000 to ≥$200,000. The family income variable was not modified for analysis.
Neighborhood disadvantage.
Neighborhood disadvantage was measured using the Area Deprivation Index (ADI) (34), which was included as part of the ABCD Study assessments of residential history (NDA: abcd_rhds01). Briefly, the ADI is a factor-based index that uses 17 socioeconomic indicators from the U.S. Census Survey (e.g., poverty, housing, employment) to characterize a given neighborhood. Parents/caregivers of participants were asked to provide up to three primary addresses, and the first address was used to derive regional U.S. Census information to determine the ADI. Data for each census region were queried from the 2011–2015 American Community Survey 5-year summary database (U.S. Census Bureau, 2016). A weighted ADI sum score that represented a participant’s level of neighborhood disadvantage was used in the statistical analyses (described further in reference 35). Greater weighted ADI sum score represented higher neighborhood disadvantage. In exploratory analyses, given emerging research on both racial disparities in toxin/pollutant exposure and their impacts on the brain (36, 37), we further assessed potential impacts of neighborhood inequities on the brain by including measures of particulate matter (PM2.5) and ground pollution, indexed by nitrogen dioxide (NO2), from participants’ residential history (the methods and analyses are described in the online supplement).
Family conflict.
Family conflict was assessed with the family conflict subscale of the Youth Family Environment Scale (NDA: abcd_fes01). This subscale consists of nine items completed by the children that assessed physical and emotional conflicts within the household (e.g., the extent to which family members become openly angry or criticize or hit each other). Participants rated each item as either “true” or “false” (coded 1 or 0, respectively), and three items with negative phrasing (e.g., “family members rarely become openly angry”) were reverse-coded for analyses. The sum score from the family conflict subscale items served as an index of family conflict and was included in the statistical analyses.
Material hardship.
Family material hardship was assessed using a material hardship questionnaire collected as part of the parent demographic survey (NDA: pdem02). The questionnaire consists of seven items related to economic insecurity (e.g., “couldn’t afford to pay rent,” “had utilities shut off due to nonpayment,” “couldn’t afford to go to the doctor”). The sum score of the material hardship items was used in the statistical analyses.
Trauma history.
Participants’ trauma history was assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children for DSM-5 (K-SADS-5). Trauma history was obtained from parent reports based on the 17-item traumatic events module of the K-SADS-5 (NDA: abcd_ptsd01). The items included events such as motor vehicle accident, natural disaster, and sexual and nonsexual assault. Endorsed items were summed for each child to create a trauma history score.
Structural brain imaging.
Structural MRI data were collected across 21 sites on Siemens Prisma, General Electric 750, and Philips 3-T scanners, using prospective motion correction when available. Detailed information on imaging protocols, parameters, and processing of the structural imaging data has been published elsewhere (38, 39). Briefly, structural MRI (T1-weighted and T2-weighted) data were preprocessed by the ABCD team using FreeSurfer, version 5.3.0 (https://surfer.nmr.mgh.harvard.edu). Images were corrected for gradient nonlinearity distortions and head motion and resampled into alignment with an averaged reference brain. The cortical surface was then reconstructed, and subcortical regions of the brain were segmented. For the present study, gray matter volume of cortical regions of interest based on the Desikan-Killiany atlas (40) and gray matter volume of subcortical regions of interest and estimated intracranial volume based on FreeSurfer segmentations (41) were used in the analyses. Participants whose MRI data failed T1 or T2 quality-control checks (NDA: mriqcrp102) or failed FreeSurfer quality control (NDA: freesqc01) were excluded from the analyses (N=832). An independent-samples t test demonstrated that racial groups differed in intracranial volume (t=19.44, df=8235, p<0.001). Thus, the gray matter volume of our a priori regions of interest (PFC, hippocampus, amygdala, and insula) was normalized as a proportion of estimated intracranial volume ([region volume/intracranial volume]×100) and averaged across left and right hemispheres. Subdivisions of the PFC based on the Desikan-Killiany atlas (i.e., frontal pole, superior frontal gyrus, rostral anterior cingulate, pars opercularis, medial orbitofrontal cortex, lateral orbitofrontal cortex, caudal middle frontal gyrus, caudal anterior cingulate, rostral middle frontal gyrus, pars orbitalis, and pars triangularis) were used as separate regions of interest given that these regions may have differing functions and thus show differing relationships. Given growing understanding of the role of the insula in threat processing (42, 43), we included the insula as another region of interest for analysis. In total, gray matter volumes of 14 regions of interest were included in the statistical analyses (NDA: abcd_smrip101; abcd_smrip201).
Statistical Analysis
Statistical analyses were conducted using SPSS, version 24.0 (IBM, Armonk, N.Y.). The number of participants available for statistical tests varied because of incomplete data on some measures. Where appropriate, t tests were corrected for unequal variances, and the Bonferroni correction for multiple comparisons was applied for each family of tests. We assessed group differences in adversity measures using chi-square tests for categorical variables (i.e., employment status) and independent-samples t tests for continuous and ordinal variables (i.e., income, educational attainment, neighborhood disadvantage, family conflict, material hardship, trauma history). A Bonferroni correction was applied to control for multiple comparisons within this family of tests (seven tests, p=0.05/7=0.007). We also conducted exploratory analyses with participant PTSD symptoms reported by the caregivers, which are detailed in the online supplement.
Next, we used 14 linear mixed-effects models to assess race-related differences in gray matter volumes of the a priori regions of interest. The models accounted for nesting of families (NDA: acpsw03) and covaried for age, gender, and scanner type (NDA: abcd_mri01) with restricted maximum likelihood estimation. The Bonferroni correction was applied to control for multiple comparisons within this family of tests (14 tests, p=0.05/14=0.0035). We used additional mixed-effects models to assess the relationship between regional gray matter volume and the measures of childhood adversity (one brain region per model, 14 models total). The models included the seven indices of adversity (i.e., educational attainment, employment status, income, neighborhood disadvantage, family conflict, material hardship, and trauma history) as independent variables and gray matter volume for each brain region as the dependent variable. We again covaried for family relatedness, age, gender, and scanner type. We conducted separate independent-samples t tests between the racial groups using the Destrieux atlas to validate the robustness of the effect across brain parcellations and covariate approaches (see Table S1 in the online supplement).
We also investigated whether accounting for childhood adversity modulated race-related differences in regional gray matter volumes, similar to prior work (39). We conducted parallel mediation analyses in the JASP statistical package (https://jasp-stats.org/) to calculate the standardized estimates of the total, direct, and indirect effects of racial group on regional gray matter volume as well as the percentage of variance mediated by the adversity metrics. Parallel mediation models used full information maximum likelihood for estimation. Participant racial group was included as the predictor variable, and metrics of adversity (educational attainment, employment status, income, neighborhood disadvantage, family conflict, material hardship, and trauma history) were included as mediators. The dependent variables for the mediation models were the residual gray matter volume values estimated from linear mixed-effects models that accounted for age, scanner, gender, and family relatedness (equivalent to the above models without including racial group). An exploratory parallel mediation analysis was also conducted to determine whether accounting for other neighborhood variables such as exposure to pollutants further explained race-related variability in gray matter volume.
Results
Race-Related Differences in Adversity
Chi-square and independent-samples t tests revealed that, on average, Black and White children in the present sample differed in parent employment status, parent educational attainment, and family income (Table 1). Specifically, White children’s parents were three times more likely to be currently employed. White children’s parents also had higher educational attainment and greater family income compared with Black children’s parents; 75.2% of White parents had a college degree, compared with 40.6% of Black parents, and 88.1% of White parents made $35,000 a year or more, compared with 46.7% of Black parents. White children also experienced less family conflict, less material hardship, less neighborhood disadvantage, and fewer traumatic events compared with Black children (Table 1). Racial differences in trauma exposure remained significant when nontraumatized individuals were removed from the analysis (t=−2.18, df=3194, p=0.03).
Race-Related Differences in Gray Matter Volume
Linear mixed-effects models revealed that Black and White children in the present sample differed in gray matter volumes in 11 of the 14 a priori regions of interest, after covarying for family relatedness, gender, age, and scanner type (Figure 1; Table 2). (An alternative visualization of the results is provided in Figure S1 in the online supplement.) White children showed greater gray matter volumes compared with Black children in the amygdala, hippocampus, frontal pole, superior frontal gyrus, rostral anterior cingulate, pars opercularis, pars orbitalis, lateral orbitofrontal cortex, caudal middle frontal gyrus, and caudal anterior cingulate and smaller gray matter volume compared with Black children in the pars triangularis (all p values <0.001). No difference was observed in gray matter volume of the insula, rostral middle frontal gyrus, or medial orbitofrontal cortex between the groups. Similar results were observed in the Destrieux parcellation (see Table S1 in the online supplement).
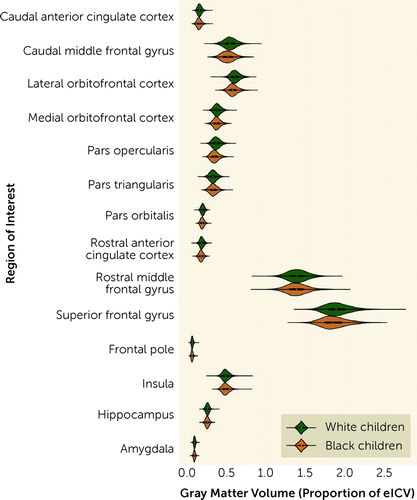
FIGURE 1. Race-related differences in regional gray matter volume in a study of childhood adversity and brain structurea
aPlots inside distributions represent box plots for each group by brain region. eICV=estimated intracranial volume.
| White American | Black American | |||||
|---|---|---|---|---|---|---|
| Region | Estimated Marginal Mean | SE | Estimated Marginal Mean | SE | t | p |
| Caudal anterior cingulate cortex | 0.173 | 0.000 | 0.168 | 0.001 | 6.00 | <0.001b |
| Caudal middle frontal gyrus | 0.558 | 0.001 | 0.536 | 0.002 | 10.53 | <0.001b |
| Lateral orbitofrontal cortex | 0.631 | 0.001 | 0.610 | 0.001 | 16.13 | <0.001b |
| Medial orbitofrontal cortex | 0.406 | 0.001 | 0.405 | 0.001 | 0.95 | 0.340 |
| Pars opercularis | 0.385 | 0.001 | 0.370 | 0.001 | 11.32 | <0.001b |
| Pars triangularis | 0.346 | 0.001 | 0.353 | 0.001 | −4.77 | <0.001b |
| Pars orbitalis | 0.210 | 0.000 | 0.207 | 0.001 | 6.85 | <0.001b |
| Rostral anterior cingulate cortex | 0.199 | 0.000 | 0.191 | 0.001 | 10.54 | <0.001b |
| Rostral middle frontal gyrus | 1.421 | 0.002 | 1.423 | 0.003 | −0.53 | 0.593 |
| Superior frontal gyrus | 1.939 | 0.002 | 1.912 | 0.004 | 7.09 | <0.001b |
| Frontal pole | 0.080 | 0.000 | 0.078 | 0.000 | 6.77 | <0.001b |
| Insula | 0.502 | 0.001 | 0.504 | 0.001 | −1.53 | 0.127 |
| Hippocampus | 0.272 | 0.000 | 0.270 | 0.001 | 4.26 | <0.001b |
| Amygdala | 0.109 | 0.000 | 0.108 | 0.000 | 4.93 | <0.001b |
TABLE 2. Race-related differences in gray matter volume (in mm3) of a priori regions of interest in a study of childhood adversity and brain structurea
Relationships Between Adversity and Gray Matter Volume
Linear mixed-effects models assessed the effects of the indices of adversity (income, education, employment, neighborhood disadvantage, material hardship, trauma history, and family conflict) on gray matter volume for each region of interest, while covarying for family relatedness, age, gender, and scanner type. Childhood adversity was associated with gray matter volume in the caudal anterior cingulate, caudal middle frontal gyrus, lateral orbitofrontal cortex, medial orbitofrontal cortex, pars opercularis, pars orbitalis, rostral anterior cingulate, rostral middle frontal gyrus, superior frontal cortex, frontal pole, insula, and amygdala (Table 3). Specifically, we observed unique effects of all adversity indices except trauma history and family conflict, which were not uniquely related to gray matter volume in any of the models. Income was the most frequent predictor, having effects on gray matter volume in eight of 14 regions.
| Material Hardship | Parental Employment | Family Income | Parental Education | Family Conflict | Neighborhood Disadvantage | Trauma History | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | b | t | b | t | b | t | b | t | b | t | b | t | b | t |
| Caudal anterior cingulate cortex | −0.001 | −2.34* | 0.002 | 1.22 | <0.001 | 2.21* | <0.001 | 0.72 | <−0.001 | −0.60 | <0.001 | 0.37 | <−0.001 | −1.03 |
| Caudal middle frontal gyrus | −0.002 | −2.48* | −0.001 | −0.14 | 0.002 | 4.54*** | <0.001 | 0.11 | −0.001 | −1.61 | <−0.001 | −1.05 | <−0.001 | −0.14 |
| Lateral orbitofrontal cortex | <−0.001 | −0.36 | 0.001 | 0.32 | 0.002 | 6.26*** | <−0.001 | −0.31 | <−0.001 | −1.34 | <−0.001 | −0.32 | 0.001 | 2.00* |
| Medial orbitofrontal cortex | <−0.001 | −0.87 | −0.001 | −0.52 | 0.001 | 2.20* | −0.001 | −1.49 | <0.001 | 1.07 | <0.001 | 0.77 | <0.001 | 0.90 |
| Pars opercularis | <0.001 | 0.09 | <−0.001 | −0.05 | 0.002 | 4.67*** | −0.001 | −2.92** | <0.001 | 0.55 | <0.001 | 0.10 | <0.001 | 0.56 |
| Pars triangularis | <−0.001 | −0.46 | −0.002 | −0.86 | <−0.001 | −0.94 | −0.001 | −1.81 | <0.001 | 1.01 | <0.001 | 2.01* | <−0.001 | −0.18 |
| Pars orbitalis | <−0.001 | −0.49 | −0.001 | −1.25 | 0.001 | 3.67*** | <0.001 | 1.06 | <0.001 | 1.22 | <−0.001 | −0.43 | <0.001 | 1.41 |
| Rostral anterior cingulate cortex | <−0.001 | −0.83 | −0.002 | −1.55 | 0.001 | 4.61*** | <0.001 | 0.63 | <0.001 | 0.33 | <0.001 | 0.30 | <0.001 | 0.71 |
| Rostral middle frontal gyrus | −0.003 | −1.92 | −0.009 | −1.44 | 0.001 | 1.51 | 0.003 | 2.88** | −0.001 | −1.26 | <0.001 | 0.94 | 0.002 | 1.79 |
| Superior frontal gyrus | −0.002 | −1.04 | <−0.001 | −0.03 | 0.005 | 5.30*** | <0.001 | 0.16 | −0.001 | −0.84 | <0.001 | 0.37 | 0.001 | 1.09 |
| Frontal pole | <−0.001 | −1.59 | −0.001 | −1.18 | <0.001 | 3.06** | <0.001 | 0.81 | <−0.001 | −0.19 | <0.001 | 1.08 | <−0.001 | −0.57 |
| Insula | <−0.001 | −0.34 | −0.003 | −1.55 | <0.001 | 1.30 | −0.001 | −2.45* | <0.001 | 0.11 | <0.001 | 3.10** | <−0.001 | −0.31 |
| Hippocampus | <−0.001 | −0.14 | 0.001 | 0.48 | <0.001 | 1.23 | <−0.001 | −0.12 | <0.001 | 0.36 | <0.001 | 1.51 | <−0.001 | −1.37 |
| Amygdala | <0.001 | 0.41 | 0.001 | 2.35* | <0.001 | 0.73 | <0.001 | 0.66 | <−0.001 | −0.44 | <−0.001 | −0.65 | <−0.001 | −1.51 |
TABLE 3. Summary of mixed-effects analyses predicting gray matter volume in a study of childhood adversity and brain structurea
We next sought to determine whether accounting for childhood adversity affected the magnitude of race-related differences in brain structure. Standardized estimates from the parallel mediation models are provided in Table 4. Standardized estimates for total and direct effects for each brain region are shown for each brain region and plotted in Figure 2. Direct effects of racial group for several brain regions were smaller than total effects, with significant partial mediation observed for the caudal anterior cingulate, caudal middle frontal gyrus, lateral orbitofrontal gyrus, pars triangularis, pars orbitalis, superior frontal gyrus, and frontal pole (Figure 3). Exploratory parallel mediation models that accounted for additional neighborhood variables of pollutant exposure showed similar effects; in these models there was no mediation for the pars triangularis or frontal pole, but full mediation was observed for the superior frontal gyrus (described in the online supplement). These findings demonstrate that racial disparities in adversity partially mediate some of the race-related differences in regional gray matter volume.
| Region | Total Effect (c) | p | Total Indirect Effect (ab) | p | Direct Effect (c') | p | Percentage Mediatedb (%) |
|---|---|---|---|---|---|---|---|
| Caudal anterior cingulate cortexc | −0.17 | <0.001 | −0.04 | 0.006 | −0.13 | <0.001 | 26.04 |
| Caudal middle frontal gyrusc | −0.29 | <0.001 | −0.09 | <0.001 | −0.20 | <0.001 | 30.58 |
| Lateral orbitofrontal cortexc | −0.45 | < 0.001 | −0.03 | 0.034 | −0.41 | <0.001 | 7.40 |
| Medial orbitofrontal cortex | −0.03 | 0.333 | −0.02 | 0.287 | −0.01 | 0.748 | — |
| Pars opercularis | −0.31 | <0.001 | 0.01 | 0.613 | −0.32 | <0.001 | 2.57 |
| Pars triangularisc | 0.13 | <0.001 | 0.06 | <0.001 | 0.08 | 0.02 | 42.42 |
| Pars orbitalisc | −0.19 | <0.001 | −0.04 | 0.008 | −0.15 | <0.001 | 21.88 |
| Rostral anterior cingulate cortex | −0.29 | <0.001 | −0.03 | 0.098 | −0.27 | <0.001 | 8.93 |
| Rostral middle frontal gyrus | 0.02 | 0.597 | −0.09 | <0.001 | 0.10 | 0.001 | — |
| Superior frontal gyrusc | −0.20 | <0.001 | −0.10 | <0.001 | −0.10 | 0.003 | 50.76 |
| Frontal polec | −0.19 | <0.001 | −0.04 | 0.006 | −0.15 | <0.001 | 23.28 |
| Insula | 0.05 | 0.116 | 0.02 | 0.155 | 0.02 | 0.501 | — |
| Hippocampus | −0.12 | <0.001 | −0.01 | 0.765 | −0.11 | <0.001 | 4.27 |
| Amygdala | −0.14 | <0.001 | −0.01 | 0.582 | −0.13 | <0.001 | 6.67 |
TABLE 4. Summary of parallel mediation analyses of race-related effects on gray matter volume accounting for adversitya
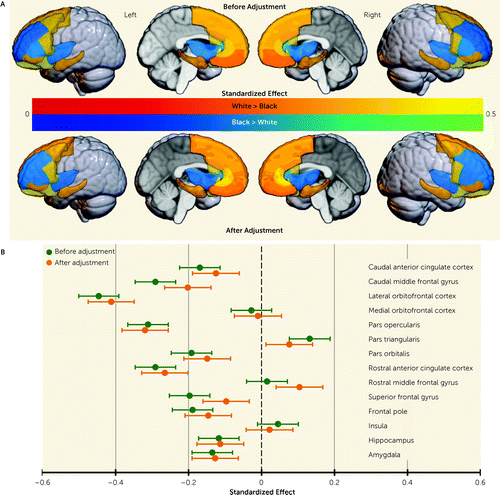
FIGURE 2. Effects of racial disparities in childhood adversity on race-related differences in brain structurea
aStandardized estimates were calculated from the parallel mediation analyses for differences in gray matter volume between Black and White children before (total effect) and after (direct effect) accounting for disparities in sociodemographic factors. Panel A is a graphical representation of estimates where White > Black (warm colors) and Black > White (cool colors) before (top) and after (bottom) accounting for racial disparities. Panel B is a plot of the standardized estimates per region for the total (green) and direct (orange) effects on gray matter volume data. Error bars indicate 95% confidence intervals.
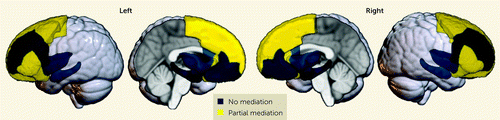
FIGURE 3. Graphical representation of parallel mediation results in a study of childhood adversity and brain structurea
aParallel mediation modeling revealed no, partial, or full mediation of race-related differences on regional gray matter volume by the adversity metrics. Blue indicates no significant total and/or indirect effect, and yellow indicates significant total, indirect, and direct effect.
Associations Between Adversity and Reported PTSD Symptoms
Given findings on PTSD from previous research, we conducted supplementary analyses on race-related differences in PTSD symptoms and the relationship with adversity, which are described in the online supplement. Black children had significantly greater PTSD symptom severity, and symptom severity was further predicted by adversity (see Table S2 in the online supplement). Accounting for adversity partially mediated race-related differences in PTSD symptoms but also attenuated correlations between regional gray matter volumes and PTSD symptom severity (see Table S3 in the online supplement).
Discussion
In this study, we investigated the neuroanatomical consequences of racial disparities in adversity during childhood. We found that, compared with White American children, Black American children endorsed more traumatic events, material hardship, and family conflict and lived in more disadvantaged neighborhoods, and their caregivers had lower income and educational attainment and were more likely to be unemployed. Greater exposure to these adversities was linked to lower gray matter volumes in the amygdala and several subregions of the PFC. Accordingly, Black children showed lower gray matter volumes in the amygdala, the hippocampus, and several subregions of the PFC compared with White children. Accounting for racial disparities in exposure to adversity partially mediated race-related differences in a number of regions, including the caudal anterior cingulate, lateral orbitofrontal gyrus, and superior frontal gyrus. However, although our findings held when other adversity disparities were considered, such as pollution exposure, there remain other structural inequities that may contribute to race-related differences in the brain, which must be investigated in future research. Taken together, our findings highlight the impact that disparities in early-life adversity have on race-related differences in the structure of neural circuitry associated with PTSD and other trauma- and stress-related disorders.
One way to conceptualize the present findings is that a significant portion of the gray matter volume differences reflect racial disparities in toxic stress. Toxic stress refers to prolonged exposure to adverse experiences that leads to excessive activation of stress response systems and an accumulation of stress hormones, which in turn disrupt the immune and metabolic regulatory systems and ultimately the developing architecture of the brain (44–46). Importantly, the effects of toxic stress may be dependent on the relative timing of stress exposure. The PFC, amygdala, and hippocampus undergo rapid development beginning in early childhood and continuing until early adulthood (47), and this development is punctuated by sensitive periods where stress may have a larger impact (48, 49). In fact, previous work suggests that exposure to adversity during these sensitive periods may have direct effects on the PFC, amygdala, and hippocampus as well as on subsequent threat responses and regulation (50–54). Moreover, our results showed that income was the most common predictor of gray matter volume disparities, aligning with previous research showing that the effects of low socioeconomic status, and specifically low income, have profound effects on neurobiological trajectories (22, 24, 25, 55–57). Taken together, early-life adversity may act as a toxic stressor that disproportionately impacts Black children as a result of their significantly greater exposure to adversity and contributes to differential neural development of key threat-processing regions.
The impacts of toxic stress may be immediate or temporally delayed, depending on the specific brain region. For example, one study examining the effect of childhood sexual abuse on regional brain development (58) found an association between abuse and lower hippocampal volume at ages 3–5 but with lower frontal cortex volume at ages 14–16. In the present study, no effects of adversity were found in the hippocampus, although effects were found in the amygdala and the prefrontal cortex, potentially reflecting the impact of differential sensitive periods of brain development in these regions. A potential delayed effect may partially explain the relatively small magnitude of racial differences in gray matter volume of threat-related regions. Specifically, it may be that the disparities in adversity do not lead to major immediate differences but will be potentiated into adulthood in either brain structure or brain function (27). Future analyses of the longitudinal ABCD data set may shed light on what potential long-term impacts these disparities may have on the brain and behavior. In sum, our findings may reflect the neuroanatomical consequences of racially disparate environments of toxic stress.
We note here that many of the observed race-related and adversity effects had relatively small effect sizes despite many findings being highly statistically significant. The ABCD Study has high statistical power for small effects, afforded by its large sample size, and these effects are likely more accurate to the general population than traditionally large effects in small sample sizes. A recent review of effect sizes in ABCD analyses (59) demonstrated that the median in-sample effect size across multiple instruments (161 variables representing all questionnaires and tasks) was 0.03. The authors found a slightly larger median effect size (0.05) when mimicking “real-world” analyses of ABCD data. Thus, the observed effects of race-related disparities on brain structure are in line with, and larger than, other observations from analyses of ABCD data.
The present findings should be considered in light of several limitations. Our analyses were limited to parent-identified Black and White participants and did not include participants with other racial identities. Although the ABCD Study is one of the largest studies of children’s brains, there was a limited amount of data on non-White and non-Black children (note that only 15.7% of the participants in the present sample were Black and only 17.6% were not Black or White). Unequal sample sizes can impact statistical group comparisons. Further, many neuroimaging studies have demographically unrepresentative samples that can impact the generalizability of research findings. Thus, we echo the recommendations in previous reports to increase representation of non-White racial/ethnic groups to address broader questions on the impact of racial and ethnic disparities across groups (60). Another limitation of the present study is the lack of longitudinal MRI data. Our analyses were focused on the impact of racial disparities on the earliest available assessment of brain structure. However, future analyses of the longitudinal MRI data in combination with potential changes in adversity may be useful to test nuanced questions about the role of adversity on race-related differences in brain development. An additional limitation is the potential role of other adversity types on race-related differences in brain structure. We focused on structural adversities but could not capture certain aspects (e.g., nutritional differences or direct toxin exposure), and our analyses did not focus on other factors, such as racial discrimination (61). Nutritional and racial discrimination data were collected 1 year after the baseline visit, precluding any meaningful interpretations with the baseline MRI data. Although we assessed pollutant exposure at the neighborhood level, more direct measures of toxin exposure, such as those available from baby teeth collected in the ABCD Study, may provide more granular information in future analyses. Recent studies demonstrate that racism and racial discrimination directly affect brain structure and function and are associated with poor mental health outcomes (28–30, 62–64), and thus future research should further explore these relationships in children. Finally, although we assessed adversity, it is unclear when these adversities occurred or for how long. Information on the timing and duration of the children’s adversity exposure could allow us to draw stronger conclusions about its effect on brain development.
In summary, we have shown that differential exposure to childhood adversity contributes to racial differences between Black American and White American children in gray matter volumes of brain regions key to emotion regulation. The disparities in gray matter volume observed in this study may be a consequence of long-term dysregulation of threat-related neural circuitry. The findings from this study thus have important implications for our understanding of the impact of socioeconomic and environmental inequalities on mental health in the United States and our understanding of racial differences in psychiatric disorder development, particularly PTSD, for which the literature on lifetime prevalence is mixed (65–70). Although more research is needed on the neurobiological consequences of racial disparities in childhood adversity, the present findings offer new insight into biological impacts of disproportionate stress exposure.
1. : The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity. Child Trends, February 12, 2018. https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity/ Google Scholar
2. : Income and poverty in the United States: 2017. Current Population Reports, No P60-263. Washington, DC, US Census Bureau, September 2018 Google Scholar
3. : Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES, and age: a review of the research. Am J Community Psychol 2007; 40:313–332Crossref, Medline, Google Scholar
4. : Investigating racial differences in clusters of adverse childhood experiences. Am J Orthopsychiatry 2020; 90:106–114Crossref, Medline, Google Scholar
5. : Racial disparities in child adversity in the US: interactions with family immigration history and income. Am J Prev Med 2016; 50:47–56Crossref, Medline, Google Scholar
6. : Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001; 116:404–416Crossref, Medline, Google Scholar
7. : Individual, family background, and contextual explanations of racial and ethnic disparities in youths’ exposure to violence. Am J Public Health 2013; 103:435–442Crossref, Medline, Google Scholar
8. : Systemic racism: individuals and interactions, institutions, and society. Cogn Res Princ Implic 2021; 6:82Crossref, Medline, Google Scholar
9. : Community violence in context risk and resilience in children and families. J Interpers Violence 2008; 23:296–315Crossref, Medline, Google Scholar
10. : The association of adverse childhood experiences with anxiety and depression for children and youth, 8 to 17 years of age. Acad Pediatr 2020; 20:600–608Crossref, Medline, Google Scholar
11. : Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci 2010; 11:651–659Crossref, Medline, Google Scholar
12. : The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017; 2:e356–e366Crossref, Medline, Google Scholar
13. : Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Soc Sci Med 2011; 73:1088–1096Crossref, Medline, Google Scholar
14. : Adverse childhood experiences and associated health outcomes: a systematic review and meta-analysis. Child Abuse Negl 2019; 97:104127Crossref, Medline, Google Scholar
15. : Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc Sci Med 2013; 90:24–31Crossref, Medline, Google Scholar
16. : Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Front Behav Neurosci 2019; 13:80Crossref, Medline, Google Scholar
17. : Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 2014; 47:578–591Crossref, Medline, Google Scholar
18. : Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci 2014; 18:580–585Crossref, Medline, Google Scholar
19. : Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin 2017; 14:471–479Crossref, Medline, Google Scholar
20. : Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 2013; 54:105–112Crossref, Medline, Google Scholar
21. : Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2015; 77:314–323Crossref, Medline, Google Scholar
22. : Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open 2020; 3:e2023774Crossref, Medline, Google Scholar
23. : The association between latent trauma and brain structure in children. Transl Psychiatry 2021; 11:240Crossref, Medline, Google Scholar
24. : Beyond family-level adversities: exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev Sci 2021; 24:e12985Crossref, Medline, Google Scholar
25. : Socioeconomic status inequalities partially mediate racial and ethnic differences in children’s amygdala volume. Stud Soc Sci Res 2020; 1:62–79Crossref, Medline, Google Scholar
26. : Race, ethnicity, family socioeconomic status, and children’s hippocampus volume. Res Health Sci 2020; 5:25–45Crossref, Medline, Google Scholar
27. : Negative life experiences contribute to racial differences in the neural response to threat. Neuroimage 2019; 202:116086Crossref, Medline, Google Scholar
28. : Experiences of discrimination are associated with greater resting amygdala activity and functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:367–378Crossref, Medline, Google Scholar
29. : Racial discrimination and white matter microstructure in trauma-exposed Black women. Biol Psychiatry 2022; 91:254–261Crossref, Medline, Google Scholar
30. : Association of racial discrimination with neural response to threat in Black women in the US exposed to trauma. JAMA Psychiatry 2021; 78:1005–1012Crossref, Medline, Google Scholar
31. : Racism and health: challenges and future directions in behavioral and psychological research. Cultur Divers Ethnic Minor Psychol 2019; 25:12–20Crossref, Medline, Google Scholar
32. : The conception of the ABCD Study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci 2018; 32:4–7Crossref, Medline, Google Scholar
33. : Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci 2018; 32:16–22Crossref, Medline, Google Scholar
34. : Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health 2003; 93:1137–1143Crossref, Medline, Google Scholar
35. : Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014; 161:765–774Crossref, Medline, Google Scholar
36. : Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci U S A 2019; 116:6001–6006Crossref, Medline, Google Scholar
37. : Adolescent Brain Cognitive Development (ABCD) Study linked external data (LED): protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci 2021; 52:101030Crossref, Medline, Google Scholar
38. : The Adolescent Brain Cognitive Development (ABCD) Study: imaging acquisition across 21 sites. Dev Cogn Neurosci 2018; 32:43–54Crossref, Medline, Google Scholar
39. : Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019; 202:116091Crossref, Medline, Google Scholar
40. : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980Crossref, Medline, Google Scholar
41. : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
42. : Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage 2012; 60:787–799Crossref, Medline, Google Scholar
43. : Brain activation during human defensive behaviour: a systematic review and preliminary meta-analysis. Neurosci Biobehav Rev 2019; 98:71–84Crossref, Medline, Google Scholar
44. : The neurobiology of stress and development. Annu Rev Psychol 2007; 58:145–173Crossref, Medline, Google Scholar
45. : The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012; 129:e232–e246Crossref, Medline, Google Scholar
46. : The Effects of Childhood Stress on Health Across the Lifespan. Atlanta, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2008 Google Scholar
47. : Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 2004; 101:8174–8179Crossref, Medline, Google Scholar
48. : Early adverse experiences and the developing brain. Neuropsychopharmacology 2016; 41:177–196Crossref, Medline, Google Scholar
49. : Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 2016; 70:217–227Crossref, Medline, Google Scholar
50. : Effects of stress throughout the lifespan on the brain, behaviour, and cognition. Nat Rev Neurosci 2009; 10:434–445Crossref, Medline, Google Scholar
51. : Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016; 41:3–23Crossref, Medline, Google Scholar
52. : Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 2014; 97:236–244Crossref, Medline, Google Scholar
53. : The effects of childhood maltreatment on brain structure, function, and connectivity. Nat Rev Neurosci 2016; 17:652–666Crossref, Medline, Google Scholar
54. : A review of adversity, the amygdala, and the hippocampus: a consideration of developmental timing. Front Hum Neurosci 2010; 3:68Medline, Google Scholar
55. : Resting state coupling between the amygdala and ventromedial prefrontal cortex is related to household income in childhood and indexes future psychological vulnerability to stress. Dev Psychopathol 2019; 31:1053–1066Crossref, Medline, Google Scholar
56. : Family income, parental education, and brain structure in children and adolescents. Nat Neurosci 2015; 18:773–778Crossref, Medline, Google Scholar
57. : Associations of family income with cognition and brain structure in USA children: prevention implications. Mol Psychiatry 2021; 26:6619–6629Crossref, Medline, Google Scholar
58. : Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008; 20:292–301Crossref, Medline, Google Scholar
59. : Recalibrating expectations about effect size: a multi-method survey of effect sizes in the ABCD Study. PLoS One 2021; 16:e0257535Crossref, Medline, Google Scholar
60. : An ecological approach to understanding the developing brain: examples linking poverty, parenting, neighborhoods, and the brain. Am Psychol 2020; 75:1245–1259Crossref, Medline, Google Scholar
61. : Prevalence of perceived racism and discrimination among US children aged 10 and 11 years: the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Pediatr 2021; 175:861–863Crossref, Medline, Google Scholar
62. : Racism and psychological and emotional injury: recognizing and assessing race-based traumatic stress. Couns Psychol 2007; 35:13–105 Crossref, Google Scholar
63. : Racial/ethnic discrimination, posttraumatic stress symptoms, and alcohol problems in a longitudinal study of Hispanic/Latino college students. J Couns Psychol 2015; 62:38–49Crossref, Medline, Google Scholar
64. : Racism as a determinant of health: a systematic review and meta-analysis. PLoS One 2015; 10:e0138511Crossref, Medline, Google Scholar
65. : Specifying race-ethnic differences in risk for psychiatric disorder in a USA national sample. Psychol Med 2006; 36:57–68Crossref, Medline, Google Scholar
66. : Racial/ethnic differences in the impact of adverse childhood experiences on posttraumatic stress disorder in a nationally representative sample of adolescents. Child Adolesc Soc Work J 2018; 36:449–457 Crossref, Google Scholar
67. : Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the United States. Med Care 2013; 51:1114–1123Crossref, Medline, Google Scholar
68. : Racial/ethnic differences in symptoms of posttraumatic stress disorder. Curr Psychiatry Rev 2016; 12:124–138 Crossref, Google Scholar
69. : Racial/ethnic variation in trauma-related psychopathology in the United States: a population-based study. Psychol Med 2019; 49:2215–2226Crossref, Medline, Google Scholar
70. : Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med 2011; 41:71–83Crossref, Medline, Google Scholar



