Coordinate-Based Network Mapping of Brain Structure in Major Depressive Disorder in Younger and Older Adults: A Systematic Review and Meta-Analysis
Abstract
Objective:
Structural neuroimaging findings in younger and older adults with major depressive disorder (MDD) are highly heterogeneous, possibly as a result of methodological limitations, lack of distinction between MDD and late-life depression (LLD), or clinical moderators. Using a novel meta-analytic network mapping approach, the authors sought to identify the circuits affected in different clinical subtypes of MDD.
Methods:
The authors identified all voxel-based and surface-based morphometry studies published through October 2020 that compared younger adults with MDD or older adults with LLD to nonpsychiatric control participants. An activation likelihood estimation (ALE) analysis and a novel coordinate-based network mapping approach were used to identify brain circuits affected in MDD and LLD. Meta-regressions examined the impact of age at onset in older patients with LLD and treatment with antidepressants in younger patients with MDD.
Results:
The authors analyzed 145 comparisons from 143 articles, including a total of 14,318 participants (MDD: N=6,362; LLD: N=535; control subjects: N=7,421). Significant ALE results confirmed previous findings implicating the left and right parahippocampus and anterior cingulate in MDD and the anterior cingulate in LLD. In contrast, coordinate-based network mapping showed differences in the frontoparietal, dorsal attention, and visual networks both in MDD and LLD. Meta-regressions showed that late onset was significantly associated with widespread structural abnormalities in LLD, and treatment with antidepressants showed a significant association with abnormalities in the anterior cingulate (Brodmann’s area 32) and dorsolateral prefrontal cortex (Brodmann’s area 9) in MDD.
Conclusions:
These findings help to clarify the shared circuitry of depression across the adult lifespan and highlight some unique circuitry relevant to late-onset depression, which may explain some of the risk for cognitive decline and dementia.
Major depressive disorder (MDD) is one of the leading causes of disability worldwide (1), with a total yearly economic burden in the United States alone estimated at over $200 billion (2). MDD is associated with chronic physical symptoms (3), increased risk of suicide (4), and dementia (5). While many adult studies of MDD typically focus on younger and midlife adults, this disorder affects people throughout the lifespan (6). Late-life depression (LLD), defined as the presence of MDD in individuals older than 55, is associated with cerebrovascular disease (7) and Alzheimer’s disease (8). However, LLD can refer to people older than 55 with a history of depression (early onset) and those older than 55 who develop depression for the first time (late onset). Some have argued that LLD with late onset is a “different” disorder that should be studied separately from MDD (8, 9). An assessment of the brain circuitry in younger patients with MDD, older patients with early-onset MDD, and older patients with late-onset MDD could help to clarify how the same depressive syndrome in these three groups may be associated with shared as well as unique neuropathology.
Considerable efforts have been dedicated to uncovering the neural mechanisms underlying MDD in structural neuroimaging studies. Meta-analyses of brain structure in MDD suggest that structural abnormalities are present in the prefrontal cortex and medial temporal lobe regions such as the hippocampus, parahippocampal gyrus, and amygdala (10–12). A large-scale analysis (N=2,148 MDD patients) of a data set from the Enhancing Neuroimaging Genetics Through Meta-Analysis reported cortical thinning in the orbitofrontal cortex (OFC), anterior and posterior cingulate, insula, and temporal lobes (13). Further, smaller volumes of the OFC (and gyrus rectus), frontal lobe, basal ganglia, thalamus, and hippocampus have been reported as loci of structural differences in MDD (14). Meta-analytic evidence suggests that both MDD and LLD affect the hippocampus, amygdala, and ventral anterior cingulate, while LLD uniquely affects the visual cortex (11). Mechanistic theories propose that LLD (and late-onset LLD in particular) is characterized by fronto-executive impairments due to cerebrovascular disease (15).
An important challenge regarding structural neuroimaging findings in MDD and LLD is the large degree of heterogeneity (8, 10) attributable to differences in demographic or clinical characteristics (16), depression “biotypes” (17), or study methodology. For instance, greater volume or thickness in several regions affected in MDD (notably cingulate, orbitofrontal, medial, and dorsolateral prefrontal cortices) is associated with remission after antidepressant treatment (18). In LLD specifically, age at onset may be associated with different etiologies and may produce different results (19). Going beyond systematic review, activation likelihood estimation (ALE) (20) is the current gold-standard approach to synthesizing whole-brain neuroimaging findings, yet it did not detect convergence in voxel-based morphometry findings in MDD (10). Given this heterogeneity in findings, a novel, more sensitive coordinate-based network mapping approach may be needed to characterize the neural circuitry underlying MDD and LLD (21–23).
We carried out a systematic review and meta-analysis of the scientific literature to identify specific networks affected in major depression in younger to middle-aged adults with MDD and older adults with LLD. Using ALE, we expected clusters of shared structural differences to emerge in the ventromedial prefrontal cortex and the hippocampus in both MDD and LLD (10, 11). Using coordinate-based network mapping, we expected large-scale impairments in the frontoparietal control network (24), the dorsal attention network (25), and possibly the default mode network (26) in both MDD and LLD. We also hypothesized impairments in the visual network in LLD but not in MDD (11). Finally, we expected that different demographic and clinical characteristics, notably age at onset of LLD and use of antidepressants in MDD, would be associated with different findings, in particular greater involvement of the aforementioned networks in late-onset than in early-onset LLD.
METHODS
Search Strategy
We searched the PubMed, MEDLINE, and Web of Science bibliographic databases through October 27, 2020 (see Table S1 in the online supplement). The search strategy included terms related to or describing MDD, LLD, and cortical thickness or voxel-based morphometry in whole-brain studies, both as text words and as MeSH terms. The protocol of this systematic review was registered in PROSPERO (CRD42020187718).
Inclusion and Exclusion Criteria
We included any case-control studies that focused on whole-brain analyses of structural differences between patients fulfilling the DSM or ICD criteria for MDD and control subjects. From this group of studies, we then classified studies whose samples had a mean age of 55 years or older in the LLD group and the remaining studies in the MDD group. Notable exclusion criteria were the presence of comorbid psychotic disorders, severe physical conditions such as cancer, and neurological disorders such as stroke, Alzheimer’s disease, and Parkinson’s disease (see section 1.2 in the online supplement).
Data Extraction and Demographic Characteristics
Two authors (P.Z. and J.A.) screened the titles and abstracts to identify eligible studies that met all the inclusion criteria and none of the exclusion criteria (see section 1.3 in the online supplement).
Activation Likelihood Estimation (ALE) Meta-Analyses
Two ALE meta-analyses were run in the GingerALE software program (20; http://www.brainmap.org/ale/), using coordinate information and sample sizes to weight study contributions. Studies reporting anatomical regions rather than coordinates were eligible for lesion network mapping but not the ALE analyses. Significance was determined using 1,000 nonparametric permutations and cluster-level inference at p<0.05 and family-wise error (FWE) correction.
Coordinate-Based Network Mapping
Going beyond ALE, we used the novel coordinate-based network mapping approach (22, 23) to test the hypothesis that MDD and LLD result in brain structure differences in several networks. Coordinate-based network mapping leverages the difference maps derived from each study’s reported coordinates into a study-specific “network map” using the coordinates of significant differences between depressed patients and control subjects in a connectivity analysis. Combined investigations of voxel-based morphometry (VBM) and functional connectivity support the existence of a strong link between VBM and resting-state connectivity in case-control studies (27–29). Thus, we used resting-state functional connectivity and morphometric similarity (30) to identify connectivity networks corresponding to each study’s reported significant differences. For each study, we extracted the coordinates or anatomical regions of interest where significant differences were found and added these seeds together to generate a study-specific seed map. The resulting seed map was then used in resting-state and morphometric similarity analyses in the unrelated Human Connectome Project 1200 sample (N=428) (see section 1.5 of the online supplement).
Z-scored study networks were thresholded (t>3) and added together to assess which brain networks are affected in >60% of MDD and LLD studies. In addition, mean Z-values from the Yeo seven-network parcellation (hereafter referred to as Yeo networks) (31) were analyzed in a mixed-effect analysis of variance (ANOVA) using a “between” factor of group (MDD, LLD) and a “within” factor of Yeo network (visual, motor, dorsal attention, ventral attention, limbic, frontoparietal, default mode) as well as a “within” factor of modality (resting state, morphometric similarity; see Figure 2; see also Table S3 in the online supplement).
Specific Effects of Early Versus Late Onset in LLD
Going beyond identifying group mean networks underlying structural impairments in LLD, we explored the effects of early and late onset (see section 1.3 of the online supplement) of depression. We identified seven late-onset LLD studies, two early-onset LLD studies, and eight LLD studies that included patients with early or late onset (“mixed-onset” studies). In our statistical comparisons, we grouped the two early-onset studies and the eight mixed-onset studies and contrasted them with the seven late-onset studies. We assessed demographic and clinical differences between the late-onset and the early- and mixed-onset groups using one-way ANOVAs. We analyzed study-specific network maps derived from morphometric similarity and resting-state connectivity in Yeo space (seven networks) using a three-way ANOVA with group (late versus early or mixed onset), Yeo networks, and modality factors. Since pervasive differences were found, we report the results in Yeo space.
Effects of Antidepressant and Time Since Onset of MDD
We used generalized linear models (GLMs) in the Glasser surface space to test for the effects of age, sex, proportion treated with antidepressants, and depression severity on the morphometric similarity and functional connectivity networks affected in the MDD and LLD groups. Four sets of GLMs were fitted, one for each depression group (MDD, LLD) and modality (morphometric similarity, functional connectivity). In each set, there were 360 GLMs, one GLM for each region in the Glasser parcellation. A false discovery rate (FDR) corrected p threshold of 0.05 was used to control for multiple comparison correction in each set of GLMs. We included Newcastle-Ottawa Scale score as a covariate to control for study quality. The following linear models were used:

RESULTS
A total of 143 articles, reporting on 145 comparisons (128 MDD comparisons [depressed participants, N=6,362; control participants, N=6,953] and 17 LLD comparisons [depressed participants, N=535; control participants, N=468]), were included in the analyses (Figure 1). Of these, 102 studies were based on VBM results, and the remaining 43 studies used surface-based cortical thickness measures (conducted in FreeSurfer). The mean age was 37.2 years (SD=6.7, range=21–50) in the 128 MDD studies and 68.4 years (SD=6.1, range=55–79) in the 17 LLD studies. Participants in all 145 studies fulfilled the criteria for a DSM or ICD diagnosis of MDD, and all studies had high scores on the modified Newcastle-Ottawa Scale, which assesses the quality of nonrandomized studies. In all LLD studies, the mean score on the Mini-Mental State Examination was >24.
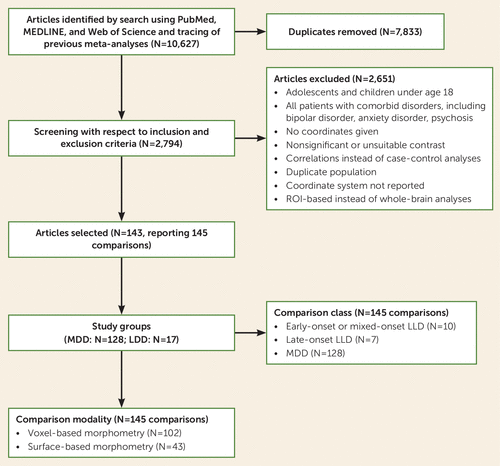
aA total of 143 articles were included, but two articles are split into separate comparisons, as they included two different age groups, resulting in 145 comparisons. MDD=major depressive disorder (in younger adults); LLD=late-life depression (in older adults); PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ROI=region of interest.
For both MDD and LLD, there were qualitative differences in brain structure in a heterogeneous set of brain areas. In MDD studies, ALE meta-analysis revealed two significant clusters (Figure 2E; see also Table S2 in the online supplement). These clusters robustly implicated medial temporal lobe regions bilaterally, including the parahippocampus (Z=5.2, pFWE<0.001) and ventral anterior cingulate (Z=4.5, pFWE<0.001, extending to the subgenual anterior cingulate cortex [ACC]), along with right striatal nuclei (putamen and caudate) and the right insular cortex and right inferior frontal gyrus (IFG) (pFWE<0.01). Using ALE meta-analysis in LLD, we identified a significant cluster in the ACC and medial prefrontal cortex (Z=3.7, pFWE<0.001) (Figure 2F; see also Table S2 in the online supplement).
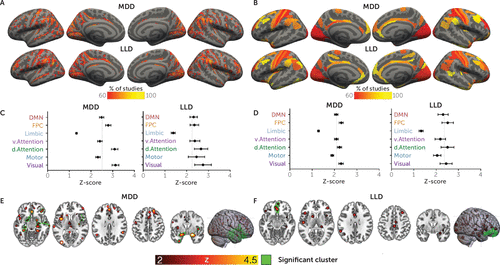
aCoordinate-based networks in major depressive disorder in younger adults (MDD) and late-life depression (LLD) derived using resting-state seed-based connectivity (panels A and C) and morphometric similarity (panels B and D) in the Human Connectome Project sample. The pattern of Yeo network involvement was very similar for both MDD and LLD for both functional and morphometric modalities. We report significantly lower Z-scores in the limbic network compared with all other networks and greater involvement of dorsal attention and visual networks compared with the motor network (Šidák-corrected p<0.05). Further, we found higher contributions of the frontoparietal control, dorsal attention, and visual networks compared with the default mode, ventral attention, and motor networks (uncorrected p<0.017). Means with standard errors of the mean are shown for Yeo networks in panels C and D. Activation likelihood estimation (ALE) results for MDD (panel E) and LLD (panel F) show a similar pattern of findings, implicating insular, inferior frontal, and anterior cingulate regions. Unlike network mapping analysis, ALE also implicated parahippocampal and striatal regions. DMN=default mode network; FPC=frontoparietal control network.
Coordinate-based network mapping captured large-scale networks implicated in both MDD and LLD. Group mean network maps in MDD and LLD were similar (Figure 2A,C), reliably implicating the inferior frontal gyrus and parietal-opercular gyri (supramarginal, angular gyrus) along with the middle frontal gyrus, frontal pole, anterior cingulate, and visual cortices in over 60% of the studies (Table 1).
| Studies With Networks in Common | Coordinates | ||||
|---|---|---|---|---|---|
| Region | N | % | x | y | z |
| Major depressive disorder (128 studies) | |||||
| Inferior frontal gyrus/temporal pole (R) | 106 | 83 | 56 | 16 | –4 |
| Supramarginal gyrus (R) | 105 | 82 | 62 | –28 | 34 |
| Late-life depression (17 studies) | |||||
| Inferior temporal gyrus (L) | 17 | 100 | –56 | –60 | 24 |
| Occipital pole (R) | 17 | 100 | 12 | –96 | 2 |
| Inferior frontal gyrus (R) | 16 | 94 | 54 | 10 | 6 |
| Dorsal anterior cingulate | 16 | 94 | 2 | 8 | 44 |
| Frontal pole (R) | 16 | 94 | 32 | 52 | 28 |
| Middle temporal gyrus (L) | 16 | 94 | –60 | –22 | –8 |
| Insula (R) | 16 | 94 | 40 | –2 | –14 |
| Middle frontal gyrus/dlPFC (L) | 16 | 94 | –34 | 34 | 38 |
| Inferior frontal gyrus (R) | 16 | 94 | 52 | 8 | 14 |
| Posterior cingulate (L) | 16 | 94 | –14 | –30 | 38 |
| Supramarginal gyrus (R) | 16 | 94 | 64 | –26 | 26 |
| Visual cortex (R) | 16 | 94 | 4 | –88 | 0 |
| Intracalcarine cortex (R) | 16 | 94 | 8 | –72 | 14 |
TABLE 1. Clusters in the functional group mean networks common to at least 80% of major depressive disorder studies and at least 90% of late-life depression studiesa
The three-way ANOVA identified a significant main effect of the Yeo networks (F=42.8, df=3, 430, p<0.001). Post hoc comparisons indicate that across MDD and LLD studies, differences between patients and control subjects were centered on the frontoparietal control, dorsal attention, and visual networks, since those networks showed significantly greater involvement than the limbic network (Figure 2B,D). Less differentiation was found with the ventral attention and default mode networks. No significant main effects of group were found (F=0.35, df=1, 143, p=0.85), and a small but significant effect of modality was found (F=4.7, df=1, 143, p=0.032). No significant two-way interactions between Yeo networks and group (MDD versus LLD, F=0.4, df=3, 430, p=0.75) or modality (morphometry and resting state) and group (F=1.0, df=1, 143, p=0.32) were found, suggesting that the involvement of the Yeo networks was the same for both MDD and LLD and that these two groups did not show different patterns in the resting-state versus morphometric similarity analyses, respectively. Further, no significant three-way interaction between Yeo networks, modality, and group was found (F=1.2, df=3, 426, p=0.29), suggesting that the pattern of Yeo network differences in the MDD and LLD groups was the same for the morphometric similarity and resting-state connectivity analyses.
A three-way ANOVA confined to the 17 LLD studies revealed a significant main effect of Yeo networks (F=14.9, df=2.5, 38, p<0.001), replicating results shown in Figure 2. This three-way ANOVA showed no significant three-way interaction (F=1.2, df=2.7, 40, p=0.33) or Yeo network–by–onset group interaction (F=1.8, df=2.5, 38, p=0.16). The ANOVA revealed a significant interaction of modality with early/mixed-onset versus late-onset group (F=4.9, df=1, 15, p=0.04) (Figure 3A). In a follow-up two-way ANOVA focusing on the morphometric similarity analysis, frontoparietal, dorsal, and ventral attention and visual networks were significantly more affected by late-onset LLD than early/mixed-onset LLD. Motor, limbic, and default mode networks were implicated at a similar level in both onset groups. The late- and mixed-onset studies did not differ in age, sex, depression severity, or use of antidepressants (data not shown; all p values for one-way ANOVAs >0.05). No main effect of group was found in the three-way ANOVA (F=1.2, df=1, 15, p=0.3) since the differences between the late- and mixed-onset groups were only found using morphometric similarity and not resting-state connectivity.
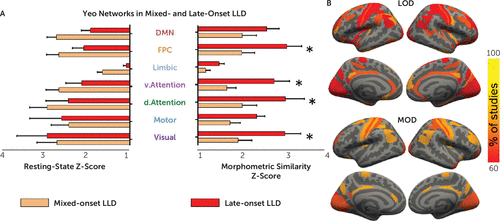
aStudies of early- and mixed-onset late-life depression (LLD) showed lower contributions of Yeo networks compared with late-onset studies (panel A). Mean Z-statistics for study-specific networks with standard errors of the mean are shown in panel A. A follow-up two-way analysis of variance of the morphometric similarity networks showed an interaction between Yeo network and group (F=3.2, df=6, 90, p=0.007). Post hoc comparisons (*pFDR<0.05) revealed significant differences in the visual, frontoparietal control, and dorsal and ventral attention networks driven by LLD onset. While 48 regions were implicated in over 60% of mixed-onset studies, 151 regions were implicated in over 60% of late-onset studies (panel B). DMN=default mode network; FPC=frontoparietal control network; LOD=late-onset depression studies; MOD=mixed-onset depression studies.
The final analysis investigating the effect of demographic and clinical factors on brain heterogeneity in younger adult MDD included 114 MDD studies, as 14 studies were excluded because of insufficient information on use of antidepressants. In MDD studies, the proportion of patients currently taking an antidepressant had a significant effect on the networks affected by structural abnormalities identified using resting-state network mapping (Figure 4C). More specifically, MDD studies that included more patients on antidepressants showed greater involvement of networks involving the dorsal and ventral ACC and dorsolateral prefrontal cortex (dlPFC) (t>2.36, df=1, 108, pFDR<0.05). By contrast, studies that included fewer patients on antidepressants found greater differences in occipitotemporal regions (t<−2.39, df=1, 108, pFDR<0.05). A sensitivity analysis included 34 first-episode studies and replicated the ACC findings (t>2.1, df=1, 27, uncorrected p<0.05; see section 2.3 in the online supplement), although it did not survive correction for multiple comparisons (pFDR>0.05). When repeating the above analyses with morphometric similarity network mapping in MDD studies, no significant effects of sex, use of antidepressant, or depression severity were found in Glasser space (pFDR>0.05).
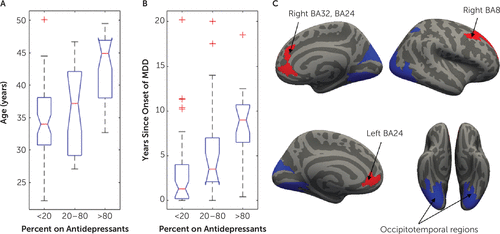
aMajor depressive disorder (MDD) studies with more participants taking an antidepressant reported a higher mean age (panel A) and a higher number of years since onset of MDD (panel B). Studies with higher antidepressant rates showed more involvement of the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (highlighted in red in panel C). Significant differences were found in the ACC (left Brodmann’s area [BA] 32: t=2.9 [all df values, 1, 108], pFDR=0.037; right BA32: t=3.2, pFDR=0.048; right posterior BA32: t=2.4, pFDR=0.045), ventral ACC (left BA24: t=2.5, pFDR =0.039; right BA24: t=2.7, pFDR =0.034), dorsolateral prefrontal cortex (right anterior ventral BA8: t=2.5, pFDR=0.038; right anterior dorsal BA8: t=2.7, pFDR=0.04; right posterior BA9: t=2.9, pFDR=0.04; right anterior BA9: t=2.7, pFDR=0.038), and left anterior agranular insular complex (t=2.6, pFDR=0.035). By contrast, studies with fewer participants taking an antidepressant showed more involvement of the fusiform and occipitotemporal gyri (highlighted in blue in panel C). Significant differences were found in the following regions from the Human Connectome Project (58) parcellation: left V2, V3, V4, V8, V3A, V3B, LO1, LO2, PIT, MT, LIPv, V6A, VMV1, VMV3, V4t, FST, V3CD, and LO3; and right MST, V6, V2, V3, V4, V8, V3A, FFC, LO1, LO2, PIT, LIPv, PH, TPOJ3, V6A, VMV1, VMV3, V4t, FST, V3CD, VMV2 (t<−2.29, df=108, pFDR<0.05).
Since an effect of the use of antidepressants was found, we assessed the effects of use of antidepressants on age, sex, depression severity, and years since the onset of depressive symptoms in MDD. Age at onset was not available for 34 MDD studies; therefore, we included this variable only for post hoc examination of clinical characteristics of different MDD subgroups. For these follow-up analyses, three groups of MDD studies were formed, according to the proportion of participants taking antidepressants (Figure 4).
Studies including more patients taking an antidepressant reported a higher mean age (Figure 4A) (F=21.4, df=2, 111, p<0.001) and a higher number of years since onset of MDD (Figure 4B) (F=10.6, df=2, 84, p<0.001). Twenty-nine of the 57 studies in which patients were not receiving antidepressants were focused on first-episode depression. In comparison, only five of 57 studies in which patients were receiving antidepressants included patients with first-episode depression. There were no significant associations between the proportion of participants taking antidepressants and depression severity (χ2=9.5, p=0.05) or sex (F=0.95, df=2, 114, p=0.39).
No significant effects of demographic, clinical, or quality control variables on networks impaired in LLD were found (morphometric similarity or resting-state networks, pFDR>0.05 in Glasser space).
DISCUSSION
Using data from 143 articles, including a total of 14,318 participants, we extracted gray matter volume and cortical thickness in younger and older adults with MDD to identify networks common to both age groups and specific regional abnormalities linked to clinical characteristics. Coordinate-based network mapping showed structural differences in frontoparietal and dorsal attention networks when patients with MDD were compared with nonpsychiatric control subjects. In older patients with LLD, late onset was associated with more extensive structural differences compared with early or mixed onset. In younger patients with MDD, use of antidepressants and time since onset of MDD were associated with differences in the anterior cingulate cortex, dlPFC, and occipital-temporal regions. By contrast, traditional ALE analyses identified a limited set of ventral anterior cingulate regions common in both MDD and LLD studies. The inferior frontal gyrus was consistently implicated in MDD using both ALE and network mapping approaches. Using ALE, but not network mapping, striatal and hippocampal regions were implicated in MDD.
ALE Results
Using ALE, we showed that studies of MDD had significant clusters localized to the medial temporal lobe, anterior cingulate, and IFG. The MDD findings agreed with previous meta-analytic evidence (10) and our hypotheses. Unthresholded ALE results in LLD showed a similar pattern (e.g., involvement of left and right parahippocampi, insula, inferior frontal gyri, striatum, and anterior and posterior cingulate), although only the anterior cingulate and medial prefrontal cortices reached statistical significance. A previous meta-analysis (11) of gray matter in LLD using a different approach than ALE (signed-differential mapping) showed similar, significant results, including reductions in the ventral anterior cingulate cortex, parahippocampus, hippocampus, amygdala, and striatum and gray matter increases in the visual cortex. While our ALE findings are largely consistent with previous meta-analytic evidence for structural abnormalities in MDD and LLD, more sensitive methods are needed to understand the underlying heterogeneity.
Coordinate-Based Network Mapping Results
In contrast to our ALE analyses, our coordinate-based network mapping approach identified impairments in the IFG, dlPFC, frontal pole, medial and lateral parietal regions, and temporal gyri in both MDD and LLD. When viewed in the context of the Yeo networks, these regions encompassed frontoparietal control and dorsal attention networks in MDD and LLD. The striking continuity between MDD and LLD in both the functional connectivity and the morphometric similarity analyses suggests that MDD affects the same networks across the adult lifespan. The frontoparietal network acts as a flexible control hub over other networks (32). Impaired frontoparietal interactions with the amygdala and the striatum may interfere with prefrontal cognitive control over emotional and motivational processes in depression. The dorsal attention network, which was also implicated in MDD in our analysis, may underlie negative attentional biases in depression (33–35). Using coordinate-based network mapping, we also found evidence of visual and parietal network involvement in both MDD and LLD studies. Visual and parietal regions are strongly connected to the IFG, dlPFC, and posterior cingulate, which may explain the presence of visual networks in our results. Thus, our coordinate-based network mapping approach provides strong evidence for a common set of executive control and dorsal/visual attention networks implicated in both MDD and LLD, suggesting that LLD follows MDD along the continuum of the lifespan.
Greater Impairment in Late-Onset LLD and Potential Mechanisms
In LLD studies, late-onset LLD showed greater impairment (i.e., higher Z-scores for the network of interest) in frontoparietal, dorsal attention, and visual networks compared with mixed-onset LLD studies despite the relatively low number of LLD studies. No consensus exists on whether early- and late-onset LLD are associated with different symptomatic or cognitive profiles (16, 36–39). However, they may have a different etiology (36, 40–43). Patients with late-onset LLD consistently show higher levels of white matter hyperintensities (16, 44), a standard marker of cerebrovascular disease (45). In light of the vascular depression hypothesis, we speculate that the increased impairment of frontoparietal and dorsal attention circuits in late-onset LLD is driven by vascular burden (15, 19). Cardiovascular damage to prefrontal circuits in late-onset LLD may also help explain the heightened risk for developing dementia in late-onset compared with early-onset LLD (5). On the other hand, stress-related atrophy of medial temporal regions may underlie early-onset LLD (15). We did not detect a medial temporal versus frontostriatal dissociation between early and late-onset LLD, however. This may be due to the connectivity profiles inherent to the coordinate-based network mapping approach. Greater involvement of frontoparietal and dorsal attention networks in our late-onset LLD group is also consistent with greater impairments in executive function reported in studies of late-onset LLD (38, 39). Late-onset LLD studies and LLD studies in general have been limited by small sample sizes. Thus, our findings provide robust evidence supporting greater impairment of executive control circuits and greater disease burden in late-onset LLD.
Potential Effects of Antidepressant Treatment and Remission
In addition, we also found network differences in young MDD patients associated with greater antidepressant treatment and a longer interval since onset of MDD. Antidepressant treatment was associated with more involvement of the rostral ACC, dorsal ACC, and dlPFC. Studies of treatment response have shown that smaller ACC volumes are associated with worse clinical outcomes (46, 47). Similarly, several functional connectivity (48, 49) and activation (50–52) studies suggest a role for the rostral ACC in response to treatment. Higher functional connectivity between the rostral ACC and the dlPFC has been deemed to be critical to depression remission, and it is targeted in clinical trials of repeated transcranial magnetic stimulation (53, 54). Our meta-analysis suggests that structural abnormalities in the dlPFC and ACC-centered networks are more prevalent in patients treated with antidepressants, supporting the clinical importance of the dlPFC and rostral ACC as treatment targets for repeated transcranial magnetic stimulation. By contrast, younger patients who were not taking antidepressants, many of whom were experiencing their first episode of MDD, showed greater structural differences in occipitotemporal networks. Unmedicated patients with MDD have been shown to have gray matter reductions in the amygdala and parahippocampal regions compared with medicated MDD patients (55) and control subjects (56). Consistent with this pattern, we found greater occipitotemporal lobe involvement in studies of unmedicated patients with MDD compared with studies with medicated patients. Since most studies with participants taking antidepressants reported gray matter atrophy in the prefrontal cortex (see section 3.5 in the online supplement), one potential interpretation is that the more notable ACC involvement in medicated studies relates to greater disease burden, given that many participants were still actively depressed despite taking antidepressants. While we controlled for depression severity, individuals with more severe MDD symptoms are more likely to receive prescriptions for medication. Recent work by our group (57) has shown that in patients with LLD, remission in the context of antidepressant treatment is associated with limited or no differences in brain structure compared with control subjects; however, a prospective study is needed to compare those who achieve remission and those whose illness remains treatment resistant, while accounting for antidepressant treatment, to more fully disambiguate whether differences relate to antidepressant use or illness severity.
Limitations
While coordinate-based network mapping increases power and sensitivity for detecting network differences driven by study subgroups, its use in this study did not implicate striatal and hippocampal regions in MDD. Coordinate-based network mapping may introduce biases inherent to connectivity profiles, such as high connectivity of occipital and parietal areas and lower connectivity of the medial temporal regions. As in previous meta-analyses of whole-brain structural differences, we only provide a synthesis of significant findings. To maximize inclusion of study subjects, we did not consider the direction of the case-control differences in brain structure in this meta-analysis. A focus on increases and decreases may provide a more nuanced network mapping for MDD and LLD. Finally, the age cutoff for LLD is somewhat arbitrary and varies across published studies; a reanalysis of the LLD studies with a more stringent cutoff of age 60 did not substantially change our results (see section 2.2 in the online supplement).
In summary, our analysis shows how coordinate-based network mapping helps overcome the heterogeneity inherent to MDD studies and highlights the involvement of frontoparietal and dorsal attention executive control networks in both MDD and LLD. Collectively, our results provide a more comprehensive understanding of brain network abnormalities in depression across the adult lifespan.
1 : Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1789–1858Crossref, Medline, Google Scholar
2 : A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv 2014; 65:977–987Link, Google Scholar
3 : The costs of depression. Psychiatr Clin North Am 2012; 35:1–14Crossref, Medline, Google Scholar
4
5 : Depression and risk of developing dementia. Nat Rev Neurol 2011; 7:323–331Crossref, Medline, Google Scholar
6 : Identifying and treating depression across the life span. Curr Psychiatr 2011; 10:20–24Google Scholar
7 : Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 2014; 14:371Crossref, Medline, Google Scholar
8 : Evidence for structural and functional alterations of frontal-executive and corticolimbic circuits in late-life depression and relationship to mild cognitive impairment and dementia: a systematic review. Front Neurosci 2020; 14:253Crossref, Medline, Google Scholar
9 : Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202:329–335Crossref, Medline, Google Scholar
10 : Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am J Psychiatry 2020; 177:422–434Link, Google Scholar
11 : Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci 2014; 39:397–406Crossref, Medline, Google Scholar
12 : Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord 2012; 140:142–148Crossref, Medline, Google Scholar
13 : Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; 22:900–909Crossref, Medline, Google Scholar
14 : Structural neuroimaging studies in major depressive disorder: meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 2011; 68:675–690Crossref, Medline, Google Scholar
15 : Mechanisms and treatment of late-life depression. Transl Psychiatry 2019; 9:188Crossref, Medline, Google Scholar
16 : Comparison of brain structural variables, neuropsychological factors, and treatment outcome in early-onset versus late-onset late-life depression. Am J Geriatr Psychiatry 2014; 22:1039–1046Crossref, Medline, Google Scholar
17 : Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23:28–38Crossref, Medline, Google Scholar
18 : Brain structural effects of antidepressant treatment in major depression. Curr Neuropharmacol 2015; 13:458–465Crossref, Medline, Google Scholar
19 : Confining the concept of vascular depression to late-onset depression: a meta-analysis of MRI-defined hyperintensity burden in major depressive disorder and bipolar disorder. Front Psychol 2019; 10:1241Crossref, Medline, Google Scholar
20 : Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30:2907–2926Crossref, Medline, Google Scholar
21 : Network localization of heterogeneous neuroimaging findings. Brain 2019; 142:70–79Crossref, Medline, Google Scholar
22 : Lesion network localization of free will. Proc Natl Acad Sci USA 2018; 115:10792–10797Crossref, Medline, Google Scholar
23 : Neuroimaging in Parkinson’s disease dementia: connecting the dots. Brain Commun 2019; 1:fcz006Crossref, Medline, Google Scholar
24 : The frontoparietal control system: a central role in mental health. Neuroscientist 2014; 20:652–664Crossref, Medline, Google Scholar
25 : Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72:603–611Crossref, Medline, Google Scholar
26 : Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. Neuroimage 2020; 206:116287Crossref, Medline, Google Scholar
27 : Altered gray matter volume and resting-state connectivity in individuals with Internet gaming disorder: a voxel-based morphometry and resting-state functional magnetic resonance imaging study. Front Psychiatry 2018; 9:77Crossref, Medline, Google Scholar
28 : Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One 2012; 7:e44758Crossref, Medline, Google Scholar
29 :
30 : Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron 2018; 97:231–247.e7Crossref, Medline, Google Scholar
31 : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106:1125–1165Crossref, Medline, Google Scholar
32 : Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013; 16:1348–1355Crossref, Medline, Google Scholar
33 : Paying attention to attention in depression. Transl Psychiatry 2019; 9:279Crossref, Medline, Google Scholar
34 : Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression. Suicide Life Threat Behav 1993; 23:139–145Crossref, Medline, Google Scholar
35 : A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci Ther 2018; 24:1004–1019Crossref, Medline, Google Scholar
36 : Early and late onset depression in old age: different aetiologies, same phenomenology. J Affect Disord 2001; 66:225–236Crossref, Medline, Google Scholar
37 : Late-life depression: the differences between early- and late-onset illness in a community-based sample. Int J Geriatr Psychiatry 2006; 21:86–93Crossref, Medline, Google Scholar
38 : A longitudinal study of differences in late- and early-onset geriatric depression: depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging Ment Health 2013; 17:1–11Crossref, Medline, Google Scholar
39 : Investigating the relationship between age of onset of depressive disorder and cognitive function. Int J Geriatr Psychiatry 2019; 34:38–46Crossref, Medline, Google Scholar
40 : What are the causes of late-life depression? Psychiatr Clin North Am 2013; 36:497–516Crossref, Medline, Google Scholar
41 : Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry 2005; 162:691–698Link, Google Scholar
42 : Differences between early and late onset adult depression. Clin Pract Epidemiol Ment Health 2011; 7:140–147Crossref, Medline, Google Scholar
43 : Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology 2004; 29:2278–2284Crossref, Medline, Google Scholar
44 : Hippocampal volume and subcortical white matter lesions in late life depression: comparison of early and late onset depression. J Neurol Neurosurg Psychiatry 2007; 78:638–640Crossref, Medline, Google Scholar
45 : Neuroimaging of cerebral small vessel disease and age-related cognitive changes. Front Aging Neurosci 2019; 11:145Crossref, Medline, Google Scholar
46 : Anterior cingulate cortex does not differ between patients with major depression and healthy controls, but relatively large anterior cingulate cortex predicts a good clinical course. Psychiatry Res 2008; 163:76–83Crossref, Medline, Google Scholar
47 : Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry 2009; 24:829–836Crossref, Medline, Google Scholar
48 : Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul 2020; 13:206–214Crossref, Medline, Google Scholar
49 : Functional connectivity of the anterior cingulate cortex in depression and in health. Cereb Cortex 2019; 29:3617–3630Crossref, Medline, Google Scholar
50 : Predicting treatment response in depression: the role of anterior cingulate cortex. Int J Neuropsychopharmacol 2018; 21:988–996Crossref, Medline, Google Scholar
51 : Rostral anterior cingulate activity in major depressive disorder: state or trait marker of responsiveness to medication? J Neuropsychiatry Clin Neurosci 2013; 25:126–133Crossref, Medline, Google Scholar
52 : Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 2001; 158:405–415Link, Google Scholar
53 : Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 2018; 391:1683–1692Crossref, Medline, Google Scholar
54 : Stanford Accelerated Intelligent Neuromodulation Therapy for treatment-resistant depression. Am J Psychiatry 2020; 177:716–726Link, Google Scholar
55 : Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci 2010; 35:337–343Crossref, Medline, Google Scholar
56 : Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 2012; 138:9–18Crossref, Medline, Google Scholar
57 : Frontal-executive and corticolimbic structural brain circuitry in older people with remitted depression, mild cognitive impairment, Alzheimer’s dementia, and normal cognition. Neuropsychopharmacology 2020; 45:1567–1578Crossref, Medline, Google Scholar
58 : A multi-modal parcellation of human cerebral cortex. Nature 2016; 536:171–178Crossref, Medline, Google Scholar



