Autoimmune Encephalitis Presenting With Malignant Catatonia in a 40-Year-Old Male Patient With COVID-19
A 40-year-old man who had previously had symptoms of and a positive test for COVID-19, but had no other previous medical or psychiatric conditions or medication, presented to the emergency unit with acute debut of agitation, grimacing, and repetitive speech and movements (verbigeration and stereotypies); his behavior was bizarre, disorganized, hyperkinetic, and uncooperative and met DSM-5 criteria for catatonia.
Twenty-two days before admission, the patient had developed COVID-19-related respiratory symptoms and fatigue, which did not require hospital care. He had tested positive for SARS-CoV-2 RNA in a nasopharyngeal swab using the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform (day 14; Figure 1A). Anosmia and ageusia were not present. During the several days before admission, he had suffered from a headache. On admission (day 22), he no longer had respiratory symptoms but he did have a fever (38.4°C). He made no eye contact, his reflexes were normal, and Babinski’s sign was absent. Treatment with antibiotics and acyclovir was initiated until the tests excluded bacterial infection and herpes encephalitis. Brain CT, MRI, and blood tests were unremarkable. The patient was lightly sedated with midazolam, followed with dexmedetomidine. Neuroleptics were not used.
Lumbar puncture showed a high red blood cell count (19,000 cells×106/L) secondary to traumatic lumbar puncture. CSF cell count indicated pleocytosis, with 23×106/L mononuclear and 8×106/L polymorphonuclear cells. Signs of blood-brain barrier disruption were present, with elevated albumin levels in CSF, at 838 mg/L; reference, <400 mg/L) and the albumin CSF/serum quotient was 15.6 (reference, <6.8). Interleukin-6 (IL-6) in CSF was elevated at 102.1 pg/mL (reference, <5 pg/mL), but CSF levels of neurofilament light chain (NFL), glial fibrillary acidic protein (GFAP), and tau protein were normal. PCR tests for SARS-CoV-2 were repeatedly negative in CSF and nasopharyngeal swabs. Antineuronal antibodies against N-methyl-d-aspartate receptor (NMDAR), glutamic acid decarboxylase, contactin-associated protein-like 2, leucine-rich, glioma inactivated 1, and ganglioside antibodies in serum and CSF were negative (Euroimmune, Lübeck, Germany).
Hours later, the patient’s state deteriorated, and his temperature rose to 39°C. He became mutistic and showed signs of autonomic instability, with recurrent episodes of fluctuating heart rate and arterial blood pressure and periods of oxygen desaturation (Figure 1B). The hypertension was difficult to treat, despite high doses of clonidine and labetalol. Plasma lactate levels varied between 0.6 and 8 mmol/L (reference, 0.8–2.0 mmol/L), but myoglobulin and creatine kinase myocardial band (CKMB) remained normal. The patient’s pupil size, reaction to light, and oculocephalic reflex were normal. Slow, horizontal roving eye movements were noted. The patient displayed decorticate posturing and increased tonus; he resisted movement of arms and jaw but had normal tonus in the legs. Hyperreflexia was present, with bilateral foot clonus and Babinski’s sign but no neck stiffness. Anesthesia was induced with propofol and clonidine to facilitate endotracheal intubation. D-Dimer was slightly elevated (1.2 mg/L; reference <0.5 mg/L), without signs of thromboembolic events. Respiration and cardiovascular function remained stable. Continuous EEG monitoring showed nonspecific slowing with left hemisphere predominance without epileptiform activity. An episode of asystole with spontaneous recovery, episodes of bradycardia of 27 bpm and repeated P waves without QRS complexes were interpreted as third-degree atrioventricular block.
Signs of autoimmune encephalitis were present, but this case did not meet the proposed criteria (1, 2). Standard radiological findings were normal, and the discrete pleocytosis and elevated protein in CSF was nonspecific. Although the diagnosis remained uncertain, para-infectious autoimmune encephalitis was still suspected. Plasmapheresis was initiated and repeated three times over 4 days. After two courses, the patient was extubated and was autonomically stable. Eye movement was normalized and hyperreflexia was less prominent, but bilateral Babinski’s sign persisted. Treatment was initiated with 1 g methylprednisolone per day.
On day 28, the patient showed a dramatic improvement. He was awake, oriented, and communicative but had no memories from the past several days. He was distracted by complex visual hallucinations of black and white figures (animals and famous people) appearing on his right side. He described them as being in a mirror (suspected polyopia). These figures were often stationary but could make gestures. He also described an experience of feeling that the world was different—strange and unreal, with brighter colors (suspected hyperchromatopsia and derealization). He had frequent episodes of failing to recognize his right hand and leg as his own and experienced their movement as unexpected (alien hand syndrome). He denied the presence of other perceptual disturbances. His understanding of Swedish, his second language, seemed intact, but his responses were mostly monosyllabic. He could name his children and give his personal identification number but was slow and made mistakes in naming the months. Mild visual object agnosia was present. Simultanagnosia was prominent, he showed deficits in isolating figures in a tangled pictorial array, and he could depict details but excluded the global features of complex pictures. He could recall one of three objects after a short delay and draw a correct clock but required three repetitions of the instructions. He had difficulty mirroring and performing fine movements. Finally, he showed no signs of visual neglect and could read text. The patient’s EEG was normal.
A second lumbar puncture showed pleocytosis, 10 mononuclear cells and 1 polymorphonuclear cell ×106/L, elevated IgG levels and IgG index, and two oligoclonal bands in CSF not represented in serum, indicating intrathecal production of antibodies. The IL-6 level in CSF was normalized. GFAP and tau remained normal, but NFL was increased to 1,030 ng/L (reference, <890 ng/L). A second MRI and a standard neurological examination on day 31 were normal. The hallucinations were less frequent. The patient described increased emotional lability and mental fatigue, with disturbed short-term memory and decision making. He also found it challenging to recognize the voices and faces of acquaintances. Serology on day 33 was strongly positive (index 8.88 S/CO [signal/cutoff]) for IgG against SARS-CoV-2 analyzed with the CE-labeled SARS-CoV-2 IgG kit with nucleoprotein-based antigen with the Abbott Architect i2000SR Analyzer at the Laboratory of Clinical Microbiology, Uppsala University Hospital, as previously described (3). [18F]fluorodeoxyglucose ([18F]FDG) PET scan on day 35 (after treatment) showed high bilateral uptake in the striatum (caudate nucleus and putamen) compared with the cortex (Figure 1C).
Using immunohistochemistry in the research lab, we detected IgG autoantibodies against mouse brain neuronal proteins in serum and CSF collected at admission (Figure 2). Neuronal labeling intensity was strongest in the CA3 in the hippocampal formation, layer V in the somatosensory cortex, and the paraventricular and reticular nucleus in the thalamus. A subset of ependymal cells located in the ventricle wall and choroid plexus revealed strong immunoreactivity of the (peri)nuclear compartment and cytoplasm. Immunoreactivity of neuropil was most intense in the caudate putamen, revealing neuronal processes and spine-like structures. Posttreatment IgG immunoreactivity in the (peri)nuclear compartment and neuropil was notably reduced, reaching the levels of reference CSF and serum.
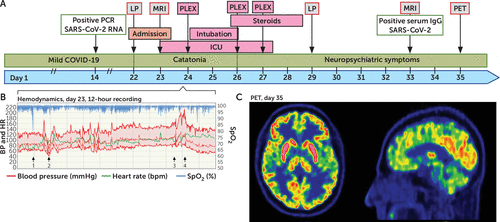
FIGURE 1. Timeline, hemodynamics, and PET findings in a patient with malignant encephalitis after infection with SARS-CoV-2a
a Panel A is a timeline of evaluations, treatments, and progression of symptoms. ICU=intensive care unit; PCR=polymerase chain reaction; PET=positron emission tomography; PLEX=plasmapheresis; LP=lumbar puncture. Panel B shows an original 12-hour chart recording taken before plasmapheresis (PLEX) (day 23) with peripheral oxygen saturation (% SpO2), arterial blood pressure (BP), and heart rate (HR). The vertical lines represent hours. The patient was initially sedated with an intravenous dexmedetomidine infusion. A hypoxic event of unknown origin was noted (arrow 1). Blood pressure and heart rate were generally variable, with episodes of hypotension associated with hyperlactatemia (arrow 2) and hypertension (arrow 3). Hypotension was treated with intravenous crystalloid fluid and noradrenalin. Induction of anesthesia and endotracheal intubation (arrow 3) was followed by hypertension (arrow 4) that persisted after treatment with bolus doses of propofol, morphine, clonidine, and labetalol. After endotracheal intubation, this treatment regimen was changed to propofol, clonidine, and morphine. Panel C shows a brain PET scan 60 minutes after injection of 209 MBq of [18F]fluorodeoxyglucose 13 days after plasmapheresis treatment. The uptake in the caudate nucleus and putamen is bilaterally more prominent than the uptake in the cortex. This observation could represent a global cortical decrease in metabolism or symmetrical hypermetabolism in the striatum. On visual inspection, there were no regions with focal cortical anomalies.
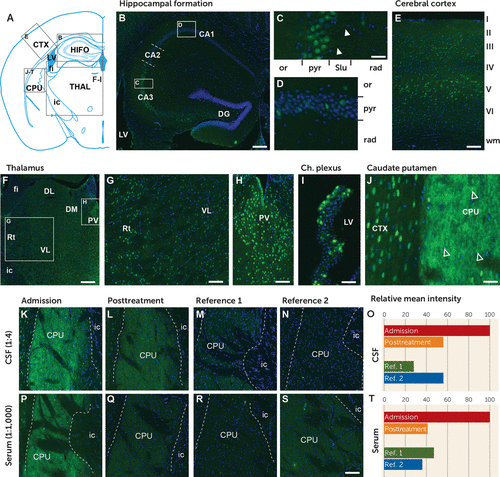
FIGURE 2. IgG immunoreactivity of mouse brain tissue from serum and CSF from a patient with malignant encephalitis after infection with SARS-CoV-2a
a Profiling of IgG immunoreactivity on mouse brain tissue. CSF (diluted 1:4) collected at the first lumbar puncture revealed a moderate to strong immunoreactivity in neuron-like cells in all investigated brain regions. Panel A shows a map over the mouse brain tissue represented in the following panels. In the hippocampal formation, the strongest immunoreactivity was observed in the CA3 pyramidal neurons (panel B) labeling the nucleus, soma, and proximal dendrites in the stratum lucidum (arrowheads in panel C). In other regions of the hippocampal formation, IgG immunoreactivity was found in a small number of neuron-like cells in the pyramidal layer, the oriens layer (panel D), and the polymorph layer of the dentate gyrus (panel B). A similar neuronal staining pattern was observed in the somatosensory cortex (panel E), with the strongest labeling of pyramidal neurons in layer V and the thalamus (panel F) and the strongest labeling of neurons in the paraventricular nucleus and reticular nucleus compared with dorsolateral and ventrolateral thalamic nuclei (panels G and H). A subpopulation of ependymal cells lining the dorsal third ventricle and choroid plexus (panel I) reveals nuclear and cytoplasm staining. The highest level of immunoreactivity was found in neurons and neuropil in the caudate putamen (panel J). Note the dotted spine-like staining pattern (arrows in panel J). CSF (panel K) and serum (panel P) from before treatment compared with after treatment (panels L and Q) revealed less of the same pattern of immunoreactivity with a remarkably reduced intensity when compared. Reference was CSF (diluted 1:4; panels M and N) and serum (diluted 1:1,000; panels R and S) from two age-matched men with bipolar I disorder in manic phase. Quantification of immunoreactivity before and after treatment is shown for CSF respective serum (panels O and T). CA1–CA3=cornu ammonis, areas 1–3; CPU=caudate putamen; CTX=cerebral cortex; DG=dentate gyrus; DL=dorsolateral thalamus; DM=dorsomedial thalamus; fi=fimbria of the hippocampus; HIFO=hippocampal formation; ic=internal capsule; LV=lateral ventricle; or=oriens layer; PV=paraventricular thalamic nucleus; pyr=pyramidal layer; rad=radiatum layer; Rt=reticular nucleus; Slu=stratum lucidum; THAL=thalamus; VL=ventrolateral thalamus.
Cases of rapid onset of encephalitis with diffuse corticospinal tract signs have been reported in conjunction with COVID-19 (4, 5). Neurological symptoms in COVID-19 patients include a few cases of catatonia (6–8), mutism (9), and autonomic dysfunction (10). Several forms of autoimmunity after COVID-19 are emerging (11), and this coronavirus may join other neurotropic viruses as a risk factor for autoimmune encephalitis.
Malignant catatonia is a more severe form of the catatonia spectrum but perhaps less recognized than the typical form with immobility, waxy flexibility, and stupor (12, 13). Neurologists in this case preferably used the term “acute encephalopathy,” and the problem of divergent nomenclature between specializations in cases of acute, global disturbance in cognition is a current topic of discussion (14). A recent review identified 124 cases of catatonia in conjunction with infections, of which 38% were viral and co-occurred with several autoimmune conditions, especially NMDAR encephalitis (15). The neurological symptom presentation in our case indicated the involvement of the corticospinal tracts and is congruent with pathological function in supratentorial structures and intact brainstem. The hallucinations and simultanagnosia appeared and regressed together, suggesting a common mechanism. Simultanagnosia and prosopagnosia indicate right hemisphere involvement, specifically the right posterior cortical network (16). Prosopagnosia is common after viral encephalitis (17). Although the MRI showed no evidence of structural damage, [18F]FDG PET findings in the striatum are consistent with increased metabolism in local neurons, glial cells, or both. Striatal hypermetabolism has previously been reported for autoimmune encephalitis caused by antibodies against NMDAR and the voltage-gated potassium channel complex, as well as in IgG4-related disease (18–23). The staining, clinical presentation, and radiology in our case are consistent with a synaptic target highly present in the striatum, where binding leads to loss of inhibition with increased metabolism in local neurons, glial cells, or both.
We conclude that malignant catatonia with potentially life-threatening autonomic instability can occur in patients with COVID-19. In our patient, this condition responded to treatment with dramatic improvement and with minimal evidence of structural brain damage. The treatment reduced autoreactive IgG in both serum and CSF but may also have other actions (24). In patients presenting with agitation, changes in behavior and speech, and autonomic instability in the weeks after infection with SARS-CoV-2, these symptoms should be recognized as a potential autoimmune manifestation of COVID-19. We are not aware of other types of encephalitis with such distinct pyramidal tract symptoms and raise the possibility that this may be a novel form of autoimmune encephalitis induced by infection with SARS-CoV-2.
1 : Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin . Lancet Psychiatry 2020 ; 7 : 93 – 108 Crossref, Medline, Google Scholar
2 : A clinical approach to diagnosis of autoimmune encephalitis . Lancet Neurol 2016 ; 15 : 391 – 404 Crossref, Medline, Google Scholar
3 : Work at inpatient care units is associated with an increased risk of SARS-CoV-2 infection: a cross-sectional study of 8679 healthcare workers in Sweden . Ups J Med Sci 2020 ; 125 : 305 – 310 Crossref, Medline, Google Scholar
4 : Neurologic features in severe SARS-CoV-2 infection . N Engl J Med 2020 ; 382 : 2268 – 2270 Crossref, Medline, Google Scholar
5 : Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China . JAMA Neurol 2020 ; 77 : 683 – 690 Crossref, Medline, Google Scholar
6 : A case of catatonia in a man with COVID-19 . Psychosomatics 2020 ; 61 : 556 – 560 Crossref, Medline, Google Scholar
7 : Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism . Brain Behav Immun 2020 ; 89 : 529 – 530 Crossref, Medline, Google Scholar
8 : Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study . Lancet Psychiatry 2020 ; 7 : 875 – 882 Crossref, Medline, Google Scholar
9 : Steroid-responsive encephalitis in coronavirus disease 2019 . Ann Neurol 2020 ; 88 : 423 – 427 Crossref, Medline, Google Scholar
10 : Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19 . J Neurol 2020 ; 267 : 2490 – 2491 Crossref, Medline, Google Scholar
11 : COVID-19 and autoimmunity . Autoimmun Rev 2020 ; 19 : 102597 Crossref, Medline, Google Scholar
12 : Catatonia in the ICU: an important and underdiagnosed cause of altered mental status: a case series and review of the literature . Crit Care Med 2014 ; 42 : e234 – e241 Crossref, Medline, Google Scholar
13 : The diagnostic criteria and structure of catatonia . Schizophr Res 2015 ; 164 : 256 – 262 Crossref, Medline, Google Scholar
14 : Updated nomenclature of delirium and acute encephalopathy: statement of ten societies . Intensive Care Med 2020 ; 46 : 1020 – 1022 Crossref, Medline, Google Scholar
15 : Catatonia and the immune system: a review . Lancet Psychiatry 2019 ; 6 : 620 – 630 Crossref, Medline, Google Scholar
16 : Neuropsychology of aesthetic judgment of ambiguous and non-ambiguous artworks . Behav Sci (Basel) 2017 ; 7 : 13 Crossref, Google Scholar
17 : Diagnosing prosopagnosia: effects of ageing, sex, and participant-stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test . Cogn Neuropsychol 2009 ; 26 : 423 – 455 Crossref, Medline, Google Scholar
18 : Striatal hypermetabolism in a case of IgG4-related disease . Nucl Med Rev Cent East Eur 2018 ; 21 (doi:
19 : Brain MRI findings in severe COVID-19: a retrospective observational study . Radiology 2020 ; 297 : E242 – E251 Crossref, Medline, Google Scholar
20 : Brain MRI findings in patients in the intensive care unit with COVID-19 infection . Radiology 2020 ; 297 : E232 – E235 Crossref, Medline, Google Scholar
21 : Striatal hypermetabolism in limbic encephalitis . J Neurol 2012 ; 259 : 1106 – 1110 Crossref, Medline, Google Scholar
22 : Semi-quantitative analysis of cerebral FDG-PET reveals striatal hypermetabolism and normal cortical metabolism in a case of VGKCC limbic encephalitis . Neuroradiol J 2017 ; 30 : 160 – 163 Crossref, Medline, Google Scholar
23 : Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types . J Neurol 2013 ; 260 : 2744 – 2753 Crossref, Medline, Google Scholar
24 : The mechanisms of action of plasma exchange . Br J Haematol 2014 ; 164 : 342 – 351 Crossref, Medline, Google Scholar



