Lyme Borreliosis and Associations With Mental Disorders and Suicidal Behavior: A Nationwide Danish Cohort Study
Abstract
Objective:
Lyme borreliosis is a tick-borne infectious disease that may confer an increased risk of mental disorders, but previous studies have been hampered by methodological limitations, including small sample sizes. The authors used a nationwide retrospective cohort study design to examine rates of mental disorders following Lyme borreliosis.
Methods:
Using Denmark’s National Patient Register and the Psychiatric Central Research Register, and including all persons living in Denmark from 1994 through 2016 (N=6,945,837), the authors assessed the risk of mental disorders and suicidal behaviors among all individuals diagnosed with Lyme borreliosis in inpatient and outpatient hospital contacts (N=12,156). Incidence rate ratios (IRRs) were calculated by Poisson regression analyses.
Results:
Individuals with Lyme borreliosis had higher rates of any mental disorder (IRR=1.28, 95% CI=1.20, 1.37), of affective disorders (IRR=1.42, 95% CI=1.27, 1.59), of suicide attempts (IRR=2.01, 95% CI=1.58, 2.55), and of death by suicide (IRR=1.75, 95% CI=1.18, 2.58) compared with those without Lyme borreliosis. The 6-month interval after diagnosis was associated with the highest rate of any mental disorder (IRR=1.96, 95% CI=1.53, 2.52), and the first 3 years after diagnosis was associated with the highest rate of suicide (IRR=2.41, 95% CI=1.25, 4.62). Having more than one episode of Lyme borreliosis was associated with increased incidence rate ratios for mental disorders, affective disorders, and suicide attempts, but not for death by suicide.
Conclusions:
Individuals diagnosed with Lyme borreliosis in the hospital setting had an increased risk of mental disorders, affective disorders, suicide attempts, and suicide. Although the absolute population risk is low, clinicians should be aware of potential psychiatric sequelae of this global disease.
Infection and inflammation have been linked to increased risk of mental disorders and suicidal behaviors (1, 2). Lyme borreliosis is one such infectious disease, which has been associated with psychiatric manifestations, including depression, suicidal ideation, obsessive-compulsive disorder, mania, psychosis, and mild to severe cognitive impairment (3–12). These have been reported among individuals with acute untreated infection (5, 6), and months to years after antibiotic therapy (7, 8). Despite the multisystem involvement of Borrelia burgdorferi infection, evidence on the relationship between mental disorders and Lyme borreliosis is inconsistent, and previous studies have been hampered by small sample sizes.
To our knowledge, the largest outpatient psychiatric study assessing persistent mental symptoms attributed to Lyme borreliosis was a retrospective psychiatric office chart review without a control comparison group, which noted that 43.5% of the 253 patients reported suicidal thoughts (12). While some controlled studies have reported higher rates of depression in children and adults after Lyme borreliosis (8–10), other controlled studies did not find elevated rates of depressive symptoms (13–15). One cross-sectional study (16) noted rates of depression among hospitalized individuals with neuroborreliosis similar to rates for Lyme arthritis, and higher rates of depressive symptoms were reported after neuroborreliosis compared with erythema migrans in a follow-up study (mean follow-up, 32 months) after antibiotic treatment (11). In contrast, other follow-up studies of Lyme borreliosis found no difference in depressive symptoms 6 months after erythema migrans (17) or 10–20 years after erythema migrans, facial palsy, or arthritis (13). Supporting an association, a controlled study reported a significantly higher rate of seropositivity for B. burgdorferi antibodies among 499 psychiatric inpatients compared with age- and gender-matched healthy participants (18). Suicidal thoughts have been less frequently studied, with two controlled studies of patients with persistent posttreatment Lyme symptoms reporting elevated rates of suicidal thoughts compared with non–medically ill control subjects (9, 10). Several of these studies were hampered by small sample size, ascertainment bias, lack of an appropriate control group, use of poorly specified or nonstandard criteria for the diagnosis of Lyme borreliosis, reliance on clinical samples, use of unvalidated measures, lack of control for confounding variables, or a cross-sectional design. Given the methodological limitations of previous studies, the relationship between acute or posttreatment Lyme borreliosis and mental disorders and suicide remains uncertain. A recent Danish cohort study using elevated production of Borrelia antibodies in CSF as a proxy diagnosis of neuroborreliosis reported no association with mental diagnoses but found that psychiatric medication prescriptions were increased during the subsequent year (19). While improving on previous research, that study relied on a laboratory proxy rather than a clinical diagnosis of neuroborreliosis and did not include the nonneurologic manifestations of Lyme borreliosis.
We conducted a nationwide, population-based cohort study comparing the rate of new-onset mental disorders among persons diagnosed with Lyme borreliosis through a hospital contact (inpatient, emergency department, or outpatient) to those with no such diagnosis, a design that bypasses many of the limitations of previous studies. Our secondary aims were to examine whether individuals who had hospital contacts with a recorded diagnosis of Lyme borreliosis had higher rates of subsequent affective disorders, suicide attempts, and death by suicide than individuals without a diagnosis of Lyme borreliosis.
Methods
The study was approved by the Danish Data Protection Agency (journal number RHP-2012–021). An anonymized data set was used for research purposes, and according to Danish legislation, informed consent from participants was not required.
Study Population
All Danish residents age 3 or older living in Denmark from 1994 through 2016 were included in our analyses. Linkage of data between various national registries was possible, as each Danish resident has a unique personal identification number. We accessed data from the Danish Civil Registration System (20), the Database for Integrated Labor Market Research (21), the National Patient Register (22), the Psychiatric Central Research Register (23), and the Register of Causes of Death (24). All registers are based on continuously updated administrative data on all residents living in Denmark. Diagnoses in the National Patient Register (22) and the Psychiatric Central Research Register (23) were recorded according to ICD-10’s diagnostic system.
Measures
Exposure variables
Lyme borreliosis was identified as recorded in the National Patient Register since 1994 using ICD-10 (see Table S1 in the online supplement); this includes all hospital contacts—inpatient, outpatient, and emergency department visits. A description of the clinical assessment, laboratory tests, and treatment of Lyme borreliosis in Denmark is available online (25). Inpatient diagnoses of Lyme borreliosis have been recorded in the register since 1994, and outpatient and emergency department diagnoses have been available since 1995. From the date of a first diagnosis, the individual was considered exposed. To assess whether neuroborreliosis was more likely to be associated with psychiatric disorders than nonneuroborreliosis, Lyme borreliosis was grouped into three mutually exclusive categories: Lyme general and other nonneurologic; other neurologic Lyme manifestations; and Lyme meningitis. The number of registered medical contacts and of new episodes of Lyme borreliosis served as indicators of severity. A medical contact was defined as a hospital-based visit with a diagnosis of Lyme borreliosis, regardless of whether it was for a new episode or a prior episode. A new episode was operationally defined as at least a 2-month interval without hospital-based contacts for Lyme borreliosis.
Outcome variables
The primary outcome variable was any mental disorder, which was defined by an individual being newly registered in the Psychiatric Central Research Register since 1994 with a mental disorder diagnosis (ICD-10 codes F00–F99). The secondary outcome variables were affective disorders, defined by ICD-10 codes F30–F39; suicide attempt, as determined by registration in the National Patient Register and the Psychiatric Central Research Register (ICD-10 codes X60–X84) or where the reason for contact was indicated to be suicide attempt; and suicide, as determined from the Cause of Death Register (24) (ICD-10 codes X60–X84, Y87.0).
Follow-Up
Participants were included from January 1, 1994. Individuals who reached age 3 or who migrated into the country were included as of the dates of those events. The follow-up ended on the date of examined outcome, death, emigration from Denmark, or December 31, 2016, whichever came first.
Statistical Analysis
The associations between Lyme borreliosis and the examined outcomes were estimated in SAS, version 9.4, using adjusted Poisson regression models to obtain incidence rate ratios and population attributable risks with 95% confidence intervals. Statistical significance was determined by a p value below 0.05 using two-sided tests. The association with Lyme borreliosis was explored further in a range of adjusted models: number of new episodes; overall number of contacts; contact setting for Lyme borreliosis, ordered according to highest level of seriousness (inpatient, emergency department, outpatient); time since first diagnosis; and age at first diagnosis.
We excluded all individuals who had been recorded as having either a mental disorder or a suicide attempt prior to 1994. From 1994 onward, individuals with a first episode of Lyme borreliosis were excluded from the Lyme borreliosis group if a mental disorder or suicide attempt occurred prior to the Lyme borreliosis diagnosis. First, we estimated the rate of having a mental disorder among individuals diagnosed with Lyme borreliosis relative to those without this diagnosis. Basic models were adjusted for calendar period, sex, and age in 5-year intervals. Fully adjusted models were further adjusted for marital status, educational level, socioeconomic status, and chronic medical comorbidity (based on Charlson comorbidity index [26] scores of 0, 1, or ≥2). In addition, we adjusted for pre–Lyme borreliosis mental disorders occurring after 1994 in the analyses of suicide attempts and suicides. Because the number of Lyme-exposed individuals who died by suicide was relatively small (N=25), we restricted these analyses to basic adjusted models.
To validate the association found between Lyme borreliosis and the primary outcome of mental disorders, we carried out additional sensitivity analyses. First, we examined the association with mental disorders in a subcohort of individuals who had full register follow-up from birth (born after 1993); this was conducted because the registry prior to 1994 did not include Lyme borreliosis as a diagnosis. Second, we tested the association in a subcohort excluding all individuals with a Charlson comorbidity index score above zero to rule out undiagnosed mental disorders associated with other somatic illnesses. Third, we tested whether different results appeared if individuals with a history of mental disorders and/or a suicide attempt before 1994 were included in analyses, as they were excluded in our primary analyses. Fourth, because tools for the diagnosis of Lyme borreliosis modestly improved over the course of the 22-year study period, we tested whether the association with mental disorders was higher in those diagnosed with Lyme borreliosis from 2005 onward as opposed to before 2005. Fifth, we tested whether rates of mental disorders differed if a Lyme borreliosis diagnosis was given before the age of 40 as compared with later. Sixth, we tested whether incidence rate ratios were elevated after Lyme borreliosis compared with the non-Lyme population in age-stratified younger and older cohorts. Seventh, to examine whether a 2-month interval between hospital contacts for Lyme borreliosis was too short a period to define a new episode, we also calculated the incidence rate ratio for mental disorders using a longer duration (6 months) between hospital contacts for Lyme borreliosis. Finally, to address specificity, we calculated the incidence rate ratios for fractures not involving the skull or spine after Lyme diagnoses.
Results
Among the 6,945,837 individuals (3,471,769 of them male) who were observed for a total of 111,952,876 person-years between 1994 and 2016, 12,616 individuals (6,528 of them male) received a hospital-based diagnosis of Lyme borreliosis (see Table S1 in the online supplement), leaving 6,933,221 individuals in the comparison group. The national cohort spanned ages 3 years to 104 years. The mean age at first hospital-based diagnosis of Lyme borreliosis was 41 years (SD=23) and the median age was 44 years (interquartile range=20–59). Among those diagnosed (see Table S1), 11,256 (90%) had general Lyme borreliosis and other nonneurologic manifestations, while 360 (3%) had Lyme meningitis and 1,000 (7%) had other neuroborreliosis manifestations. Among those with Lyme borreliosis, 831 were subsequently registered with a mental disorder (see characteristics in Table S2 in the online supplement), 324 with an affective disorder, 67 with suicide attempt, and 25 with suicide.
Any Mental Disorder
The incidence rate of a new mental disorder among individuals with a Lyme borreliosis diagnosis was 733 per 100,000 person-years (95% CI=683, 782), compared with 567 (95% CI=565, 568) among individuals with no such diagnosis. The fully adjusted model showed that the rate of mental disorder diagnoses was 28% higher (incidence rate ratio [IRR]=1.28, 95% CI=1.20, 1.37) among individuals with Lyme borreliosis compared with those with no such diagnosis (Figure 1). A dose-response relationship was noted with respect to number of episodes (one episode: IRR=1.24, 95% CI=1.15, 1.33; two or more episodes: IRR=1.79, 95% CI=1.44, 2.22) (Table 1 and Figure 2). Compared with individuals with no history of Lyme borreliosis, the first 6 months after the first diagnosis was associated with a 96% higher rate of mental disorder diagnoses (IRR=1.96, 95% CI=1.53, 2.52), which fell gradually over time; however, even 5 years after first diagnosis, the IRR remained elevated (IRR=1.19; CI=1.09, 1.31; p<0.0001). Elevated rates were noted for those whose first Lyme borreliosis diagnosis was before age 40 and were highest in the age range of 20–29 years (IRR=1.72; CI=1.40, 2.12). No significant differences between rates of mental disorders were noted when we assessed for severity of Lyme borreliosis by the clinical setting where the initial diagnosis was made.
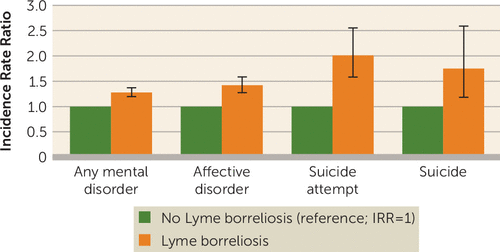
a Estimates for any mental disorder, affective disorder, and suicide attempt were adjusted for calendar period, sex, age group, civil status, educational level, socioeconomic status, and chronic medical comorbidity. The model examining the outcome of suicide attempt was further adjusted for pre-Lyme mental disorders. Because there were few observed events, the estimate for suicide was adjusted for calendar period, sex, and age group. Error bars indicate 95% confidence interval. IRR=incidence rate ratio.
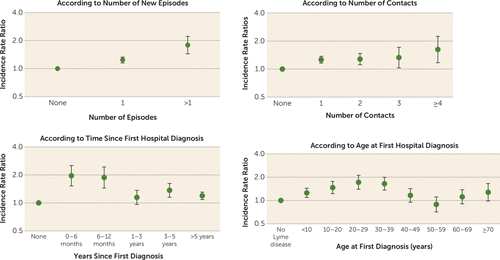
a Estimates for any mental disorder were adjusted for calendar period, sex, age group, civil status, educational level, socioeconomic status, and chronic medical comorbidity. Error bars indicate 95% confidence interval.
| Basic Adjusted Modela | Fully Adjusted Modela | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases With Diagnosis | Incidence Rate per 100,000 Person-Years | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Number of episodes (defined as more than 2 months between hospital contacts) | |||||||
| No Lyme | 633,973 | 567 | 565, 568 | 1 | 1 | ||
| 1 | 748 | 712 | 661, 763 | 1.16 | 1.08, 1.24 | 1.24 | 1.15, 1.33 |
| >1 | 83 | 989 | 776, 1,202 | 1.63 | 1.32, 2.03 | 1.79 | 1.44, 2.22 |
| Number of contacts for hospital services | |||||||
| No Lyme | 633,973 | 567 | 565, 568 | 1 | 1 | ||
| 1 | 534 | 719 | 658, 780 | 1.17 | 1.07, 1.27 | 1.26 | 1.16, 1.37 |
| 2 | 202 | 742 | 639, 844 | 1.21 | 1.05, 1.39 | 1.28 | 1.11, 1.47 |
| 3 | 59 | 749 | 558, 940 | 1.22 | 0.95, 1.58 | 1.33 | 1.03, 1.71 |
| ≥4 | 36 | 888 | 598, 1,179 | 1.48 | 1.06, 2.05 | 1.62 | 1.17, 2.25 |
| Time since first diagnosis | |||||||
| No Lyme | 633,973 | 567 | 565, 568 | 1 | 1 | ||
| <1 year | 61 | 1,053 | 789, 1,318 | 1.82 | 1.42, 2.34 | 1.96 | 1.53, 2.52 |
| 1–2 years | 56 | 1,010 | 746, 1,275 | 1.74 | 1.34, 2.26 | 1.88 | 1.45, 2.44 |
| 2–3 years | 125 | 618 | 510, 727 | 1.06 | 0.89, 1.27 | 1.15 | 0.96, 1.37 |
| 3–4 years | 131 | 753 | 624, 882 | 1.27 | 1.07, 1.51 | 1.37 | 1.15, 1.62 |
| >4 years | 458 | 710 | 645, 775 | 1.12 | 1.02, 1.22 | 1.19 | 1.09, 1.31 |
| Age at first diagnosis | |||||||
| No Lyme | 633,973 | 567 | 565, 568 | 1 | 1 | ||
| <10 years | 202 | 903 | 778, 1,027 | 1.35 | 1.17, 1.55 | 1.25 | 1.09, 1.44 |
| 10–19 years | 117 | 1,254 | 1,027, 1,481 | 1.92 | 1.60, 2.30 | 1.47 | 1.22, 1.76 |
| 20–29 years | 89 | 1,063 | 842, 1,283 | 1.7 | 1.38, 2.10 | 1.72 | 1.40, 2.12 |
| 30–39 years | 106 | 754 | 611, 898 | 1.22 | 1.01, 1.48 | 1.65 | 1.36, 1.99 |
| 40–49 years | 94 | 496 | 396, 597 | 0.83 | 0.68, 1.02 | 1.16 | 0.95, 1.42 |
| 50–59 years | 78 | 394 | 306, 481 | 0.67 | 0.53, 0.83 | 0.89 | 0.71, 1.11 |
| 60–69 years | 88 | 630 | 498, 762 | 1.09 | 0.89, 1.35 | 1.11 | 0.90, 1.37 |
| ≥70 years | 57 | 869 | 644, 1,095 | 1.57 | 1.21, 2.04 | 1.28 | 0.98, 1.66 |
| Setting type | |||||||
| No Lyme | 633,973 | 567 | 565, 568 | 1 | 1.0 | ||
| Inpatient | 531 | 737 | 675, 800 | 1.2 | 1.10, 1.31 | 1.3 | 1.15, 1.37 |
| Emergency department | 103 | 721 | 582, 860 | 1.18 | 0.98, 1.44 | 1.4 | 1.11, 1.64 |
| Outpatient | 197 | 726 | 624, 827 | 1.17 | 1.02, 1.34 | 1.3 | 1.14, 1.50 |
TABLE 1. Association between Lyme borreliosis and mental disorders in Denmark (1994–2016), basic and fully adjusted incidence rate ratios
Affective Disorders
A 42% higher rate of affective disorders (IRR=1.42, 95% CI=1.27, 1.59) was noted among individuals with Lyme borreliosis compared with individuals with no such history (Figure 1). The rate of affective disorders by number of episodes of Lyme borreliosis was 1.37 (95% CI=1.22, 1.54) after one episode and 2.07 (95% CI=1.49, 2.87) after two or more episodes (Table 2). The highest rate of affective disorders was noted 6–12 months after diagnosis of Lyme borreliosis (IRR=2.59, 95% CI=1.77, 3.81), yet the rate remained elevated 5 or more years after first diagnosis (IRR=1.34, 95% CI=1.16, 1.55). The highest rate of affective disorders among individuals with Lyme borreliosis occurred in the age range of 10–19 years (IRR=2.15, 95% CI=1.59, 2.90).
| Basic Adjusted Modela | Fully Adjusted Modela | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases With Affective Diagnosis | Incidence Rate per 100,000 Person-Years | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Number of episodes (more than 2 months between) | |||||||
| No Lyme | 218,358 | 189 | 189, 190 | 1 | 1 | ||
| 1 | 288 | 261 | 231, 292 | 1.19 | 1.06, 1.34 | 1.37 | 1.22, 1.54 |
| >1 | 36 | 407 | 274, 541 | 1.85 | 1.33, 2.56 | 2.07 | 1.49, 2.87 |
| Number of contacts for hospital services | |||||||
| No Lyme | 218,358 | 189 | 189, 190 | 1 | 1 | ||
| 1 | 210 | 269 | 233, 306 | 1.21 | 1.06, 1.39 | 1.37 | 1.20, 1.57 |
| 2 | 77 | 270 | 209, 330 | 1.26 | 1.00, 1.57 | 1.47 | 1.17, 1.84 |
| 3 | 22 | 268 | 156, 380 | 1.23 | 0.81, 1.86 | 1.5 | 0.99, 2.28 |
| ≥4 | 15 | 354 | 175, 534 | 1.65 | 0.99, 2.73 | 1.85 | 1.12, 3.07 |
| Time since first diagnosis | |||||||
| No Lyme | 218,358 | 189 | 189, 190 | 1 | 1 | ||
| <1 year | 16 | 267 | 136, 398 | 1.36 | 0.84, 2.23 | 1.53 | 0.94, 2.50 |
| 1–2 years | 26 | 453 | 279, 627 | 2.3 | 1.56, 3.38 | 2.59 | 1.77, 3.81 |
| 2–3 years | 47 | 224 | 160, 288 | 1.13 | 0.85, 1.50 | 1.29 | 0.97, 1.72 |
| 3–4 years | 49 | 270 | 195, 346 | 1.32 | 1.00, 1.75 | 1.51 | 1.14, 2.00 |
| >4 years | 186 | 273 | 234, 312 | 1.16 | 1.01, 1.34 | 1.34 | 1.16, 1.55 |
| Age at first diagnosis | |||||||
| No Lyme | 218,358 | 189 | 189, 190 | 1 | 1 | ||
| <10 years | 56 | 237 | 175, 299 | 1.18 | 0.91, 1.53 | 1.84 | 1.42, 2.40 |
| 10–19 years | 42 | 419 | 292, 546 | 2.04 | 1.50, 2.75 | 2.15 | 1.59, 2.90 |
| 20–29 years | 37 | 400 | 271, 529 | 1.94 | 1.40, 2.67 | 1.55 | 1.13, 2.15 |
| 30–39 years | 54 | 363 | 266, 460 | 1.67 | 1.28, 2.18 | 1.73 | 1.32, 2.25 |
| 40–49 years | 46 | 233 | 166, 300 | 1.06 | 0.79, 1.41 | 1.21 | 0.91, 1.62 |
| 50–59 years | 33 | 161 | 106, 216 | 0.68 | 0.49, 0.96 | 0.81 | 0.58, 1.14 |
| 60–69 years | 42 | 293 | 205, 382 | 1.23 | 0.91, 1.67 | 1.41 | 1.04, 1.91 |
| ≥70 years | 14 | 209 | 99, 318 | 0.87 | 0.51, 1.46 | 0.97 | 0.58, 1.64 |
| Setting type | |||||||
| No Lyme | 218,358 | 189 | 189, 190 | 1 | 1 | ||
| Inpatient | 209 | 277 | 239, 314 | 1.29 | 1.12, 1.47 | 1.5 | 1.31, 1.72 |
| Emergency department | 47 | 313 | 223, 402 | 1.43 | 1.07, 1.90 | 1.71 | 1.28, 2.27 |
| Outpatient | 68 | 239 | 182, 296 | 1.03 | 0.81, 1.30 | 1.11 | 0.88, 1.41 |
TABLE 2. Association between Lyme borreliosis and affective disorders in Denmark (1994–2016), basic and fully adjusted incidence rate ratios
Suicide Attempt
A higher rate of suicide attempt was found among individuals diagnosed with Lyme borreliosis (IRR=2.01, 95% CI=1.58, 2.55) than among those with no such diagnosis (Figure 1). Regarding temporal effects, only the period 3 years after Lyme borreliosis diagnosis was associated with an increased rate of suicide attempt (IRR=2.45, 95% CI=1.87, 3.22) (Table 3). With respect to age, those diagnosed with Lyme borreliosis before age 10 had the highest IRR for later suicide attempt, at 4.01 (95% CI=2.58, 6.21), while those older than 30 had an IRR of 2.85 (95% CI=1.77, 4.59).
| Basic Adjusted Modela | Fully Adjusted Modela | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases With Suicidal Behavior | Incidence Rate per 100,000 Person-Years | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Suicide attempt | |||||||
| Number of episodes (more than 2 months between) | |||||||
| No Lyme | 58,144 | 50 | 50, 50 | 1 | 1 | 1 | |
| 1 | 61 | 54 | 41, 68 | 1.18 | 0.91, 1.51 | 1.96 | 1.52, 2.52 |
| >1 | 6 | 66 | 13, 119 | 1.56 | 0.70, 3.47 | 2.71 | 1.22, 6.03 |
| Number of contacts to hospital services | |||||||
| No Lyme | 58,144 | 50 | 50, 50 | 1 | 1 | 1 | |
| 1 | 46 | 58 | 41, 75 | 1.26 | 0.95, 1.69 | 2.03 | 1.52, 2.71 |
| >1 | 21 | 50 | 29, 72 | 1.09 | 0.71, 1.67 | 1.97 | 1.28, 3.02 |
| Time since first diagnosis | |||||||
| No Lyme | 58,144 | 50 | 50, 50 | 1 | 1 | 1 | |
| <3 years | 15 | 45 | 22, 68 | 0.93 | 0.56, 1.54 | 1.23 | 0.74, 2.04 |
| ≥3 years | 52 | 59 | 43, 75 | 1.31 | 1.00, 1.72 | 2.45 | 1.87, 3.22 |
| Age at first diagnosis | |||||||
| No Lyme | 58,144 | 50 | 50, 50 | 1 | 1 | 1 | |
| <10 years | 17 | 71 | 37, 105 | 3.08 | 1.98, 4.77 | 4.01 | 2.58, 6.21 |
| 10–30 years | 27 | 137 | 86, 189 | 0.94 | 0.66, 1.34 | 1.34 | 0.94, 1.91 |
| >30 years | 23 | 30 | 17, 42 | 0.98 | 0.61, 1.58 | 2.85 | 1.77, 4.59 |
| Setting type | |||||||
| No Lyme | 58,144 | 50 | 50, 50 | 1 | 1 | 1 | |
| Inpatient | 43 | 56 | 39, 73 | 1.18 | 0.88, 1.60 | 2.02 | 1.50, 2.72 |
| Emergency department | 12 | 78 | 34, 123 | 1.76 | 1.00, 3.10 | 3.02 | 1.72, 5.32 |
| Outpatient | 12 | 41 | 18, 65 | 0.95 | 0.54, 1.68 | 1.48 | 0.84, 2.61 |
| Suicideb | |||||||
| Number of episodes (more than 2 months between) | |||||||
| No Lyme | 12,644 | 11 | 11, 11 | 1 | |||
| 1 | 22 | 20 | 11, 28 | 1.68 | 1.10, 2.55 | ||
| >1 | 3 | 33 | −4, 70 | 2.52 | 0.81, 7.82 | ||
| Number of contacts for hospital services | |||||||
| No Lyme | 12,644 | 11 | 11, 11 | 1 | |||
| 1 | 16 | 20 | 10, 30 | 1.73 | 1.06, 2.83 | ||
| >1 | 9 | 21 | 7, 35 | 1.77 | 0.92, 3.40 | ||
| Age at first diagnosis | |||||||
| No Lyme | 12,644 | 11 | 11, 11 | 1 | |||
| <50 years | 11 | 14 | 6, 22 | 1.71 | 0.95, 3.10 | ||
| ≥50 years | 14 | 33 | 16, 50 | 1.77 | 1.05, 2.99 | ||
| Time since first diagnosis | |||||||
| No Lyme | 12,644 | 11 | 11, 11 | 1 | |||
| <3 years | 9 | 27 | 9, 45 | 2.41 | 1.25, 4.62 | ||
| ≥3 years | 16 | 18 | 9, 27 | 1.51 | 0.93, 2.47 | ||
| Setting type | |||||||
| No Lyme | 12,644 | 11 | 11, 11 | 1 | |||
| Inpatient | 17 | 22 | 12, 32 | 1.87 | 1.16, 3.01 | ||
| Emergency department | 3 | 19 | −3, 42 | 1.64 | 0.53, 5.09 | ||
| Outpatient | 5 | 17 | 2, 32 | 1.47 | 0.61, 3.53 | ||
TABLE 3. Association between Lyme borreliosis and suicide attempt and suicide in Denmark (1994–2016), basic and fully adjusted incidence rate ratios
Death by Suicide
When we adjusted for period, sex, and age group, individuals diagnosed with Lyme borreliosis were found to have a 75% higher rate of suicide (IRR=1.75, 95% CI=1.18, 2.58) compared with those with no such diagnosis. A higher IRR was also noticed within 3 years of first diagnosis (IRR=2.41, 95% CI=1.25, 4.62) (Table 3).
Population Attributable Risks
Population attributable risks associated with mental disorders, affective disorders, suicide attempt, and suicide were low: 0.03% (95% CI=0.02, 0.04), 0.04% (95% CI=0.03, 0.06), 0.11% (95% CI=0.06, 0.16), and 0.08% (95% CI=0.02, 0.16), respectively.
Analysis by Neurologic Classification
Compared with the population not diagnosed with Lyme borreliosis, those classified specifically with neuroborreliosis did not have a significantly increased rate of mental disorders, affective disorders, or death by suicide; however, the rate of suicide attempt was increased (IRR=2.40, 95% CI=1.00, 5.77, N=5) (see Table S3 in the online supplement). When we combined the two neurologic classifications (meningitis and other neurologic manifestations), there was no significant difference in rates of mental disorders compared with the population not diagnosed with Lyme borreliosis (IRR=1.17, 95% CI=0.91, 1.52), or in rates of affective disorders (IRR=1.29, 95% CI=0.82, 2.03). Also, there was no significant difference in the rates of mental disorders between those with Lyme meningitis and those with other neurologic manifestations (IRR=0.99, 95% CI=0.59, 1.66, p=0.980).
Sensitivity Analysis
Compared with our primary analysis, similar rate ratios of mental disorders were found 1) when we restricted the sample to individuals with complete follow-up data since birth (IRR=1.32, 95% CI=1.12, 1.66); 2) when we restricted the sample to individuals without other somatic comorbidities as measured by a Charlson comorbidity index score of zero (IRR=1.29, 95% CI=1.20, 1.39); and 3) when we did not exclude those with a history of mental disorders and/or suicide attempt before 1994 (IRR=1.26, 95% CI=1.18, 1.35) (see Table S4 in the online supplement). Further, those diagnosed with Lyme borreliosis later than 2004 did not have a significantly different rate of mental disorders compared with those diagnosed before 2005 (p=0.186).
We further examined the impact of age. Individuals diagnosed with Lyme borreliosis before age 40 had a higher rate of mental disorders (IRR=1.33, 95% CI=1.15, 1.53, p<0.0001) compared with those age 40 or older. In age-stratified analyses, compared with individuals without a Lyme borreliosis diagnosis, the IRR for mental disorders for individuals with Lyme borreliosis younger than age 40 was 1.36 (95% CI=1.24, 1.49), whereas it was 1.27 (95% CI=1.13, 1.41) for individuals age 40 or older.
When we redefined a new episode to require at least a 6-month interval between clinical contacts for Lyme borreliosis, the IRR for mental disorders was 1.25 (95% CI=1.17, 1.34) for one episode and 2.05 (95% CI=1.55, 2.72) for two or more episodes. There were no statistically significant interactions between sex and Lyme borreliosis for any of the four outcomes.
Finally, compared with those without Lyme borreliosis, those with Lyme borreliosis had a small increased rate of fractures not involving the skull or spine after a hospital-based diagnosis of Lyme borreliosis (IRR=1.14, 95% CI=1.09, 1.20) (see Table S5 in the online supplement).
Discussion
Our study represents the first nationwide epidemiologic investigation of mental disorders and suicidal behaviors after Lyme borreliosis. The results reveal that rates of any mental disorder, affective disorder, suicide attempt, and suicide are each higher after the diagnosis of Lyme borreliosis compared with no history of Lyme borreliosis. As the number of episodes of Lyme borreliosis increased, the rates of mental disorders, affective disorders, and suicidal behaviors increased. Temporal proximity to diagnosis also increased the rates of mental disorders, affective disorders, and suicide. Rates of mental disorders remained significantly higher after adjustment for important confounding variables, including age, sex, calendar period, marital status, socioeconomic status, and medical comorbidity. These results support the early clinical reviews suggesting that Lyme borreliosis is associated with psychiatric manifestations (3, 4). The population attributable risks demonstrate that although mental disorders and suicidal behaviors may be increased after Lyme borreliosis, hospital-based cases of Lyme borreliosis are not a major contributor to the overall frequency of mental disorders or suicide in the general population.
A 42% higher rate of affective disorders and a 75% higher rate of suicide (in basic adjusted analyses) among people with Lyme borreliosis are of concern. Notably, the rate for affective disorders was highest during the first year after diagnosis and highest for completed suicide during the first 3 years after diagnosis. Although the IRR for suicide was elevated, our study also clarifies that, in Denmark, the absolute rate for suicide after Lyme borreliosis was low, accounting for 25 fatalities over a 22-year period, representing less than 0.2% of all suicides. Our findings are consistent with findings from previous studies, which demonstrated an increased rate of depression and suicidality after infection in general (1, 2) and after spirochetal and B. burgdorferi infections in particular (8–12, 27). The absence of an association with neuroborreliosis is also consistent with previous studies (13–15, 19). However, a recent study raised the question of whether mental disorders may emerge in the first year after a laboratory diagnosis of neuroborreliosis, as psychiatric prescriptions were elevated during that period, presumably for pain and/or psychiatric indications (19).
Infection with B. burgdorferi can occur at any time in the life span; however the incidence peaks in childhood, with a second peak in later middle age (28, 29). Similarly, the peak incidence of many major mental disorders occurs earlier in life, with nearly three-fourths starting by age 24 (30). Compared with individuals without Lyme borreliosis, we found the highest incidence rate ratios for mental disorders after Lyme borreliosis during each of the first four decades of life (<10, 10–19, 20–29, 30–39 years). Compared with those age 40 or older, our younger cohort had an approximately 33% higher rate of mental disorders after diagnosis of Lyme borreliosis. Our age-stratified analyses furthermore demonstrated increased incidence rate ratios in both the younger and older Lyme cohorts when compared with the non-Lyme population. This suggests that the mechanisms by which Borrelia infection may influence mental disorders are not limited to specific factors associated with childhood.
Our study demonstrated an increased rate of mental disorders after hospital-based diagnosis of Lyme borreliosis. The strength of this association further increased with number of episodes and temporal proximity to the original diagnosis. Still, our findings should not be considered specific to Lyme borreliosis infection, as hospital-based diagnoses of infections have previously been reported to be associated with an increased risk of mental disorders and suicidal behaviors (2, 31). To address specificity, we additionally examined the rate of fractures not involving the skull or spine (a non–mental health outcome) after exposure to Lyme borreliosis. Compared with the incidence rate ratios for such fractures after Lyme borreliosis, the incidence rate ratios were two-, three-, and sevenfold higher for mental disorders, affective disorders, and suicide attempts, respectively, after Lyme borreliosis, suggesting a higher risk of mental disorders than the examined proxy of a non-mental disorder. The 14% increased rate of fractures after hospital-based diagnosis of Lyme borreliosis might not be surprising if individuals receive prescriptions for psychiatric medications after hospitalization, as reported recently for neuroborreliosis (19), given that use of psychotropic medications is associated with an increase in risk of fractures (32).
Although our study design cannot establish causality, plausible mechanisms may link Lyme borreliosis to mental disorders and suicide. Infections and autoimmune diseases have been shown to increase the risk of subsequent mental disorders (1, 2). Inflammation, which has been associated with psychiatric disorders and suicidal behaviors (33, 34), is a natural component of the host response to Borrelia infection (29). Ongoing immune activation may result from either persistent infection or postinfectious processes. Elevated biomarkers of inflammation and immune activation have been demonstrated in posttreatment Lyme borreliosis, including IL-23 (35), CCL19 (36), and CRP (37). Microglial activation—a marker of central inflammatory processes—was demonstrated in a positron emission tomography brain imaging study of posttreatment Lyme borreliosis (38). Autoimmunity may play a role as well, as B. burgdorferi–triggered cross-reactive antibodies can target peripheral and central neural tissue (39, 40), and repeated infection may trigger a more robust antineuronal antibody and neuronal activation response (41). Dysbiosis, due either to the disease itself or to the impact of antibiotics, may play a role in infection-related mood disorders (42) and in posttreatment Lyme borreliosis (43). Psychological factors may also contribute to mood disorders and suicide, possibly due to having a chronic painful or debilitating illness associated with uncertainty, economic stressors, and disability.
We hypothesized that patients with neuroborreliosis might have higher rates of mental disorders than the “general Lyme and other nonneurologic” group, largely because CNS infection with B. burgdorferi may lead to encephalitis, which can have psychiatric manifestations (5, 6). However, higher rates of mental disorders were not found in the neuroborreliosis group. Possible explanations include the following. First, if clinicians used the general code for Lyme borreliosis when diagnosing neurologic manifestations instead of the more specific code for neuroborreliosis, our ability to test this hypothesis with a registry-based data set would have been impaired. Nonspecific codes would also be used for individuals with Lyme borreliosis who have neuropsychiatric or other atypical manifestations of neuroborreliosis (44), as clinicians may not recommend a lumbar puncture. Third, a diagnosis of neuroborreliosis may lead clinicians not to record an evolving psychiatric diagnosis, as the neurologic diagnosis would be considered primary. Finally, the “general Lyme and other nonneurologic” group may experience increased mental distress due to other factors, such as the chronic pain that accompanies Lyme arthritis and posttreatment Lyme disease syndrome (45).
It is important to emphasize that the vast majority of mental disorders are unrelated to infection with B. burgdorferi. Nevertheless, in the case of, for example, an individual from a Lyme-endemic area with a new-onset affective disorder that is accompanied by a recent history of multisystem nonspecific signs and symptoms, such as an expanding rash or a viral-like syndrome followed by severe fatigue, asymmetric or migrating arthritis, myalgias, neuropathies, and/or cognitive problems, the medical history should be expanded to include Lyme borreliosis as one of the potential general medical conditions to rule out (45–47).
Borrelia genospecies and strains can differ by geographic region. Our report focuses on Lyme borreliosis in Denmark, where the predominant genospecies of Borrelia sensu lato consist of Borrelia afzelii and Borrelia garinii, similar to the rest of Europe. Our findings may therefore be generalizable to Europe but not to the United States, where B. burgdorferi sensu stricto causes virtually all cases of Lyme borreliosis. Because B. burgdorferi sensu stricto elicits a more robust host inflammatory response than the other genospecies (48), rates of mental disorders and suicidal behavior after Lyme borreliosis could potentially be higher in the United States than those reported for Denmark.
Strengths and Limitations
The strengths of this study are several. First, our population-based cohort study was large, and the data were collected prospectively, covering 22 years of follow-up with little or no loss to follow-up. Second, the data in the analyses were adjusted for time-varying important and well-known risks for mental disorders and suicide. Third, the inclusion of various temporal and severity covariates adds strength to the examined associations. Fourth, because diagnoses of mental disorders are not recorded in the Danish National Patient Register, our study made use of the Psychiatric Central Research Register, thereby relying on a registered mental disorder diagnosis rather than inference based on other methods such as use of prescription medications.
First among the study’s limitations is that because our sample identification relied on the National Danish Registry, which only records diagnoses made in the hospital setting, our results may not be generalizable to less severe cases of Lyme borreliosis that do not require hospital contact, as their care is provided in the community setting by general practitioners. This limitation also applies to mental disorders, as less severe mental disorders may be managed entirely in the community setting. Second, because our study design entailed counting mental disorders that emerged only after Lyme borreliosis, cases for which a mental disorder was a prodromal manifestation of borrelia infection would have been missed. Third, we cannot know for certain whether a clinical contact after 2 months marked a new episode or was related to persistent or recurrent symptoms from prior infection; it seems likely, however, that this marked a new episode, given the similar findings in the sensitivity analysis where we redefined a new episode as at least 6 months between hospital contacts. Fourth, given the predominance of nonspecific coding, it is not possible to assess whether rates differed by system involvement. Fifth, we were not able to address the question of whether delay in detection and treatment played a mediating role in the rate of subsequent mental disorders or suicidality. Sixth, because until 1994 the Danish Registries used ICD-8, which did not have a code for Lyme borreliosis, our study design excluded all individuals who had a history of mental disorders prior to 1994, as before that year, we were unable to determine associations between Lyme borreliosis and subsequent mental disorders. Sensitivity analysis that did not exclude individuals with mental disorders prior to 1994 revealed incidence rate ratios for mental disorders after Lyme borreliosis nearly identical to those in the original, more restrictive study sample; this was also the case for the sensitivity analysis, which included only the younger cohort of those born between 1994 and 2016.
Conclusions
In this nationwide registry-based retrospective cohort study, individuals with hospital contacts for Lyme borreliosis, compared with the general population without Lyme borreliosis, had increased rates of mental disorders, affective disorders, suicide attempts, and suicide. Although the absolute population risk is low, clinicians should be aware of potential psychiatric sequelae of this global disease.
1. : Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study . JAMA Psychiatry 2013 ; 70 : 812 – 820 Crossref, Medline, Google Scholar
2. : A nationwide cohort study of the association between hospitalization with infection and risk of death by suicide . JAMA Psychiatry 2016 ; 73 : 912 – 919 Crossref, Medline, Google Scholar
3. : [Lyme borreliosis in neurology and psychiatry] . Fortschr Med 1990 ; 108 : 191 – 193, 197 (German) Medline, Google Scholar
4. : Lyme disease: a neuropsychiatric illness . Am J Psychiatry 1994 ; 151 : 1571 – 1583 Link, Google Scholar
5. : Borrelia burgdorferi central nervous system infection presenting as an organic schizophrenialike disorder . Biol Psychiatry 1999 ; 45 : 795 Crossref, Medline, Google Scholar
6. : A woman in her 50s with manic psychosis . Tidsskr Nor Laegeforen 2012 ; 132 : 537 – 539 Crossref, Medline, Google Scholar
7. : Borrelia burgdorferi in the nervous system: the new “great imitator” . Ann N Y Acad Sci 1988 ; 539 : 56 – 64 Crossref, Medline, Google Scholar
8. : Role of psychiatric comorbidity in chronic Lyme disease . Arthritis Rheum 2008 ; 59 : 1742 – 1749 Crossref, Medline, Google Scholar
9. : Depressive symptoms and suicidal ideation among symptomatic patients with a history of Lyme disease vs two comparison groups . Psychosomatics 2018 ; 59 : 481 – 489 Crossref, Medline, Google Scholar
10. : A controlled study of cognitive deficits in children with chronic Lyme disease . J Neuropsychiatry Clin Neurosci 2001 ; 13 : 500 – 507 Crossref, Medline, Google Scholar
11. : Chronic symptoms are common in patients with neuroborreliosis: a questionnaire follow-up study . Acta Neurol Scand 2002 ; 106 : 205 – 208 Crossref, Medline, Google Scholar
12. : Suicide and Lyme and associated diseases . Neuropsychiatr Dis Treat 2017 ; 13 : 1575 – 1587 Crossref, Medline, Google Scholar
13. : Evaluation of study patients with Lyme disease, 10–20-year follow-up . J Infect Dis 2001 ; 183 : 453 – 460 Crossref, Medline, Google Scholar
14. : Quality of life, fatigue, depression, and cognitive impairment in Lyme neuroborreliosis . J Neurol 2015 ; 262 : 2572 – 2577 Crossref, Medline, Google Scholar
15. : Neurocognitive functions and brain atrophy after proven neuroborreliosis: a case-control study . BMC Neurol 2015 ; 15 : 139 Crossref, Medline, Google Scholar
16. : Estimation of cognitive and affective disorders occurrence in patients with Lyme borreliosis . Ann Agric Environ Med 2017 ; 24 : 33 – 38 Crossref, Medline, Google Scholar
17. : Standardized symptom measurement of individuals with early Lyme disease over time . Arch Clin Neuropsychol 2017 ; 32 : 129 – 141 Crossref, Medline, Google Scholar
18. : Higher prevalence of antibodies to Borrelia burgdorferi in psychiatric patients than in healthy subjects . Am J Psychiatry 2002 ; 159 : 297 – 301 Link, Google Scholar
19. : Assessment of the risk of psychiatric disorders, use of psychiatric hospitals, and receipt of psychiatric medication among patients with Lyme neuroborreliosis in Denmark . JAMA Psychiatry 2021 ; 78 : 177 – 186 Crossref, Medline, Google Scholar
20. : The Danish civil registration system . Scand J Public Health 2011 ; 39 ( suppl ): 22 – 25 Crossref, Medline, Google Scholar
21. : The Danish integrated database for labor market research: towards demystification for the English speaking audience . DRUID Working Papers 10-16, DRUID, Copenhagen Business School, Department of Industrial Economics, and Strategy/Aalborg University, Department of Business Studies, 2010 Google Scholar
22. : The Danish National Patient Register . Scand J Public Health 2011 ; 39 ( suppl ): 30 – 33 Crossref, Medline, Google Scholar
23. : The Danish Psychiatric Central Research Register . Scand J Public Health 2011 ; 39 ( suppl ): 54 – 57 Crossref, Medline, Google Scholar
24. : The Danish Register of Causes of Death . Scand J Public Health 2011 ; 39 ( suppl ): 26 – 29 Crossref, Medline, Google Scholar
25. : [Lyme borreliosis: clinic, diagnostics, and treatment in Denmark, 2nd ed]. Danish Society for Clinical Microbiology, Danish Society for Infectious Diseases, and Danish Neurological Society, February 2014 (Danish) http://dskm.dk/onewebmedia/Borrelia%20klaringsrapport%202.udgave%202014.pdf Google Scholar
26. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation . J Chronic Dis 1987 ; 40 : 373 – 383 Crossref, Medline, Google Scholar
27. : Clinical and neuropsychological characteristics of general paresis misdiagnosed as primary psychiatric disease . BMC Psychiatry 2016 ; 16 : 230 Crossref, Medline, Google Scholar
28. : Changes in Lyme neuroborreliosis incidence in Denmark, 1996 to 2015 . Ticks Tick Borne Dis 2020 ; 11 : 101549 Crossref, Medline, Google Scholar
29. : Lyme borreliosis . Nat Rev Dis Primers 2016 ; 2 : 16090 Crossref, Medline, Google Scholar
30. : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication . Arch Gen Psychiatry 2005 ; 62 : 593 – 602 Crossref, Medline, Google Scholar
31. : Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study . Acta Psychiatr Scand 2017 ; 135 : 97 – 105 Crossref, Medline, Google Scholar
32. : Association of mental disorders and related medication use with risk for major osteoporotic fractures . JAMA Psychiatry 2017 ; 74 : 641 – 648 Crossref, Medline, Google Scholar
33. : The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders . Neuroscience 2015 ; 300 : 141 – 154 Crossref, Medline, Google Scholar
34. : Inflammation: depression fans the flames and feasts on the heat . Am J Psychiatry 2015 ; 172 : 1075 – 1091 Link, Google Scholar
35. : Elevated levels of IL-23 in a subset of patients with post–Lyme disease symptoms following erythema migrans . Clin Infect Dis 2014 ; 58 : 372 – 380 Crossref, Medline, Google Scholar
36. : CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study . Clin Vaccine Immunol 2016 ; 23 : 757 – 766 Crossref, Medline, Google Scholar
37. : Expression of C-reactive protein and serum amyloid A in early to late manifestations of Lyme disease . Clin Infect Dis 2016 ; 63 : 1399 – 1404 Crossref, Medline, Google Scholar
38. : Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [11C]DPA-713 PET . J Neuroinflammation 2018 ; 15 : 346 Crossref, Medline, Google Scholar
39. : Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms . Brain Behav Immun 2010 ; 24 : 1018 – 1024 Crossref, Medline, Google Scholar
40. : Cross-reactivity between Borrelia burgdorferi flagellin and a human axonal 64,000 molecular weight protein . J Infect Dis 1993 ; 167 : 1372 – 1378 Crossref, Medline, Google Scholar
41. : Anti-lysoganglioside and other anti-neuronal autoantibodies in post-treatment Lyme disease and erythema migrans after repeat infection . Brain Behav Immun Health 2020 ; 2 : 100015 (doi:
42. : From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways . Mol Psychiatry 2016 ; 21 : 738 – 748 Crossref, Medline, Google Scholar
43. : A distinct microbiome signature in posttreatment Lyme disease patients . mBio 2020 ; 11 : 1 – 13 Crossref, Google Scholar
44. : Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid . Clin Infect Dis 2006 ; 43 : 704 – 710 Crossref, Medline, Google Scholar
45. : Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease . Front Med (Lausanne) 2020 ; 7 : 57 Crossref, Medline, Google Scholar
46. : Chronic neurologic manifestations of Lyme disease . N Engl J Med 1990 ; 323 : 1438 – 1444 Crossref, Medline, Google Scholar
47. : Neurocognition in post-treatment Lyme disease and major depressive disorder . Arch Clin Neuropsychol 2019 ; 34 : 466 – 480 Crossref, Medline, Google Scholar
48. : Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii . J Infect Dis 2009 ; 200 : 1936 – 1943 Crossref, Medline, Google Scholar



