Hormonal Treatments for Major Depressive Disorder: State of the Art
Abstract
Major depressive disorder is a common psychiatric disorder associated with marked suffering, morbidity, mortality, and cost. The World Health Organization projects that by 2030, major depression will be the leading cause of disease burden worldwide. While numerous treatments for major depression exist, many patients do not respond adequately to traditional antidepressants. Thus, more effective treatments for major depression are needed, and targeting certain hormonal systems is a conceptually based approach that has shown promise in the treatment of this disorder. A number of hormones and hormone-manipulating compounds have been evaluated as monotherapies or adjunctive treatments for major depression, with therapeutic actions attributable not only to the modulation of endocrine systems in the periphery but also to the CNS effects of hormones on non-endocrine brain circuitry. The authors describe the physiology of the hypothalamic-pituitary-adrenal (HPA), hypothalamic-pituitary thyroid (HPT), and hypothalamic-pituitary-gonadal (HPG) axes and review the evidence for selected hormone-based interventions for the treatment of depression in order to provide an update on the state of this field for clinicians and researchers. The review focuses on the HPA axis–based interventions of corticotropin-releasing factor antagonists and the glucocorticoid receptor antagonist mifepristone, the HPT axis–based treatments of thyroid hormones (T3 and T4), and the HPG axis–based treatments of estrogen replacement therapy, the progesterone derivative allopregnanolone, and testosterone. While some treatments have largely failed to translate from preclinical studies, others have shown promising initial results and represent active fields of study in the search for novel effective treatments for major depression.
Major depressive disorder is a common psychiatric disorder that is associated with marked suffering, morbidity, mortality, and cost (1, 2). The lifetime prevalence of major depression in adults in the United States is estimated to be 17% (3); prevalence is higher in women than men (21% and 13%, respectively) (4), and 12-month prevalence is approximately 7% (5). In 2010 the economic cost of depression in the United States was estimated to be $210 billion (2). The World Health Organization projects that worldwide, major depression will be the leading cause of disease burden by 2030 (6). While numerous treatments for major depression exist, many patients do not respond adequately to traditional antidepressants. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, one-third of patients with major depression experienced a remission of depressive symptoms with their first antidepressant trial of citalopram, and only two-thirds of patients overall cumulatively experienced a remission after four sequential treatments (7). Thus, more effective treatments for major depression are needed. Targeting certain hormonal systems is a conceptually based approach that has shown promise in the treatment of major depression.
Alterations in hormones and endocrine function may play an important role in mechanisms underlying the pathophysiology of major depression. Primary abnormalities in the adrenal, thyroid, and gonadal axes are associated with alterations in mood, and medications that target endocrine function are often accompanied by mood-related and cognitive effects. These clinical observations prompted exploration of the roles that hormones may play in the pathophysiology of major depression and of potential treatment approaches targeting specific hormonal systems. A number of hormones and hormone-manipulating compounds have been evaluated as monotherapies or adjunctive treatments for major depression, with therapeutic actions attributable not only to the modulation of endocrine systems in the periphery but also to the CNS effects of hormones on non-endocrine brain circuitry. Here we review the evidence for clinically relevant interventions for the treatment of depression that are based on the hypothalamic-pituitary-adrenal (HPA), hypothalamic-pituitary thyroid (HPT), and hypothalamic-pituitary-gonadal (HPG) axes.
Hypothalamic-Pituitary-Adrenal Axis
Overview of Physiology
The HPA axis is a critical endocrine system that orchestrates the stress response, a complex set of behavioral, neuroendocrine, autonomic, and immune responses enabling adaptation to aversive psychological and physiological stimuli (8). The main components of the HPA axis are the paraventricular nucleus (PVN) of the hypothalamus, the anterior lobe of the pituitary gland, and the adrenal cortex.
The principal central initiator of HPA axis activity is corticotropin-releasing factor (CRF), also termed corticotropin-releasing hormone (9), a peptide secreted by the PVN in response to stress (Figure 1). CRF is released into the hypothalamo-hypophyseal portal system and stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary into the systemic circulation. Another hypothalamic peptide, arginine-vasopressin (AVP), either can be released into the hypophyseal portal system like CRF or can be released from axon terminals in the posterior pituitary. AVP synergistically enhances ACTH release in combination with CRF, an action that may be especially relevant in states of chronic stress (10). Once released into the peripheral circulation, ACTH acts on the adrenal cortex, stimulating the synthesis and secretion of glucocorticoids (e.g., cortisol) into the systemic circulation. A cortisol-binding globulin, transcortin, is found in plasma, and when cortisol is bound to transcortin, it is unable to interact with its receptors (11). AVP is also produced locally in the adrenal medulla and can stimulate release of cortisol, underscoring its role as a positive HPA regulator (12).
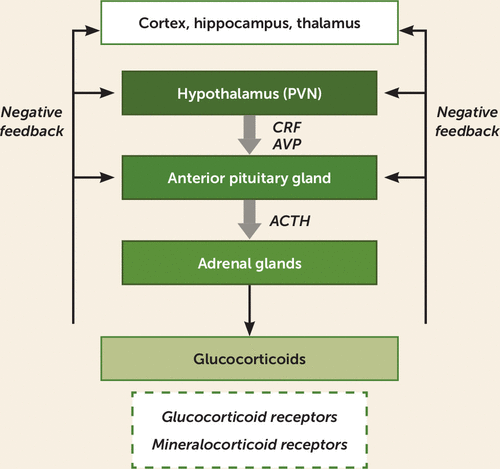
FIGURE 1. Hypothalamic-pituitary-adrenal (HPA) axisa
a In the HPA axis, the paraventricular nucleus of the hypothalamus (PVN) releases corticotropin-releasing factor (CRF) and, to a lesser extent, arginine-vasopressin (AVP). These factors stimulate the anterior pituitary to release adrenocorticotropic hormone (ACTH) into the peripheral circulation, which stimulates the adrenal glands to release glucocorticoids (e.g., cortisol). Glucocorticoids act on both glucocorticoid receptors and mineralocorticoid receptors. Negative feedback occurs at the level of the pituitary, the hypothalamus, and higher brain structures (e.g., cortex, hippocampus, and periventricular thalamus).
In the periphery and the brain, cortisol acts via two distinct modes of signaling: membrane-bound glucocorticoid receptors that activate rapid protein kinase signaling, and the more classic, protracted nuclear receptor signaling, in which intracellular glucocorticoid receptors translocate to the nucleus to transcriptionally regulate gene expression (13, 14). Two receptors bind cortisol: the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). The MR has a higher affinity for cortisol than the GR, and this greater sensitivity to low cortisol concentrations allows it to regulate physiological fluctuations in HPA activity (e.g., the circadian rhythm of cortisol release). The MR is also thought to play an important role in inhibiting HPA axis activity (15) that is associated with adaptive coping and resilience to stress (16). The lower-affinity GR is better suited to detecting high cortisol concentrations, such as those released during the stress response. Although the expression of the MR is restricted to limbic brain regions, the GR is expressed much more widely, highlighting the broad impact that cortisol can have in modulating brain function.
When intracellular glucocorticoid receptors are activated, they translocate to the nucleus and bind to glucocorticoid response elements on DNA to activate or repress gene expression to mediate the diverse set of responses to stress and to efficiently terminate the stress response, a concept known as feedback inhibition. Glucocorticoid receptor–mediated negative feedback occurs at multiple levels and via several mechanisms within the CNS and pituitary (Figure 1). At the level of the hypothalamus and pituitary, both rapid inhibition via fast, membrane-bound signaling (17, 18) and slower nuclear receptor–mediated transcriptional repression of the genes that produce CRF and ACTH exist (19). The GR also can down-regulate its own activity in response to cortisol via transcriptional induction of the chaperone protein FK506 binding protein 5 (FKBP5), which sequesters the GR in the cytoplasm, preventing it from entering the nucleus (20). Glucocorticoid receptors also mediate feedback inhibition more indirectly via limbic areas such as the hippocampus (21), paraventricular thalamus (22), and prefrontal cortex (23), which likely play a larger role in the processing of psychological versus physiological stressors (21). The regulation of negative feedback within the HPA axis is complex but critically important in modulating adaptive responses to stress, that is, creating a system that can quickly respond to a stressor, and then efficiently terminate, reset, and await the next event.
In addition to the classic endocrine functions of the HPA axis, the hypothalamic peptides (CRF, and to a lesser extent AVP) also regulate neural circuits relevant to stress, anxiety, and mood processing. CRF is a member of a family of peptides and is expressed not only in the PVN but also in limbic regions, including the central nucleus of the amygdala, cerebrocortical areas, and the brainstem (24). CRF’s two predominant receptors (CRFR1 and CRFR2) also have significant extrahypothalamic expression. A binding protein, CRFBP, is thought to modulate CRF activity by preventing its access to its receptors. Taken together, the extrahypothalamic roles of the CRF system are critically important, and alterations in these systems have been implicated in the pathogenesis of affective and anxiety disorders.
HPA Abnormalities in Major Depression
Early work on the pathophysiology of depression suggested hyperactivity of the HPA axis and impairment in its sensitivity to negative feedback regulation in major depression, although more recent investigations suggest a much more complex picture. Alterations at every level of the HPA system have been reported, although these reports have not always been replicated, and the various reported alterations may occur only in subsets of individuals with major depression. In general, patients with major depression are reported to exhibit hyperactivity of the HPA axis, with impaired sensitivity to negative feedback; however, subsets of patients with hypocortisolemia have been reported (25). It is noteworthy that HPA axis overactivity appears to be particularly enriched in the melancholic and psychotic depression subtypes (26–28).
While HPA axis abnormalities have been studied in large numbers of patients (e.g., a meta-analysis including over 18,000 individuals [29]), results are heterogeneous overall and effect sizes modest. That said, the most prominently reported HPA axis alterations associated with depression include 1) blunted cortisol circadian rhythms with elevated levels late in the day (29); 2) negative feedback insensitivity, as characterized by a failure to suppress morning cortisol after the administration of dexamethasone (30); 3) increased release of cortisol in response to exogenously administered ACTH (31); 4) blunted ACTH in response to CRF administration (32); 5) exaggerated ACTH and cortisol responses in the combined dexamethasone-CRF test (33, 34); 6) increased cerebrospinal fluid CRF concentrations (35, 36); and 7) down-regulation of brain CRHR1 receptors and mRNA, hypothesized to reflect compensatory changes to persistently high CRF concentrations (37). Additionally, genetic studies have identified single-nucleotide polymorphisms (SNPs) in genes relevant to the stress response, modulators of glucocorticoid function (e.g., GR, FKBP5), and the CRF system (e.g., CRFR1, CRFR2, CRFBP) that are associated with major depression and/or potentially related to treatment response (38–40).
HPA Axis–Based Treatments for Major Depression
A number of strategies have attempted to modulate HPA function and/or extrahypothalamic targets as an approach to treat major depression. Several strategies showed initial promise in small clinical trials but were subsequently abandoned. These strategies included cortisol synthesis inhibitors in patients with major depression with or without hypercortisolemia, glucocorticoids in patients with major depression with hypocortisolemia, and vasopressin receptor antagonists. For example, the cortisol synthesis inhibitor metyrapone showed positive antidepressant effects as an augmentation agent in several small trials (Ns ranging from six to nine) (41–44) and one larger double-blind inpatient study (N=63) (45); however, a larger multisite randomized controlled trial (N=165) was negative (46). Stimulation of MRs (e.g., with the MR agonist fludrocortisone) is also a strategy under investigation (47). Treatments focused on the CRF and GR systems have been the most extensively studied, and here we discuss these approaches in more depth: CRFR1 antagonists, driven by highly promising preclinical data but which have so far not proved effective in humans, and the GR antagonist mifepristone, which shows promise in the treatment of major depression with psychotic features and remains an active area of investigation.
CRFR1 antagonists.
The potential of CRFR1 antagonists generated considerable interest given the preclinical and clinical evidence implicating excess CRF production in the pathophysiology of major depression. Preclinical studies showed that central injection or overexpression of CRF in certain brain areas such as the amygdala generates anxious and depressive phenotypes, and that CRFR1 antagonists block these behavioral manifestations (48–50). The human studies suggesting hyperactivity of the CRF system in major depression and its resolution with antidepressant treatment (51) bolstered the rationale for targeting this system directly. Despite initial positive results (52), this line of research has suffered from a series of disappointments. Two early compounds were abandoned because of hepatotoxicity (R-121919 and PF-00572778), and subsequent double-blind, placebo-controlled trials yielded negative results or were stopped early because of lack of efficacy (ONO-2333 Ms [N=278] [50], CP-316,311 [N=123] [53], BMS-562086 [N=260 for generalized anxiety disorder (54); trial ended unpublished for major depression]). Although the current CRFR1 antagonists do not appear to have efficacy as a monotherapy for major depression, there appears to be value in further studying novel compounds with different pharmacokinetic profiles in subpopulations selected for functional genetic alterations in the CRF system (38). Moreover, the lack of a positron-emission tomography ligand to assess receptor occupancy of the CRFR1 antagonist has precluded an understanding of whether the doses used were sufficient to adequately test the efficacy of this strategy. In addition, these compounds have never been tested in combination with other antidepressants. Strategies targeting the other major CRF receptor (CRF2) and the CRF binding protein could also be useful. According to ClinicalTrials.gov, no current CRF antagonist trials are actively recruiting patients.
GR antagonists.
GR antagonists have been tested primarily in depression with psychotic features for the following reasons: 1) patients with Cushing’s syndrome and patients receiving high-dose exogenous glucocorticoids often have marked mood and psychotic symptoms; 2) effective treatment of the endocrine abnormality in these patients frequently reverses these symptoms; and 3) HPA axis abnormalities are enriched in patients with major depression with psychotic features (39). Preclinical data from animal models of depression supported the use of GR antagonists as augmenting agents, enhancing both the speed of effect and the overall efficacy of SSRIs (55). Small early studies with ketoconazole, an antifungal medication that also antagonizes GR receptors and inhibits cortisol synthesis, showed mixed results: antidepressant effects in an open study (43) of multiple cortisol synthesis inhibitors (N=17); antidepressant effects in hypercortisolemic but not eucortisolemic patients in a double-blind study (N=20) (56); and no significant antidepressant effects in a double-blind study (N=16) (57). Mifepristone, which antagonizes both the GR and the progesterone receptor, has been the most rigorously tested GR antagonist. The mifepristone studies are unusual in that participants received the medication for 7 days, and symptoms were measured 1 week later, 1 month later, and even later, when patients were on standard antidepressant monotherapy. Although results from individual clinical trials have been somewhat inconsistent (see Table S1 in the online supplement), a summary of seven clinical trials, five of them double-blind (Ns ranging from five to 433), suggests that mifepristone has efficacy in reducing psychotic symptoms, the primary outcomes of the trials. Two double-blind trials showed a significant difference in the proportion of responders to mifepristone compared with placebo (response was defined as a reduction of 50% in score on the positive symptom subscale of the Brief Psychiatric Rating Scale [BPRS] [58] and a 30% reduction in the full BPRS score [59]). Depressive symptoms were considered as secondary outcomes in these trials and often did not show a separation from placebo (60).
Notably, mifepristone’s effectiveness appears to be optimized when it attains a plasma level of ∼1600 ng/mL, which equates to roughly 1200 mg/day orally (61). Thus, inadequate dosing may explain the results of some earlier trials that did not show significant differences from placebo (58). Indeed, a recent study combining and reanalyzing the data from five placebo-controlled trials (mifepristone, N=833; placebo, N=627) showed that when patients with psychotic major depression achieved therapeutic plasma levels, a robust improvement in psychotic symptoms was observed, starting at 28 days and lasting through 56 days, the last time point examined, with a number needed to treat of 7 (compared with 48 for patients with low plasma mifepristone levels) (62). Patients with high mifepristone plasma levels also showed a reduction in depressive symptoms relative to patients in the placebo group, while patients with low mifepristone plasma levels did not (62). These are intriguing findings that should be replicated in clinical trials specifically designed to test this hypothesis. Although mifepristone is associated with some gastrointestinal side effects and headache, very few patients discontinued because of side effects. These data are a promising start and represent a nontraditional dosing advantage of a time-limited treatment with persisting benefit (at least 56 days). Multiple studies of mifepristone are listed on ClinicalTrials.gov, typically as an adjunctive treatment in depression (to ECT in nonpsychotic depression [NCT00285818] and to a mood stabilizer in bipolar depression [NCT00043654]), and also as a monotherapy for alcohol use disorder (NCT02179749) (63). Neurocognitive symptoms associated with psychiatric disorders are another interesting potential application, as patients with bipolar disorder treated with mifepristone show improvements in spatial working memory (64). Of note, other GR antagonists with better selectivity for the GR receptor are being developed and tested, which underscores the level of interest and activity in exploring the therapeutic use of GR antagonists.
Summary of HPA axis–targeted interventions.
Overall, the strongest support for HPA axis interventions in the treatment of depression is for the use of GR antagonists for the treatment of psychotic symptoms in psychotic depression (Table 1). While variation in response in individual mifepristone clinical trials occurred, combined reanalysis of all trial data supports efficacy, especially when plasma levels are considered. Despite considerable preclinical data, the clinical trial literature does not support the use of currently available CRFR1 antagonists for the treatment of major depression or other psychiatric disorders. Other HPA-based treatments (e.g., cortisol synthesis inhibitors, glucocorticoids, and vasopressin receptor antagonists) have been tested in small clinical trials, and together the evidence does not support their use in clinical practice.
| Intervention | Disorder/Population | Level of Evidence |
|---|---|---|
| CRF1 antagonists | Major depression | Strong evidence of no benefit |
| GR antagonists (e.g., mifepristone) | Major depression with psychotic features | Moderate evidence of efficacy for psychotic symptoms if minimum plasma level achieved; additional prospective studies needed |
| T3 augmentation of antidepressants | Treatment-resistant major depression | Moderate evidence of efficacy; efficacy of augmentation with SSRIs requires demonstration in placebo-controlled trials |
| T3 for acceleration of antidepressant effect with TCAs | Major depression | Strong evidence of efficacy |
| T3 for acceleration of antidepressant effect with SSRIs | Major depression | Strong evidence of no benefit |
| Estrogen replacement therapy (or combined hormone replacement therapy) | Perimenopausal women with major depression and physical menopause symptoms | Moderate evidence of efficacy |
| Estrogen replacement therapy (or combined hormone replacement therapy) | Perimenopausal women without major depression (prevention) | Weak evidence of benefit |
| Estrogen replacement therapy (or combined hormone replacement therapy) | Postmenopausal women with major depression | Poor as monotherapy, preliminary evidence as adjunct to SSRIs in geriatric depression |
| Oral contraceptives | PMDD | Moderate evidence of efficacy for drospirenone-containing oral contraceptives, weak evidence of efficacy for other oral contraceptives, despite being considered second-line treatment after SSRIs |
| Allopregnanolone stabilization | PMDD | Moderate evidence of efficacy |
| Allopregnanolone enhancement | Postpartum depression | Strong evidence of efficacy |
| Testosterone replacement therapy | Depressive symptoms secondary to clinical hypogonadism | Strong evidence of efficacy |
| Testosterone augmentation | Subthreshold depressive symptoms in men without clinical hypogonadism | Preliminary evidence of efficacy |
| Testosterone augmentation | Treatment-resistant major depression in men without clinical hypogonadism | Strong evidence of no benefit |
TABLE 1. Summary of evidence for hormonal treatments for major depressive disordera
Hypothalamic-Pituitary-Thyroid (HPT) Axis
Overview of Physiology
Thyroxine (T4) and triiodothyronine (T3) are the two primary thyroid hormones, and they are responsible for regulation of metabolism and protein synthesis throughout the body (65). Thyrotropin-releasing hormone (TRH), primarily released from neurons that originate in the hypothalamic PVN, regulates the release of thyroid-stimulating hormone (TSH) from the anterior pituitary gland (Figure 2). TSH stimulates the production of T4 and, to a lesser degree, T3 from the thyroid gland. Serum free T4 and free T3 levels regulate pituitary TSH release through negative feedback. T4 is primarily converted by the tissue deiodinases into the more biologically active T3 and the inactive metabolite reverse T3. Thyroid hormones are highly protein bound, and it is only the free fraction that is biologically available. Effects of thyroid hormones are primarily mediated by their binding to nuclear receptors that transcriptionally regulate gene expression. Thyroid receptors have two predominant isoforms, TRα1 and TRβ1, with varying sensitivity to T4 and to T3. TRα1 is the predominant isoform in the brain, and while it is activated by both T3 and T4, it has heightened sensitivity to T4 compared with TRβ1. T3 and T4 enter the brain either directly across the blood-brain barrier or indirectly across the choroid plexus epithelium into cerebrospinal fluid (CSF) (66, 67). The transport across the blood-brain barrier happens via a number of identified and unidentified transporter proteins, such as MCT8 or OATP1C1. Although both T3 and T4 are transported from circulation into the CNS, T4 is thought to be transported in preference to T3 (66, 67). In the brain, deiodinase 2 in astrocytes is the primary enzyme that converts T4 into T3, which is critical for the local generation of T3 that interacts with neurons. Deiodinase 3, another CNS thyroid enzyme, is selectively expressed in neurons. Deiodinase 3 inactivates both T4 and T3 by deiodination (66, 68). Thyroid hormones in the CSF help maintain the brain interstitial levels of thyroid hormones. T4 is carried in the CSF via a number of transport proteins, including transthyretin (67). Transthyretin is secreted into the CSF by the choroid plexus and is the primary CSF carrier of T4. Lower levels of transthyretin have been reported in the CSF of depressed patients compared with control subjects, and this has been suggested to lead to a state of “brain hypothyroidism” despite normal peripheral thyroid hormone levels (69). This concept remains speculative, because in animal studies, rodents with absent transthyretin maintain normal T3 and T4 levels in brain as a result of additional transport systems (70).
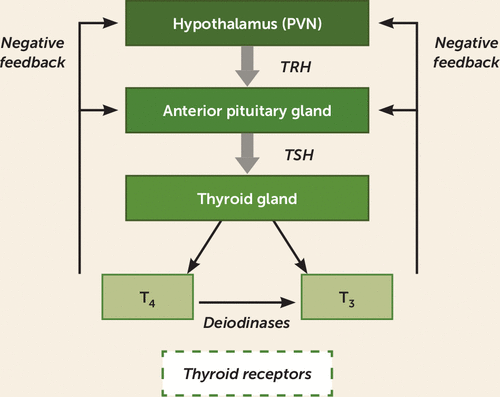
FIGURE 2. Hypothalamic-pituitary-thyroid (HPT) axisa
a In the HPT axis, the paraventricular nucleus of the hypothalamus (PVN) releases thyroid-releasing hormone (TRH), which stimulates the anterior pituitary to release thyroid-stimulating hormone (TSH) into the peripheral circulation. The thyroid gland then releases T4 and T3 into the circulation. Circulating T4 can also be converted to T3 via deiodinases. These hormones act on thyroid receptors in target organs and as a means of regulating negative feedback at the level of the hypothalamus and pituitary.
HPT Abnormalities in Major Depression
Clinical hypothyroidism is frequently associated with depressive symptoms (71), and subclinical hypothyroidism (defined as elevated serum TSH with normal serum T3 and T4 levels) is commonly reported in treatment-resistant depression (72, 73). However, most patients with depression do not have biochemical evidence of thyroid dysfunction (69, 74). Other reported abnormalities in depression include TSH levels in the “normal” but high range, low T3 levels, elevated T4 levels, elevated reverse T3 levels, a blunted TSH response to TRH, positive antithyroid antibodies, and elevated CSF TRH concentrations (69). Abnormalities in the HPT axis in depression have mostly been demonstrated in cross-sectional studies; data from larger longitudinal prospective studies are scarce, and findings have been inconsistent (75, 76). The literature suggests a link between subtle thyroid dysfunction and major depression, but more conclusive data are needed (77).
HPT Axis–Based Treatments for Major Depression
T3, T4, TRH, and TSH have all been investigated as potential treatments for major depression. Aside from T3, hormones of the thyroid axis either appear to lack efficacy or have not been well-studied in major depression. T4 has been studied, with promising results, in bipolar disorder, particularly in rapid-cycling bipolar disorder. A number of small placebo-controlled studies have evaluated intravenous and oral TRH administration, and the majority have not demonstrated efficacy for TRH in the treatment of depression (71). A 1970 study in women reported rapid augmentation of response with a tricyclic antidepressant (TCA) after intravenous administration of TSH compared with placebo (78), but no subsequent clinical trials have been reported.
Triiodothyronine (T3).
The bulk of evidence suggests that T3 has clinical utility in depression for two purposes: to accelerate antidepressant response when used with tricyclic antidepressants, and as an augmentation agent to treat depression in patients with insufficient response to antidepressant monotherapy.
Acceleration of antidepressant effect with tricyclic antidepressants
Research investigating this effect began with TCAs before the approval of the use of SSRIs, which occurred in the late 1980s. T3 treatment initiated within 5 days of initiating TCA treatment produced a faster onset of antidepressant response compared with the TCA alone. Evidence for this accelerated response comes primarily from a meta-analysis of six double-blind placebo-controlled studies (N=125) of T3 use with imipramine and amitriptyline (79). In the pooled analysis, T3 significantly accelerated the clinical response compared with placebo, with a moderate effect size (0.58). These trials were of short duration (less than 4 weeks), and the time to response was generally within 7–14 days in the T3 plus TCA treatment, compared with 21–28 days for the placebo plus TCA comparator. The typical dosage of T3 used in these studies was 25 μg/day. In three of the studies, T3 was discontinued after 2–4 weeks, but in all three studies, participants remained well after T3 discontinuation. Of note, the effect size for accelerated response with T3 in the meta-analysis increased as the percentage of women participating in the study increased, suggesting that women may be more likely than men to benefit from the addition of T3, a finding that warrants further investigation.
Evidence from randomized controlled trials indicates that this acceleration of antidepressant response is not generalizable to all classes of antidepressants: T3 accelerates responses to TCAs but does not appear to have this effect with SSRIs. A meta-analysis of four placebo-controlled randomized controlled trials of patients with major depression (N=444) found no evidence for a quicker response onset when T3 was used in combination with SSRIs (80). Reasons for the discrepancy between the effects of T3 with TCAs compared with SSRIs remain unknown.
Augmentation strategy for inadequate response to antidepressant monotherapy
Evidence for T3 augmentation efficacy with TCAs comes from a meta-analysis of eight controlled trials (N=292) in patients with major depression who did not experience remission with TCA treatment alone (81). Four of these studies used a randomized double-blind design; of the other four, three were unblinded studies with retrospective cohorts and one was a double-blind trial in which each patient served as his or her own control. Patients receiving T3 augmentation were twice as likely to respond to TCAs than those receiving placebo augmentation, with a 23% improvement in response rates. Improvements in depression scores were moderately large (effect size, 0.62). However, across studies, there was inconsistent evidence of efficacy, and study quality varied. Although the two larger double-blind randomized controlled trials (N=33 and N=38) were robustly positive in favor of T3 augmentation, pooled results of the four double-blind randomized controlled trials were not significant (p=0.29). One negative randomized controlled trial included in this analysis was particularly problematic because of a 2-week crossover design and unequal baseline depression severity in the randomized groups. This may partly explain the negative pooled results for the four double-blind randomized controlled trials. Among the trials included in the meta-analysis, only one established relative treatment resistance on the basis of an antidepressant treatment duration of 6 weeks, which is now considered the minimum duration for determining lack of response. In addition to the controlled trials, additional support for the use of T3 as augmentation with TCAs comes from five positive open-label studies with reported response rates greater than 50% (71).
Notably, no placebo-controlled trials have demonstrated the efficacy of T3 augmentation with SSRIs or serotonin-norepinephrine reuptake inhibitors in treatment-resistant depression. The only placebo-controlled trial to have examined this hypothesis was a small trial with a treatment duration of 2 weeks that reported negative results (82). Support for this approach for patients with treatment-resistant depression comes primarily from level 3 of STAR*D, in which response to T3 augmentation (up to 50 μg/day; N=73) was compared with lithium augmentation (up to 900 mg/day; N=69) or a new antidepressant monotherapy (mirtazapine or nortriptyline). All participants who entered level 3 had not experienced remission of depression after at least two different medication treatments. These participants did not respond with prospective citalopram treatment in level 1 and with medication switch (bupropion, sertraline, or venlafaxine) or medication augmentation (bupropion or buspirone) in level 2 (83). Remission rates were 25% with T3 augmentation and 16% with lithium augmentation after a mean treatment duration of about 10 weeks. This difference was not statistically significant. T3 had superior tolerability, as lithium was more frequently associated with side effects and greater rates of discontinuation.
The efficacy of T3 augmentation in depression was also demonstrated in a network meta-analysis of 48 randomized controlled trials investigating the efficacy of 11 different augmentation agents in treatment-resistant depression. These trials consisted of direct comparisons between the drugs as well as comparisons with placebo. Six trials of thyroid hormone augmentation (T3 or T4) were included. The odds of remission in treatment-resistant depression were three times greater with thyroid hormone augmentation compared with placebo, with comparable tolerability (84). Given the tremendous variability of the design of the T3 trials and their failure to establish treatment resistance, the results of the network analysis should be viewed cautiously.
A meta-analysis of four randomized controlled trials found no evidence that adding T3 to SSRIs enhanced the antidepressant effect of SSRI treatment in depressed patients who were not antidepressant treatment resistant (80). While the STAR*D findings suggest that T3 is beneficial as an augmentation agent with SSRIs in treatment-resistant depression, this benefit remains to be demonstrated in randomized placebo-controlled trials.
T3 has been reported overall to be safe and tolerable. Although a small number of participants in clinical trials have experienced side effects such as sweating, tremor, nervousness, and palpitations, T3 administration has been well tolerated without serious side effects (85). Thyroid monitoring guidelines proposed by Rosenthal et al. (65) for T3 augmentation recommend assessing TSH, free T4, and free T3 levels at baseline, at 3 months, and then every 6–12 months. Because hyperthyroidism can induce bone resorption leading to an increased risk of fractures, monitoring bone density every 2 years is recommended with use of thyroid hormones in postmenopausal women.
Thyroxine (T4).
Limited evidence from small open-label studies using T4 as an augmentation agent in major depression exists, but the efficacy of T4 remains to be established in placebo-controlled randomized controlled trials (86). While T4 is understudied in unipolar depression, supraphysiological doses of T4 (250–500 μg/day) have been investigated as maintenance treatment in rapid-cycling bipolar disorder as well as in treatment-resistant bipolar depression, with generally favorable results (87). Bauer and Whybrow (88) were the first to conduct an open-label trial of adjunctive supraphysiological doses of T4, in 11 patients with treatment-refractory rapid-cycling bipolar disorder, with improvement in both depressive and manic symptoms, and this finding was replicated in additional open-label studies (87). Recently, the first comparative double-blind placebo-controlled trial of T4 and T3 as adjunctive treatments was conducted in 32 patients with treatment-resistant rapid-cycling bipolar disorder (89). Participants in the T4 group spent significantly less time in a depressed or mixed state and greater time euthymic, whereas there were no significant differences with T3 and placebo (89).
Supraphysiologic doses of T4 have also been studied in open-label trials as adjunctive therapy for treatment-resistant bipolar depression, and beneficial effects have been noted on depressive symptoms, with response rates around 50% (87). A randomized double-blind placebo-controlled study of 300 μg of T4 as an adjunct to mood stabilizers and/or antidepressants in bipolar depression (N=62) showed improvements in depression, but the overall results were not statistically significant because of a high placebo response rate (90). Significant improvement in depression compared with placebo, however, was noted in a secondary analysis of female participants.
Overall, these studies show promising evidence for adjunctive use of supraphysiological doses of T4 in rapid-cycling bipolar disorder (87), while more research is needed for use of T4 in acute bipolar depression.
HPT axis: conclusions.
Evidence from randomized controlled trials with TCAs and from STAR*D suggests clinical benefits of T3 as an augmentation agent with antidepressants in patients with major depression who have not responded to antidepressant monotherapy. This conclusion is consistent with practice guidelines for the pharmacological treatment of major depression from the American Psychiatric Association, the Canadian Network for Mood and Anxiety Treatments, and the World Federation of Societies of Biological Psychiatry, which recommend augmentation of antidepressants with thyroid hormones as a treatment option in cases where monotherapy has failed (91–93). Clinicians also recommend using thyroid hormone supplementation in patients with depression who do not respond, especially in patients with TSH levels in the high normal range (94). Researchers should aim to demonstrate, in future placebo-controlled trials, the efficacy of T3 as an augmentation agent with SSRIs in treatment-resistant depression. In addition to use as augmentation, evidence from multiple randomized controlled trials supports use of T3 for acceleration of antidepressant response with TCAs, with the important caveat that this acceleration of antidepressant response has not been observed in randomized controlled trials with SSRIs. Also, promising evidence supports adjunctive use of supraphysiological doses of T4 in rapid-cycling bipolar disorder, and emerging evidence supports use of T4 in bipolar depression.
Hypothalamic-Pituitary-Gonadal (HPG) Axis: Ovarian Hormones
Overview of Physiology
In men and women, the major hypothalamic driver of the HPG axis is the pulsatile secretion of gonadotropin-releasing hormone (GnRH), released from neurons residing in the medial preoptic area (Figure 3A). In response to GnRH, the anterior pituitary releases luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the bloodstream, stimulating the ovaries to produce estradiol and progesterone, and the testicles to produce testosterone (Figure 3B). As is common in endocrine systems, these hormones regulate axis activity via feedback inhibition, with estradiol, progesterone, and testosterone inhibiting HPG axis activity at both the level of the hypothalamus and the pituitary (95). The function of these sex hormones is also subject to modulation by binding to albumin and more specific binding proteins, which control hormone access to tissues and receptors (96). In women, a complex feedback interplay regulates the approximately 28-day menstrual cycle (Figure 3C), characterized by 1) a follicular phase of approximately 14 days in which LH and FSH levels are relatively low and estradiol steadily climbs; 2) a short ovulation phase triggered by a burst of GnRH release and rapid rise of LH, triggering the release of an ovarian follicle; and 3) a 14-day luteal phase in which LH and FSH return to relatively low levels and the corpus luteum of the ovary produces increased estradiol and progesterone, stimulating proliferation of the endometrial lining. In the absence of pregnancy, estradiol and progesterone levels decline near the end of the luteal phase, and menses proceeds. As discussed below, periods of significant hormonal change, including the monthly end of the luteal phase, and more specific endocrine events, including puberty, pregnancy, and menopause, are associated with mood changes and increased risk for affective disorders.
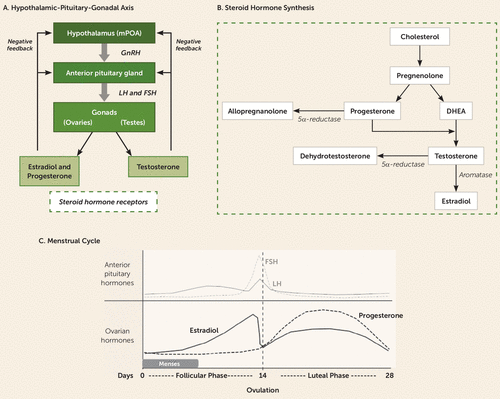
FIGURE 3. Hypothalamic-pituitary-gonadal (HPG) axis, steroid hormone synthesis, and the menstrual cyclea
a As shown in panel A, in the HPG axis, the medial preoptic area of the hypothalamus (mPOA) releases gonadotropin-releasing hormone (GnRH), which stimulates the anterior pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the peripheral circulation. These factors stimulate the gonads to produce sex hormones, with the ovaries producing estradiol and progesterone and the testes producing testosterone. Panel B summarizes steroid hormone synthesis: Cholesterol is the precursor molecule to sex hormones, which can undergo a variety of enzymatic transformations. DHEA=dehydroepiandrosterone. As shown in panel C, after the menstrual cycle begins at day 0 with the occurrence of menses, estradiol gradually climbs during the follicular phase (days 0 through 14 of a 28-day cycle). Ovulation is triggered by the spike in anterior pituitary production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). During the luteal phase (days 14 through 28), progesterone and, to a lesser extent, estradiol rise. The production of both hormones then begins to fall toward the end of the luteal phase, a time of vulnerability for premenstrual dysphoric disorder symptoms. In the absence of pregnancy, falling ovarian hormone levels lead to the shedding of the uterine lining with menses, beginning the cycle again.
The actions of estradiol are primarily mediated by two nuclear estrogen receptors (ERs), ERα and ERβ, which modulate gene transcription by binding to estrogen response elements on DNA (97). There is also some evidence for fast-acting membrane-bound estrogen receptor signaling (98). ERs are expressed in the brain in males and females with largely similar distributions, although with higher receptor numbers in females (99). ERα is thought primarily to mediate reproductive functions given its high expression in a number of hypothalamic nuclei (100). Preclinical gene knockout studies suggest that ERβ may regulate estradiol-mediated effects on HPA activity and mood, via its expression in the PVN and limbic system (100).
In addition to HPG axis regulation, progesterone also has important CNS effects. Like estradiol, progesterone acts both through classic nuclear receptor signaling (progesterone receptors [PRs]) and also likely via fast-acting membrane-bound receptors (101). PRs are found in the hypothalamus, hippocampus, cortex, and amygdala, and in general PR activation is thought to oppose the actions of estradiol (102). Progesterone has been largely considered neuroprotective, in part via PR-mediated stimulation of growth factors and activation of glial cells (101). Although a relatively large number of animal studies and small clinical trials have examined progesterone’s effects in the context of stroke and traumatic brain injury, two recent large randomized controlled trials showed no protective effects after brain injury (103, 104). Within psychiatry, there has been a greater focus on the actions of the progesterone metabolite allopregnanolone. Allopregnanolone is a neuroactive steroid that acts as a positive allosteric modulator at synaptic and extrasynaptic GABAA receptors. It has been implicated as a mediator of many of the central effects attributed to progesterone and is being actively investigated in clinical trials (see next section).
Ovarian Hormone Abnormalities in Major Depression
Considerable clinical evidence demonstrates that fluctuations in ovarian hormones are associated with increased risk of depressive states. Women experience depression at nearly twice the rate of men, a difference that is apparent only during the reproductive years (105). Some women experience severe affective symptoms during the late luteal phase of their monthly cycle as progesterone and its metabolite, allopregnanolone, progressively rise, then rapidly fall (see Figure 3C), a syndrome termed premenstrual dysphoric disorder (PMDD) (106). An increased risk of mood symptoms and major depressive disorder occurs during other periods of ovarian hormone withdrawal, including the postpartum period and menopause. These findings are complemented by preclinical studies showing enhanced depressive-like behaviors in rodents during low-estradiol times of the cycle (107), increased behavioral despair following ovariectomy that can be rescued with estradiol administration (108), and the ability of estradiol to enhance the release of monoamines (109).
However, the impact of ovarian hormones on mood is complex, as demonstrated in the literature on oral contraceptives, which are commonly prescribed for pregnancy prevention or to normalize irregular menstrual cycles. Although oral contraceptives have long been linked with the potential to alter mood, there has been significant controversy over whether they worsen mood, improve mood, have more complex mood effects, or are mood neutral. Two large prospective studies examining health records of more than 1 million Danish women over 10 years showed that oral contraceptive use is associated with increased risk for subsequent antidepressant treatment, depression diagnosis, and suicidal acts, with significant risk for all hormone preparations (larger effects in progestin-only products, transdermal patches, and vaginal rings compared with combination oral preparations), as well as pronounced effects in the adolescent age group (110, 111). In contrast, a smaller cross-sectional study described an increased risk of mood disorders with progestin-only oral contraceptives but a decreased risk of mood disorders when women using combined estrogen-progestin oral contraceptives were compared with those taking no contraception (112). A systematic review of studies of progestin-only contraception, however, reported no consistent evidence of association with depressive symptoms across 26 studies, most of which were deemed by the authors as low quality or at significant risk of bias (113). A randomized controlled trial of 178 women from a community sample (<9% meeting criteria for a depressive disorder or currently on antidepressants) described an even more nuanced picture, in which oral contraceptives were associated with a worsening of mood symptoms in the intermenstrual phase in a subset of women, but an improvement in mood during the premenstrual phase (114). Despite these changes in mood symptoms, no difference was detected between oral contraceptives and placebo in the emergence of clinical depression as assessed with the Montgomery-Åsberg Depression Rating Scale (114). The literature is further complicated by differences in dosing schedules (e.g., monophasic versus triphasic oral contraceptive preparations), and it has been suggested that regimens with more constant hormone distributions may carry less risk of adverse mood effects (115).
The impact of oral contraceptives may also depend on the endocrine context. For example, women with polycystic ovary syndrome, a disorder characterized by polycystic ovaries, elevated androgen output, and irregular menstruation, have higher rates of depression and anxiety (116), and oral contraceptives are associated with an improvement in depressive symptoms and health-related quality of life (117). Taken together, these complicated data, which are limited by high interstudy variability in hormone preparation, dosing schedule, population studied, and mood measures, suggest that ovarian hormones can have a significant impact on mood but point to an intricate underlying biology that is sensitive to age, timing within the menstrual cycle, and administered hormone (estrogens versus progestins).
Ovarian Hormone Treatments for Major Depression
The ovarian hormone treatment literature is complicated by differences in study populations, with some trials examining affective symptoms in euthymic women who receive hormones for medical indications and other trials that directly study hormone-based interventions in psychiatrically ill populations (generally women with major depression, postpartum depression, or PMDD). Many different ovarian hormone–based strategies have been evaluated for their effects on depressed mood. Treatment guidelines suggest that suppression of ovulation (118) via oral contraceptives (with specific evidence for those containing the antiandrogenic progestin, drospirenone, with a 4-day pill-free interval [119, 120], and mixed evidence for other oral contraceptives [121]) and GnRH agonists (e.g., leuprolide [122]) may be effective in the treatment of PMDD, although compared with SSRIs, these treatments are considered second and third line, respectively (123).
An interesting literature reports on the use of selective estrogen receptor modulators (SERMs) in psychiatric disorders. SERMs (e.g., tamoxifen, raloxifene) act as estrogen receptor agonists in some tissues and antagonists in others, and it was hoped that they would provide some of the benefits of estrogen replacement therapy with reduced risks (e.g., endometrial and breast cancer risk). Their pharmacology, however, is complex, and SERMs also carry significant risks. For example, while tamoxifen decreases ER-positive breast cancer risk as a result of ER antagonism, it carries a risk of uterine cancer as a result of endometrial ER agonism (124). Raloxifene received a U.S. Food and Drug Administration (FDA) black box warning for increased risk of deep vein thrombosis, pulmonary embolism, and death due to stroke in at-risk postmenopausal women. While the evidence for SERMs in the treatment of major depression is equivocal, evidence is developing for use of SERMs as an adjunctive treatment for mania (125) and psychosis (126) in women and men. These antimanic and antipsychotic actions are thought to result from SERM inhibition of the protein kinase C second messenger cascade, and a number of placebo-controlled clinical trials are under way to examine their use for acute mania and schizophrenia.
Oxytocin, which is not an ovarian hormone per se, but is a neuropeptide that plays important roles in postpartum bonding and social cognition more broadly, has also been studied in major depression. Although preclinical work has suggested that oxytocin is a promising target, clinical trials have not yielded convincing results for the treatment of major depression or postpartum depression, although it is still being actively studied in these disorders.
Here we will focus in more depth on the use of estrogen replacement therapy, given its clinical relevance in current psychiatric practice, as well as the emerging study of the progesterone-derived neurosteroid allopregnanolone in PMDD, postpartum depression, and a variety of other psychiatric conditions.
Estrogen replacement therapy (ERT) or hormone replacement therapy (HRT; estrogen and progestin) in peri- or postmenopausal major depression.
Hormone replacement therapy is FDA approved to treat the vasomotor symptoms (hot flashes) and vulvovaginal atrophy associated with menopause. Women who have received a hysterectomy may take oral estrogen replacement therapy, whereas women with an intact uterus must take hormone replacement therapy (estrogen plus a progestin) to mitigate endometrial cancer risk posed by unopposed estrogen. Clinical trials examining mood in peri- and postmenopausal women treated with hormone replacement have yielded mixed results for several reasons. One complicating factor is the difference in psychiatric symptomatology across studies, with the majority of studies assessing one or two mood symptoms in psychiatrically asymptomatic women (often excluding those with major depressive disorder), and a minority of studies examining effects in women meeting criteria for major depressive disorder or dysthymia (109). A second complicating factor is treatment timing in relationship to menopause onset. Perimenopause appears to be a distinct neuroendocrine state compared with postmenopause, with different ERT/HRT risks and benefits, depending on the timing of hormone administration. This differential risk was initially described for cardiovascular risk in the exhaustive analyses of the large Women’s Health Initiative study and in subsequent trials (127). In the Early Versus Late Postmenopausal Treatment With Estradiol (ELITE) trial, a cardioprotective effect of ERT/HRT was observed in early menopausal women (<6 years after menopause), but a risk-enhancing effect was found in women >10 years after menopause (128). ERT/HRT timing is important not only for understanding the general medical risks of treatment, but it also appears to influence the efficacy of these treatments for depressive symptoms. Additional complicating factors in studies of perimenopausal women include the spontaneous return of ovarian function (adding temporary, unpredictable endogenous sources of estradiol) and the co-occurring symptoms of menopause (e.g., hot flashes and sleep disturbance), which can have their own impact on mood and may be responsive to HRT (129).
Relatively few studies have directly tested ERT/HRT as an antidepressant monotherapy in peri- or postmenopausal women with major depression, comprising only five of the 24 trials assessing mood that were included in a recent meta-analysis (109). Of these five trials conducted in depressed, unmedicated women, two were considered to be at high risk of bias. Both potentially biased studies were conducted in younger postmenopausal women and included one positive trial (N=129) with high attrition rates (32% in the HRT group and 57% in the placebo group) (130) and one negative trial that had baseline differences in presence of prior episodes of major depression (N=57; HRT did not separate from placebo, although both groups improved) (131). Of the three higher-quality studies in depressed women, two (132, 133) showed antidepressant efficacy of ERT (transdermal patches) compared with placebo in perimenopausal women (N=34 and N=50). The third examined a mixed population of peri- and postmenopausal women and showed that increased estradiol levels (spontaneously occurring or due to ERT) were associated with depression improvement in perimenopausal women (N=72) but not postmenopausal women, although both groups improved symptomatically compared with the placebo group (129).
ERT has also been evaluated as an adjunctive treatment to SSRIs. In a retrospective analysis of a multisite randomized controlled trial of fluoxetine in participants with geriatric depression, women who were receiving ERT (not as a randomized intervention, N=72) showed greater improvement on fluoxetine than those who were not receiving ERT (N=295) (134). Small studies prospectively assessing ERT in conjunction with an antidepressant in postmenopausal women have shown either no effect (135) or an acceleration, but not an augmentation effect (136). These studies warrant cautious interpretation given the limitations of a high dropout rate (one-third of the sample [135]) and baseline differences in age and depression severity between treatment groups (136). While the evidence base is relatively small, these studies suggest that ERT/HRT may have some antidepressant efficacy in perimenopausal women, with less convincing data for postmenopausal women.
Studies examining mood after ERT/HRT in women without psychiatric illness comprise 19 of the 24 trials described in the recent meta-analysis (109). The overall grade of the evidence in this meta-analysis was a C (i.e., low-quality evidence), and in these 19 trials evaluating mood in nondepressed women, there was little evidence of benefit, particularly in women without other physical symptoms of menopause (109). In contrast to the conclusion of the meta-analysis, two more recent trials suggested some benefit or protective effects of ERT/HRT in these groups. The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women in the community over 4 years who received oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo. Women with clinical depression, defined as a score >28 on the Beck Depression Inventory, were excluded, but women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms (effect size, 0.49) and anxiety (effect size, 0.26) were observed in the oral estrogen plus progesterone group compared with the placebo group over the course of 4 years of treatment, whereas the transdermal estrogen group did not separate from placebo (137). Another study of 172 euthymic peri- and postmenopausal women found that those in early menopausal transition (but not those in late transition or postmenopausal) treated with 12 months of transdermal estrogen plus oral progesterone had a lower risk of developing depressive symptoms (138). These benefits were moderated by the number of stressful life events experienced over the preceding 6 months, with greater antidepressant effects in those women with the highest number of stressful life events (138).
Taken together, this complex literature suggests with some confidence that ERT/HRT interventions are most likely to be successful when implemented early in the transition to menopause. The most clear-cut indication for the use of HRT is for perimenopausal women experiencing depression who also are experiencing significant physical symptoms of menopause (e.g., hot flashes, vaginal dryness), for which time-limited HRT is already FDA approved. The use of ERT/HRT in late or postmenopausal women has little evidence for efficacy and is associated with increased risk for cardiovascular events (as opposed to a protective cardiac risk profile in perimenopausal women) (127), and therefore should be avoided. The more ambiguous cases are those of perimenopausal women who are depressed but do not have FDA-approved symptoms for HRT (some evidence for antidepressant efficacy has been reported in this group [109]). Although some studies suggest that HRT is a preventive strategy for developing depression in perimenopausal women (138), more evidence is needed (139).
Progesterone and its neurosteroid derivative, allopregnanolone.
Progesterone and its metabolite allopregnanolone have been implicated in hormone-related mood disorders, including PMDD and postpartum depression, resulting in multiple clinical trials targeting this system. Allopregnanolone is a neurosteroid that is converted from progesterone by α-reductase enzymes (Figure 3B). At physiologic concentrations, allopregnanolone acts as a positive allosteric modulator of the GABAA receptor, increasing conductance through synaptic and extrasynaptic GABAA brain receptors with a potency similar to lorazepam (140). At high concentrations, it can also directly activate GABAA receptors (141). Fluctuations in progesterone (e.g., the rapid decrease during the postpartum period, or the increase over the course of the luteal phase of the menstrual cycle) are paralleled by changes in allopregnanolone levels and have been implicated in postpartum depression and PMDD (142). Preclinical studies have shown allopregnanolone to have significant antianxiety and antidepressant properties, as well as the ability to suppress HPA axis activity (reviewed in 142). Because exogenously administered allopregnanolone has poor bioavailability, clinical trials have relied on allopregnanolone-like small molecules (e.g., brexanolone, ganaxolone, sepranolone) delivered intravenously or, to a lesser extent, on α-reductase enzyme modulators.
Treatment studies in PMDD have focused on preventing the increase in allopregnanolone that occurs during the mid to late luteal phase, when women are symptomatic. This approach (i.e., decreasing allopregnanolone) is somewhat counterintuitive given the preclinical data describing its antidepressant-like properties. However, preclinical data suggest that allopregnanolone may paradoxically promote anxiety-like behavior in some contexts (e.g., adolescence), which may be related to changing subunit compositions of the GABAA receptor (143). Women with PMDD have been hypothesized to possess a differential sensitivity to allopregnanolone, likened to subsets of patients in whom benzodiazepines can cause paradoxical agitation (144). Although the mechanism is not entirely clear, there has been some success in PMDD trials in which the actions of allopregnanolone are inhibited. A small crossover study (16 women with PMDD, 16 healthy control subjects) showed that the 5α-reductase inhibitor dutasteride, which prevents the conversion of progesterone to allopregnanolone, decreased PMDD symptoms and had no mood effects in the healthy control group (145). Dutasteride was well tolerated, and while it prevented the luteal phase increase in plasma allopregnanolone, it did not significantly change luteal plasma progesterone levels. A second trial with 60 women with PMDD evaluated a novel compound, sepranolone, which acts as a steroid antagonist at the GABAA receptor. Women who received five daily subcutaneous injections of sepranolone exhibited significant improvement in PMDD symptoms compared with women who received placebo, and the medication was well tolerated (146).
In contrast to PMDD, strategies for the treatment of postpartum depression have focused on augmenting allopregnanolone signaling to stabilize the reduction in this neurosteroid that occurs in the postpartum period. There is considerable excitement about the recent success of brexanolone, an intravenous stabilized form of allopregnanolone, which was recently approved by the FDA for the treatment of postpartum depression. A small open-label pilot study (147) and a small placebo-controlled randomized controlled trial (N=10–11 per group) (148), followed by two larger multisite trials (together totaling 246 patients), showed that a 60-hour infusion of brexanolone, where dosing is designed to approximate the range of third-trimester allopregnanolone levels, resulted in significant reductions in depressive symptoms in women with severe postpartum depression (149). Remission was rapid, with separation from placebo evident by 24 hours, and the effect of this single treatment was sustained up to 30 days, the longest interval assessed. Given that SSRIs, which can take several weeks to be effective, are the current first-line treatment for postpartum depression, the rapid antidepressant effects of brexanolone may provide symptom stabilization during the critical early phases of the postpartum period. Limitations to intravenous brexanolone include side effects of dizziness, syncope, and sedation, which necessitate administration in a supervised inpatient setting for the full 60-hour infusion.
Neurosteroids are also being evaluated in men and women with other neuropsychiatric disorders, including treatment-resistant major depression, bipolar depression (150), the negative symptoms of schizophrenia (151), posttraumatic stress disorder (152), and epilepsy (153). Thus, neurosteroids represent an active area of drug development for psychiatry, with promising data for disorders associated with clear hormonal underpinnings (e.g., PMDD and postpartum depression) and a less established, but growing, evidence base for other psychiatric disorders.
Ovarian hormone treatment: conclusions.
Ovarian hormones have provided the basis for several treatments, some of which are in active clinical use and others that are promising but require more evidence. The use of time-limited ERT/HRT (<5 years) is reasonable in perimenopausal women with physical symptoms of menopause (vasomotor symptoms, vaginal dryness) in addition to depression. A trial of ERT/HRT, either alone or in conjunction with an SSRI, may also be reasonable in perimenopausal women with depression who do not have significant physical symptoms. It is not currently recommended that ERT/HRT be prescribed in euthymic perimenopausal women for depression prophylaxis, although there are some data to support a positive impact on mood in this population. For PMDD, the first-line treatment remains SSRIs, but oral contraceptives are a reasonable second-line treatment. There are strong positive data for GnRH agonists (e.g., leuprolide), which act by suppressing the HPG axis, although they should be considered only after a patient has not obtained adequate benefit from other treatments, including psychotherapy. Use of neuroactive steroids, that is, manipulation of the allopregnanolone system, presents an active, exciting area of new treatment development, which includes both inhibition of the system for the treatment of PMDD and augmentation of allopregnanolone for treating postpartum depression. Intravenous brexanolone, recently FDA approved for postpartum depression, presents an exciting treatment opportunity for severe peripartum depression because of its rapidity of action. Finally, SERMs, such as tamoxifen and raloxifene, and allopregnanolone derivatives are being investigated in men and women for a variety of disorders, including schizophrenia and bipolar disorder. While these treatments are not suitable for routine clinical use, the field is moving rapidly, and in the near future there will likely be a better evidence base for clinical decision making in relation to their use.
Hypothalamic-Pituitary-Gonadal (HPG) Axis: Testicular Hormones
Overview of Physiology
In men, as in women, hypothalamic GnRH stimulates the pituitary gland to secrete LH and FSH, although the targets of these gonadotropins are sex specific (Figure 3A). In males, LH stimulates Leydig cells to produce testosterone, and FSH acts on Sertoli cells to stimulate sperm production. In addition to the gonads and adrenal glands, active biosynthesis of testosterone occurs in the brain. This can be de novo synthesis from cholesterol or conversion from dehydroepiandrosterone (DHEA) or progesterone (Figure 3B) (154). Testosterone binds to androgen receptors, which, like estrogen receptors, have both nuclear actions to regulate gene expression and rapid, second-messenger-dependent membrane signaling (154). Some 1%−4% of testosterone circulates in the free, unbound form, 33%–54% circulates bound with low affinity to serum albumin, and the remainder is primarily bound to sex hormone–binding globulin (155). Both unbound and albumin-bound testosterone are considered biologically active (155). Serum total testosterone levels <270 ng/dL are considered low, and values between 270 and 300 ng/dL may or may not be considered low, depending on the laboratory.
DHEA and its sulfate ester, DHEA-S, are among the most abundant steroid hormones in the human body. They are produced in the adrenal cortex and the gonads as well as the brain, suggesting important functions as a neurosteroid, although these functions are poorly understood (156). In addition, DHEA can serve as a precursor to testosterone and estrogen. DHEA is known to have antiglucocorticoid and anti-inflammatory effects and has also been implicated in neuroprotection and catecholamine synthesis (156).
Testicular HPG Axis Abnormalities in Major Depression
Depressive symptoms are common in men with clinical hypogonadism, and a number of studies suggest an association of lower testosterone levels with depressive symptoms in men (157). Men with major depressive disorder have lower total and free testosterone levels than nondepressed control subjects in both middle age and later years (158, 159). Depressive symptoms are common in men referred with borderline testosterone levels (160), and middle-aged nondepressed men in the community with low testosterone levels have a higher likelihood of developing depression (161). The prevalence of categorically low testosterone levels in major depression or treatment-resistant major depression has not yet been studied systematically in the general outpatient psychiatric population (157). At this time, there is no strong evidence supporting routine screening for low testosterone levels in depressed men in the absence of other clinical signs of hypogonadism (157, 162).
HPG Axis–Based Treatments for Major Depression
The interpretation of clinical trials assessing the efficacy of testosterone in the treatment of depression is complicated by the heterogeneity of patient populations in regard to 1) presence or absence of testosterone deficiency syndrome (unequivocally low testosterone levels in the presence of symptoms such as low libido, erectile dysfunction, lethargy, etc.); 2) testosterone level range: normal versus low or borderline-low levels; 3) major depression versus subthreshold depressive symptoms or dysthymia; 4) presence of antidepressant treatment resistance; and 5) HIV status.
Three meta-analyses have been conducted investigating testosterone efficacy for depressive symptoms, and all three have reported a significant positive effect compared with placebo (163–165). The largest of these meta-analyses (164) included 27 randomized controlled trials in which testosterone treatment was utilized and depressive symptoms were monitored using a validated scale. Testosterone treatment was associated with a significant reduction in depressive symptoms compared with placebo, although the effect size was small, with a Hedges’ g of 0.21 (95% CI=0.10, 0.32). Only eight of the trials included participants with a diagnosed depressive disorder. The remainder included populations such as healthy men, older men, patients with AIDS wasting syndrome, and patients with cognitive disorders. While these meta-analyses indicate that testosterone appears to have a small antidepressant effect, their validity is questionable given the heterogeneous patient populations and study designs. These meta-analyses pooled subjects treated with differing interventions (testosterone or DHEA), subjects with differing depressive diagnoses (major depression, dysthymia, subthreshold depressive symptoms, and no depression diagnosis), subjects with variable severity of depressive symptoms, subjects with variable levels of serum testosterone, subjects with and without symptoms of testosterone deficiency, and subjects with comorbid medical disorders such as HIV, Alzheimer’s disease, and metabolic syndrome. Given this heterogeneity, the overall positive results of these meta-analyses do not provide strong support for the use of testosterone in depressive disorders in general. Evidence for efficacy must be established separately for different patient populations.
There is evidence to suggest that testosterone replacement therapy improves depressive symptoms in men with testosterone deficiency syndrome. For example, in an 8-month prospective placebo-controlled trial involving 106 men with testosterone deficiency syndrome and depression of moderate severity, testosterone replacement monotherapy was associated with significant improvement in depression scores compared with placebo (166). A meta-analysis of 87 randomized controlled trials of testosterone therapy in hypogonadal men reported that testosterone replacement therapy improved depressive symptoms significantly, with a standardized mean difference of −0.23 (95% CI=−0.44, −0.01) (167).
However, in the absence of clinical hypogonadism, testosterone is not indicated for the treatment of major depression. Five randomized placebo-controlled clinical trials utilizing testosterone as a treatment for depression in men with major depression have been conducted (168–172) (the studies are summarized in Table S2 in the online supplement). A small 8-week pilot study of men with treatment-resistant major depression was robustly positive in favor of testosterone (170), but four subsequent randomized controlled trials in the treatment of major depression were all negative, including the largest trial, with 100 participants (168, 169, 171, 172). These latter trials were mostly conducted in men with low or borderline-low testosterone levels (levels ≤350 ng/dL), using testosterone as an adjunct to antidepressants in treatment-resistant major depression. The duration of the testosterone trials ranged from 6 to 12 weeks. Taken together, the results from these trials argue against testosterone’s efficacy as an augmentation agent in treatment-resistant major depression.
Although results in major depression have been disappointing, two small randomized controlled trials of testosterone monotherapy conducted in men with subthreshold depression (dysthymia or minor depression) reported significant and robust improvement in symptoms. One of these trials was conducted in men with midlife-onset dysthymic disorder (N=23) with low or low-normal testosterone levels (173), and the other was conducted in men with dysthymia or minor depression (N=33) with low testosterone levels (174). The results are far from conclusive but suggest that testosterone may be an effective treatment for depressive symptoms in nonmajor depression.
Testosterone has also been investigated as a treatment for depressive symptoms in patients with HIV and AIDS. Testosterone deficiency is a common endocrine abnormality associated with HIV infection in men, and testosterone levels decline as the illness advances. Although preliminary studies suggested that testosterone may be beneficial for depression in these patients (175, 176), a subsequent larger randomized controlled trial (N=123) comparing testosterone, fluoxetine, and placebo in HIV-positive patients with depressive disorders reported no significant differences in mood improvement among the three groups (177).
In addition to potential clinical benefits, it should also be taken into account that use of testosterone therapy, especially long-term use, can increase the risk of prostate cancer, polycythemia, and venous thromboembolism (178). Breast cancer, polycythemia, prostate cancer, and elevated serum prostate-specific antigen are considered absolute contraindications to testosterone therapy (178).
DHEA has been studied as an antidepressant treatment and has shown efficacy in two small randomized controlled trials. In contrast to testosterone trials, which were conducted only in men, these trials included male and female participants. One trial was restricted to patients with major depression (179), and the other included participants with midlife-onset major and minor depression (180). In addition, in one placebo-controlled trial, DHEA showed efficacy for treatment of nonmajor depression in HIV-positive patients (181).
In conclusion, preliminary evidence suggests that testosterone improves depressive symptoms in men with testosterone deficiency syndrome. Small trials subject to effect size inflation have suggested possible efficacy in men with low and low-normal testosterone levels who have nonmajor depression (dysthymia and subthreshold depressive symptoms). However, the bulk of the evidence suggests that testosterone does not have efficacy in treatment-resistant major depression as an adjunct to antidepressants, and its routine use would not be clinically appropriate. Conclusive evidence is lacking regarding testosterone’s antidepressant efficacy in HIV-positive patients. In addition to testosterone, there is also preliminary evidence of DHEA’s efficacy in midlife major and minor depression.
Conclusions and Future Directions
A long history of clinical experience and preclinical investigation has implicated three major endocrine systems—the HPA, HPT, and HPG axes—in the pathophysiology of mood disturbances and major depression. Since the late 1960s, these observations have prompted clinical trials testing hormones, peptides, and small molecules that target these systems, in an effort to produce novel antidepressants. Although these efforts have resulted in some successes, there have also been a significant number of disappointments and failures to replicate early successes in larger cohorts, for example, with CRFR1 antagonists. Some of these difficulties may relate to the heterogeneous nature of depression and the likely existence of subtypes within the larger clinical construct of major depression. Indeed, older schemas of depression subtypes (e.g., melancholic or psychotic) have been somewhat helpful in identifying populations enriched for HPA axis abnormalities, and the search for more modern ways of subdividing patients (using the Research Domain Criteria, SNPs, inflammatory markers, neuroimaging biotypes, etc.) is an active area of study. Future studies might use features of endocrine alterations to specifically enrich study populations, moving in the direction of personalized medicine. Other special considerations when evaluating these treatments include age and sex. While sex may be an obvious selection criterion for sex-specific disorders (e.g., postpartum depression or PMDD), sex may also influence other hormonal systems and their sensitivity to manipulation. For example, there are some data to suggest that thyroid-based interventions, such as T3 acceleration of TCAs, have greater effects in women, although this hypothesis has not yet been directly tested. Finally, endocrine status is an important factor to consider in patient selection, for example, whether the patient has a primary endocrine syndrome that includes depressed mood, a subclinical endocrine abnormality in the setting of depressed mood, or major depression without primary endocrine disturbances. Depressive symptoms frequently occur as part of a syndromal hormonal alteration, such as in Addison’s disease, Cushing’s syndrome, hypothyroidism, and hypogonadism. When the primary endocrine disturbance is corrected, affective and cognitive symptoms frequently normalize along with the other signs and symptoms of the syndrome. When depressive symptoms coexist with subclinical alterations in hormonal axes, in general it is unclear whether the depressive symptoms are caused by the subclinical alterations or occur independently of them. It is also important to recognize that hormonal treatments for major depression can be effective in the absence of any apparent endocrine abnormality, and most of the trials reviewed here have utilized high pharmacological doses (instead of low physiologic replacement doses) in individuals who do not have apparent endocrine deficiencies. Antidepressant mechanisms of hormonal treatments are not solely related to their actions on “classical” endocrine axes. Hormone receptors are distributed throughout the CNS, often within brain circuitry related to emotion and cognition, and the actions of hormones there are likely independent of their traditionally described endocrine roles.
Despite the inconsistency and lack of convincing evidence for some treatments, we identify some actionable clinical conclusions (Table 1), as well as exciting research avenues. Relatively well established interventions, other than correcting obvious endocrine syndromes, include use of T3 with TCAs for acceleration and augmentation of antidepressants and use of T3 augmentation with SSRIs in treatment-resistant depression; ERT/HRT in perimenopausal or early postmenopausal women who are also experiencing physical complaints related to menopause (e.g., hot flashes, vulvovaginal atrophy); and oral contraceptives as second-line therapy after SSRIs in PMDD. Rapidly developing areas of highly promising research include mifepristone for the treatment of major depression with psychotic features and progesterone-derived neurosteroid modulation to decrease or stabilize allopregnanolone to treat PMDD and to enhance allopregnanolone signaling in postpartum depression, particularly as a rapid-acting option when waiting for an SSRI to take effect. Given the evidence, we believe that several of the strategies discussed here could be useful for patients who have not responded to first-line treatments. However, it is important to underscore that while potentially useful, these medications (with the exception of intravenous brexanolone for postpartum depression) do not have FDA-approved indications for major depression. Over the next several years, the field will have a better sense of whether these exciting preliminary findings can be replicated in larger samples and applied to clinical practice.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5). Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76:155–162Crossref, Medline, Google Scholar
3 : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
4 : Sex and depression in the National Comorbidity Survey, I: lifetime prevalence, chronicity, and recurrence. J Affect Disord 1993; 29:85–96Crossref, Medline, Google Scholar
5 : The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105Crossref, Medline, Google Scholar
6 : The increasing burden of depression. Neuropsychiatr Dis Treat 2011; 7(suppl 1):3–7Medline, Google Scholar
7 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
8 : The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006; 8:383–395Medline, Google Scholar
9 : Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213:1394–1397Crossref, Medline, Google Scholar
10 : Arginine vasopressin (AVP): a review of its historical perspectives, current research, and multifunctional role in the hypothalamo-hypophysial system. Pituitary 2016; 19:345–355Crossref, Medline, Google Scholar
11 : Regulation of cortisol bioavailability: effects on hormone measurement and action. Nat Rev Endocrinol 2012; 8:717–727Crossref, Medline, Google Scholar
12 : Vasopressin stimulates cortisol secretion from human adrenocortical tissue through activation of V1 receptors. J Clin Endocrinol Metab 1993; 76:1522–1528Medline, Google Scholar
13 : Recent advances in the molecular mechanisms determining tissue sensitivity to glucocorticoids: novel mutations, circadian rhythm, and ligand-induced repression of the human glucocorticoid receptor. BMC Endocr Disord 2014; 14:71Crossref, Medline, Google Scholar
14 : Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011; 14:398–406Crossref, Medline, Google Scholar
15 : The acute effects of a mineralocorticoid receptor (MR) agonist on nocturnal hypothalamic-adrenal-pituitary (HPA) axis activity in healthy controls. Psychoneuroendocrinology 2007; 32:859–864Crossref, Medline, Google Scholar
16 : Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol 2016; 28 (doi: 10.1111/jne.12379)Crossref, Medline, Google Scholar
17 : Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010; 151:4811–4819Crossref, Medline, Google Scholar
18 : Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci 2010; 30:6106–6115Crossref, Medline, Google Scholar
19 : Glucocorticoid signaling: an update from a genomic perspective. Annu Rev Physiol 2016; 78:155–180Crossref, Medline, Google Scholar
20 : Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol 2001; 124:152–165Crossref, Medline, Google Scholar
21 : The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 2008; 149:5482–5490Crossref, Medline, Google Scholar
22 : Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 2006; 147:4917–4930Crossref, Medline, Google Scholar
23 : Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 2011; 31:10506–10515Crossref, Medline, Google Scholar
24 : CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004; 44:525–557Crossref, Medline, Google Scholar
25 : Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry 2007; 62:479–486Crossref, Medline, Google Scholar
26 : The clinical use of the dexamethasone suppression test in DSM-III affective disorders: correlation with the severe depressive subtypes of melancholia and psychosis. J Psychiatr Res 1987; 21:185–194Crossref, Medline, Google Scholar
27 : Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry 2006; 60:275–281Crossref, Medline, Google Scholar
28 : HPA axis in major depression: cortisol, clinical symptomatology, and genetic variation predict cognition. Mol Psychiatry 2017; 22:527–536Crossref, Medline, Google Scholar
29 : Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011; 73:114–126Crossref, Medline, Google Scholar
30 : Cognitive functioning and cortisol suppression in recurrent major depression. PsyCh J 2013; 2:167–174Crossref, Medline, Google Scholar
31 : The ACTH stimulation test before and after clinical recovery from depression. Psychiatry Res 1987; 20:325–336Crossref, Medline, Google Scholar
32 : The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160:1–12Crossref, Medline, Google Scholar
33 : Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression: a potential biomarker? Biol Psychiatry 2007; 62:47–54Crossref, Medline, Google Scholar
34 : Time-dependent effects of dexamethasone plasma concentrations on glucocorticoid receptor challenge tests. Psychoneuroendocrinology 2016; 69:161–171Crossref, Medline, Google Scholar
35 : Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann N Y Acad Sci 1996; 780:96–105Crossref, Medline, Google Scholar
36 : CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 1987; 144:873–877Link, Google Scholar
37 : Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004; 24:1478–1485Crossref, Medline, Google Scholar
38 : Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry 2017; 82:866–874Crossref, Medline, Google Scholar
39 : Anna-Monika Award Lecture, DGPPN Kongress, 2013: The role of the hypothalamic-pituitary-adrenal (HPA) axis in the pathogenesis of psychotic major depression. World J Biol Psychiatry 2015; 16:2–11Crossref, Medline, Google Scholar
40 : Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. American J Med Gen B Neuropsychiatr Genet 2012;159B:429–436Crossref, Medline, Google Scholar
41 : Treatment of major depression with metyrapone and hydrocortisone. J Affect Disord 1995; 33:123–128Crossref, Medline, Google Scholar
42 : The relationship between the effects of metyrapone treatment on depressed mood and urinary steroid profiles. Psychoneuroendocrinology 1996; 21:277–286Crossref, Medline, Google Scholar
43 : Neuroendocrine responses to inhibitors of steroid biosynthesis in patients with major depression resistant to antidepressant therapy. Can J Psychiatry 1998; 43:279–286Crossref, Medline, Google Scholar
44 : Effect of metyrapone supplementation on imipramine therapy in patients with treatment-resistant unipolar depression. Pol J Pharmacol 2004; 56:849–855Medline, Google Scholar
45 : Metyrapone as additive treatment in major depression: a double-blind and placebo-controlled trial. Arch Gen Psychiatry 2004; 61:1235–1244Crossref, Medline, Google Scholar
46 : Antidepressant augmentation with metyrapone for treatment-resistant depression (the ADD study): a double-blind, randomised, placebo-controlled trial. Lancet Psychiatry 2016; 3:117–127Crossref, Medline, Google Scholar
47 : Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res 2010; 44:339–346Crossref, Medline, Google Scholar
48 : The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919 in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005; 180:215–223Crossref, Medline, Google Scholar
49 : Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol 2004; 499:135–146Crossref, Medline, Google Scholar
50 : Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today 2010; 15:371–383Crossref, Medline, Google Scholar
51 : Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy: corticotrophin-releasing factor, beta-endorphin, and somatostatin. Br J Psychiatry 1991; 158:59–63Crossref, Medline, Google Scholar
52 : Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000; 34:171–181Crossref, Medline, Google Scholar
53 : A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry 2008; 165:617–620Link, Google Scholar
54 : Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety 2010; 27:417–425Crossref, Medline, Google Scholar
55 : Glucocorticoid receptor antagonists hasten and augment neurochemical responses to a selective serotonin reuptake inhibitor antidepressant. Biol Psychiatry 2007; 62:1228–1235Crossref, Medline, Google Scholar
56 : Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol Psychiatry 1999; 45:1070–1074Crossref, Medline, Google Scholar
57 : Limited efficacy of ketoconazole in treatment-refractory major depression. J Clin Psychopharmacol 1999; 19:466–470Crossref, Medline, Google Scholar
58 . Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology 2006; 31:628–636Crossref, Medline, Google Scholar
59 : Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry 2006; 60:1343–1349Crossref, Medline, Google Scholar
60 : Development of new psychopharmacological agents for depression and anxiety. Psychiatr Clin North Am 2015; 38:379–393Crossref, Medline, Google Scholar
61 : A multisite trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemp Clin Trials 2009; 30:284–288Crossref, Medline, Google Scholar
62 : Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry 2018; 84:46–54Crossref, Medline, Google Scholar
63 : Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 2015; 125:3193–3197Crossref, Medline, Google Scholar
64 : A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biol Psychiatry 2012; 72:943–949Crossref, Medline, Google Scholar
65 : T3 augmentation in major depressive disorder: safety considerations. Am J Psychiatry 2011; 168:1035–1040Link, Google Scholar
66 : Thyroid hormones, T3 and T4, in the brain. Front Endocrinol (Lausanne) 2014; 5:40Crossref, Medline, Google Scholar
67 : Thyroxine (T4) transfer from blood to cerebrospinal fluid in sheep isolated perfused choroid plexus: role of multidrug resistance-associated proteins and organic anion transporting polypeptides. Front Neurol 2017; 8:214Crossref, Medline, Google Scholar
68 Bernal J: Thyroid hormones in brain development and function, in Endotext [Internet]. Edited by Feingold KR, Anawalt B, Boyce A, et al. South Dartmouth, Mass, MDText.com, Inc, 2000–Google Scholar
69 : The link between thyroid function and depression. J Thyroid Res 2012; 2012:590648Crossref, Medline, Google Scholar
70 : The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol 2008; 20:1101–1114Crossref, Medline, Google Scholar
71 : Hormone treatment of depression. Dialogues Clin Neurosci 2011; 13:127–138Medline, Google Scholar
72 : Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993; 54:47–54Medline, Google Scholar
73 : Clinical and subclinical hypothyroidism in patients with chronic and treatment-resistant depression. Aust N Z J Psychiatry 1996; 30:246–252Crossref, Medline, Google Scholar
74 : Prevalence of depression in patients affected by subclinical hypothyroidism. Panminerva Med 2010; 52:277–282Medline, Google Scholar
75 : Thyroid hormones and depression: the Health in Men study. Am J Geriatr Psychiatry 2011; 19:763–770Crossref, Medline, Google Scholar
76 : Subclinical thyroid dysfunction and depressive symptoms among the elderly: a prospective cohort study. Neuroendocrinology 2016; 103:291–299Crossref, Medline, Google Scholar
77 : Peripheral thyroid dysfunction in depression. World J Biol Psychiatry 2006; 7:131–137Crossref, Medline, Google Scholar
78 : Enhancement of imipramine by thyroid stimulating hormone: clinical and theoretical implications. Am J Psychiatry 1970; 127:191–199Link, Google Scholar
79 : Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001; 158:1617–1622Link, Google Scholar
80 : Simultaneous initiation (coinitiation) of pharmacotherapy with triiodothyronine and a selective serotonin reuptake inhibitor for major depressive disorder: a quantitative synthesis of double-blind studies. Int Clin Psychopharmacol 2009; 24:19–25Crossref, Medline, Google Scholar
81 : Triiodothyronine augmentation in the treatment of refractory depression: a meta-analysis. Arch Gen Psychiatry 1996; 53:842–848Crossref, Medline, Google Scholar
82 : Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry 2006; 51:791–793Crossref, Medline, Google Scholar
83 : A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 2006; 163:1519–1530Link, Google Scholar
84 : Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 2015; 76:e487–e498Crossref, Medline, Google Scholar
85 : Liothyronine for depression: a review and guidance for safety monitoring. Innov Clin Neurosci 2017; 14:24–29Medline, Google Scholar
86 : A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990; 32:241–251Crossref, Medline, Google Scholar
87 : Thyroid functions and bipolar affective disorder. J Thyroid Res 2011; 2011:306367Crossref, Medline, Google Scholar
88 : Rapid cycling bipolar affective disorder, II: treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study. Arch Gen Psychiatry 1990; 47:435–440Crossref, Medline, Google Scholar
89 : Adjunctive thyroid hormone treatment in rapid cycling bipolar disorder: a double-blind placebo-controlled trial of levothyroxine (L-T4 ) and triiodothyronine (T3 ). Bipolar Disord 2018; 20:594–603Crossref, Medline, Google Scholar
90 : Supraphysiologic doses of levothyroxine as adjunctive therapy in bipolar depression: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2014; 75:162–168Crossref, Medline, Google Scholar
91 : World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 2013; 14:334–385Crossref, Medline, Google Scholar
92 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 3rd ed. Washington, DC, American Psychiatric Association, 2010 (https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf)Google Scholar
93 : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder, Section 3: Pharmacological Treatments. Can J Psychiatry 2016; 61:540–560Crossref, Medline, Google Scholar
94 : Antidepressant-resistant depression in patients with comorbid subclinical hypothyroidism or high-normal TSH levels. Am J Psychiatry 2018; 175:598–604Link, Google Scholar
95 : 60 years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. J Endocrinol 2015; 226:T41–T54Crossref, Medline, Google Scholar
96 : Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 2016; 230:R13–R25Crossref, Medline, Google Scholar
97 : Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997; 277:1508–1510Crossref, Medline, Google Scholar
98 : Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 2002; 16:70–84Crossref, Medline, Google Scholar
99 : Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology 1992; 131:381–388Crossref, Medline, Google Scholar
100 : Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 1997; 388:507–525Crossref, Medline, Google Scholar
101 : Non-genomic mechanisms of progesterone action in the brain. Front Neurosci 2013; 7:159Crossref, Medline, Google Scholar
102 : Progesterone receptors: form and function in brain. Front Neuroendocrinol 2008; 29:313–339Crossref, Medline, Google Scholar
103 : A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med 2014; 371:2467–2476Crossref, Medline, Google Scholar
104 : Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 2014; 371:2457–2466Crossref, Medline, Google Scholar
105 : Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 2014; 35:320–330Crossref, Medline, Google Scholar
106 : Mood, sexuality, hormones, and the menstrual cycle, II: hormone levels and their relationship to the premenstrual syndrome. Psychosom Med 1983; 45:503–507Crossref, Medline, Google Scholar
107 : Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 2002; 41:306–315Crossref, Medline, Google Scholar
108 : Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci USA 1998; 95:13941–13946Crossref, Medline, Google Scholar
109 : Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety 2015; 32:539–549Crossref, Medline, Google Scholar
110 : Association of hormonal contraception with suicide attempts and suicides. Am J Psychiatry 2018; 175:336–342Link, Google Scholar
111 : Association of hormonal contraception with depression. JAMA Psychiatry 2016; 73:1154–1162Crossref, Medline, Google Scholar
112 : The use of hormonal contraceptive agents and mood disorders in women. J Affect Disord 2012; 140:92–96Crossref, Medline, Google Scholar
113 : The relationship between progestin hormonal contraception and depression: a systematic review. Contraception 2018; 97:478–489Crossref, Medline, Google Scholar
114 : Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology 2017; 76:135–143Crossref, Medline, Google Scholar
115 : Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care 2016; 21:347–355Crossref, Medline, Google Scholar
116 : High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017; 32:1075–1091Crossref, Medline, Google Scholar
117 : Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab 2016; 101:2966–2974Crossref, Medline, Google Scholar
118 : Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Arch Women Ment Health 2016; 19:953–958Crossref, Medline, Google Scholar
119 : Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception 2005; 72:414–421Crossref, Medline, Google Scholar
120 : Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol 2005; 106:492–501Crossref, Medline, Google Scholar
121 : An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception 2012; 85:437–445Crossref, Medline, Google Scholar
122 : Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338:209–216Crossref, Medline, Google Scholar
123 : Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep 2015; 17:87Crossref, Medline, Google Scholar
124 : Risk-reducing medications for primary breast cancer: a network meta-analysis. Cochrane Database Syst Rev 2019; 4:CD012191Medline, Google Scholar
125 : Tamoxifen: a protein kinase C inhibitor to treat mania: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychopharmacol 2016; 36:272–275Crossref, Medline, Google Scholar
126 : A placebo-controlled study of raloxifene added to risperidone in men with chronic schizophrenia. Acta Med Iran 2015; 53:337–345Medline, Google Scholar
127 : Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trials. JAMA 2017; 318:927–938Crossref, Medline, Google Scholar
128 : Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016; 374:1221–1231Crossref, Medline, Google Scholar
129 : Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab 2011; 96:E1044–E1054Crossref, Medline, Google Scholar
130 : Influence of a continuous combined HRT (2 mg estradiol valerate and 2 mg dienogest) on postmenopausal depression. Climacteric 2004; 7:301–311Crossref, Medline, Google Scholar
131 : Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry 2004; 55:406–412Crossref, Medline, Google Scholar
132 : Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000; 183:414–420Crossref, Medline, Google Scholar
133 : Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001; 58:529–534Crossref, Medline, Google Scholar
134 : Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry 1997; 5:97–106Crossref, Medline, Google Scholar
135 : Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause 2006; 13:202–211Crossref, Medline, Google Scholar
136 : Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res 2007; 41:338–343Crossref, Medline, Google Scholar
137 : Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med 2015; 12:e1001833Crossref, Medline, Google Scholar
138 : Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry 2018; 75:149–157Crossref, Medline, Google Scholar
139 : Should hormone therapy be used to prevent depressive symptoms during the menopause transition? JAMA Psychiatry 2018; 75:125–126Crossref, Medline, Google Scholar
140 : Neurosteroids, stress, and depression: potential therapeutic opportunities. Neurosci Biobehav Rev 2013; 37:109–122Crossref, Medline, Google Scholar
141 : Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther 2007; 116:7–19Crossref, Medline, Google Scholar
142 : Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl) 2014; 231:3557–3567Crossref, Medline, Google Scholar
143 : Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci 2007; 10:469–477Crossref, Medline, Google Scholar
144 : GABAA receptor-modulating steroids in relation to women’s behavioral health. Curr Psychiatry Rep 2015; 17:92Crossref, Medline, Google Scholar
145 : 5(alpha)-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology 2016; 41:1093–1102Crossref, Medline, Google Scholar
146 : Effects of GABA active steroids in the female brain with focus on the premenstrual dysphoric disorder. J Neuroendocrinol 2018; 30 (doi: 10.1111/jne.12553)Crossref, Medline, Google Scholar
147 : Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol 2017; 32:32Crossref, Google Scholar
148 : Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 2017; 390:480–489Crossref, Medline, Google Scholar
149 : Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018; 392:1058–1070Crossref, Medline, Google Scholar
150 : A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology 2014; 39:2867–2873Crossref, Medline, Google Scholar
151 : Proof-of-concept randomized controlled trial of pregnenolone in schizophrenia. Psychopharmacology (Berl) 2014; 231:3647–3662Crossref, Medline, Google Scholar
152 : A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacology (Berl) 2017; 234:2245–2257Crossref, Medline, Google Scholar
153 : Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 2017; 58:558–564Crossref, Medline, Google Scholar
154 : On the effects of testosterone on brain behavioral functions. Front Neurosci 2015; 9:12Crossref, Medline, Google Scholar
155 : A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38:302–324Crossref, Medline, Google Scholar
156 : Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 2009; 30:65–91Crossref, Medline, Google Scholar
157 : Low serum testosterone in outpatient psychiatry clinics: addressing challenges to the screening and treatment of hypogonadism. Sex Med Rev 2018; 6:69–76Crossref, Medline, Google Scholar
158 : Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology 2006; 31:1029–1035Crossref, Medline, Google Scholar
159 : Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry 2017; 25:425–437Crossref, Medline, Google Scholar
160 : High rates of depression and depressive symptoms among men referred for borderline testosterone levels. J Sex Med 2015; 12:1753–1760Crossref, Medline, Google Scholar
161 : Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004; 61:162–167Crossref, Medline, Google Scholar
162 : The effect of testosterone levels on mood in men: a review. Psychosomatics 2013; 54:509–514Crossref, Medline, Google Scholar
163 : Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry 2014; 26:19–32Medline, Google Scholar
164 : Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiatry 2019; 76:31–40Crossref, Medline, Google Scholar
165 : Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract 2009; 15:289–305Crossref, Medline, Google Scholar
166 : Effect of testosterone replacement therapy on cognitive performance and depression in men with testosterone deficiency syndrome. World J Mens Health 2016; 34:194–199Crossref, Medline, Google Scholar
167 : Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open 2017; 7:e015284Medline, Google Scholar
168 : Safety and efficacy of testosterone gel 1% augmentation in depressed men with partial response to antidepressant therapy. J Geriatr Psychiatry Neurol 2005; 18:20–24Crossref, Medline, Google Scholar
169 : Parallel-group placebo-controlled trial of testosterone gel in men with major depressive disorder displaying an incomplete response to standard antidepressant treatment. J Clin Psychopharmacol 2010; 30:126–134Crossref, Medline, Google Scholar
170 : Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003; 160:105–111Link, Google Scholar
171 : Intramuscular testosterone supplementation to selective serotonin reuptake inhibitor in treatment-resistant depressed men: randomized placebo-controlled clinical trial. J Clin Psychopharmacol 2005; 25:584–588Crossref, Medline, Google Scholar
172 : The sexual effects of testosterone replacement in depressed men: randomized, placebo-controlled clinical trial. J Sex Marital Ther 2006; 32:267–273Crossref, Medline, Google Scholar
173 : Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol 2009; 29:216–221Crossref, Medline, Google Scholar
174 : A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016Crossref, Medline, Google Scholar
175 : Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab 2000; 85:60–65Medline, Google Scholar
176 : A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry 2000; 57:141–147Crossref, Medline, Google Scholar
177 : Testosterone versus fluoxetine for depression and fatigue in HIV/AIDS: a placebo-controlled trial. J Clin Psychopharmacol 2004; 24:379–385Crossref, Medline, Google Scholar
178 : Testosterone therapy: review of clinical applications. Am Fam Physician 2017; 96:441–449Medline, Google Scholar
179 : Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 1999; 156:646–649Abstract, Google Scholar
180 : Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry 2005; 62:154–162Crossref, Medline, Google Scholar
181 : Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry 2006; 163:59–66Link, Google Scholar



