Low-Dose Testosterone Augmentation for Antidepressant-Resistant Major Depressive Disorder in Women: An 8-Week Randomized Placebo-Controlled Study
Abstract
Objective:
Low-dose testosterone has been shown to improve depression symptom severity, fatigue, and sexual function in small studies in women not formally diagnosed with major depressive disorder. The authors sought to determine whether adjunctive low-dose transdermal testosterone improves depression symptom severity, fatigue, and sexual function in women with antidepressant-resistant major depression. A functional MRI (fMRI) substudy examined effects on activity in the anterior cingulate cortex (ACC), a brain region important in mood regulation.
Methods:
The authors conducted an 8-week randomized double-blind placebo-controlled trial of adjunctive testosterone cream in 101 women, ages 21–70, with antidepressant-resistant major depression. The primary outcome measure was depression symptom severity as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary endpoints included fatigue, sexual function, and safety measures. The primary outcome of the fMRI substudy (N=20) was change in ACC activity.
Results:
The participants’ mean age was 47 years (SD=14) and their mean baseline MADRS score was 26.6 (SD=5.9). Eighty-seven (86%) participants completed 8 weeks of treatment. MADRS scores decreased in both study arms from baseline to week 8 (testosterone arm: from 26.8 [SD=6.3] to 15.3 [SD=9.6]; placebo arm: from 26.3 [SD=5.4] to 14.4 [SD=9.3]), with no significant difference between groups. Improvement in fatigue and sexual function did not differ between groups, nor did side effects. fMRI results showed a relationship between ACC activation and androgen levels before treatment but no difference in ACC activation with testosterone compared with placebo.
Conclusions:
Adjunctive transdermal testosterone, although well tolerated, was not more effective than placebo in improving symptoms of depression, fatigue, or sexual dysfunction. Imaging in a subset of participants demonstrated that testosterone did not result in greater activation of the ACC.
In patients with major depressive disorder, nonresponse to treatment with selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) is common, particularly in women, occurring in about 70% of patients despite adequate dosing (1). Additional well-tolerated augmentation strategies are needed, particularly ones that do not cause or exacerbate symptoms such as fatigue and sexual dysfunction. Modest preclinical and clinical data suggest that transdermal testosterone, in dosages designed to raise levels into or near the physiologically normal range for women (10%−20% of male levels), is a candidate for such a therapy.
Preclinical and animal models are consistent with the hypothesis that androgens are modulators of mood (2–4). Testosterone affects brain function directly as well as by aromatization of testosterone to estradiol and conversion to the potent androgen dihydrotestosterone (5–10). Additionally, the testosterone metabolite 5α-androstane-3α,17β-diol (3α-diol) is a neuroactive steroid that has been shown to exert important GABAergic-related effects on affective symptoms. Preclinical models also suggest androgen effects on central serotonin neurotransmission, which is relevant to the modulation of core depression symptoms (11).
There are also clinical data that suggest a beneficial effect of testosterone on mood in hypogonadal men with refractory depression (12) and from small randomized placebo-controlled trials in women not selected for major depression. In the latter studies, conducted in women after bilateral oophorectomy with sexual dysfunction (13) or in women with hypopituitarism (14), low-dose testosterone administration improved mood, fatigue (14), and sexual dysfunction (15). Our preliminary data in women with treatment-resistant depression (N=9) treated with transdermal testosterone in an 8-week open-label trial (16) demonstrated that 67% achieved categorical response (defined as a decrease ≥50% from baseline on Montgomery-Åsberg Depression Rating Scale [MADRS] score), and 33% achieved remission (defined as a MADRS score ≤10) after 8 weeks of therapy. Moreover, in our studies and in other investigations, in total evaluating more than 2,000 women followed for as long as 1 year, low-dose transdermal testosterone was found to be extremely well tolerated, without significant hyperandrogenic or metabolic side effects (17).
Based on these data, we hypothesized that low-dose adjunctive testosterone would result in greater improvement in depression symptom severity in women with antidepressant-resistant major depression compared with placebo. We additionally hypothesized that testosterone would be well tolerated and, compared with placebo, would improve fatigue and sexual dysfunction—specific symptoms that are commonly associated with major depression and with many medications used to treat major depression. In addition, because use of compounded and male-branded testosterone products by women is common (18), we sought to establish whether adjunctive transdermal testosterone was safe and well tolerated for women with major depression. Finally, we also explored whether adjunctive low-dose testosterone would increase activation of the subgenual and dorsal anterior cingulate cortex (ACC), a brain region important in the regulation of mood.
Methods
Participants
The protocol was approved by the Partners Human Research Committee and the Butler Hospital Institutional Review Board, and written informed consent was obtained from all participants before any procedures were performed. Inclusion criteria were female sex, age between 21 and 75 years, a primary diagnosis of major depressive disorder by DSM-IV criteria using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) (19), a MADRS score ≥12, and current treatment with an antidepressant (a first, second, or third trial in the current depressive episode) at an adequate dosage for at least 8 weeks, with sufficient source documentation to confirm a high level of confidence in treatment details, using the MGH Antidepressant Treatment Response Questionnaire (20). In addition, a baseline free testosterone level no higher than the third quartile of the normal range was required. Exclusion criteria included significant suicide or homicide risk, history of psychotic features, or bipolar disorder as assessed by the SCID-IV. Medical exclusions included untreated hypothyroidism, current use of androgens, and a history of a hormone-responsive cancer. Sixty-five participants were recruited and studied at the Massachusetts General Hospital site, and 36 at the Butler Hospital site.
Study Design
The study was a two-site 8-week randomized placebo-controlled parallel-groups trial. Participants were assigned, in a randomized 1:1 ratio, to receive low-dose adjunctive testosterone cream (21) or identical-appearing placebo made by the same manufacturer. The starting dosage of 10 mg/day was chosen to target the upper-normal range of testosterone for young women (21). Dosage titration was performed by an unblinded study monitor and was based on serum levels of free testosterone. Placebo sham dosage adjustments were made to maintain investigator blinding. Testosterone cream was applied as an adjunct to ongoing stable pharmacotherapy.
Psychiatric assessments, hormone level measurements, and dosage adjustments were performed as outlined in Table 1. To ensure reliability and quality control, ratings at Massachusetts General Hospital were supervised by psychiatrists and psychologists at Massachusetts General Hospital and Butler Hospital, using the same standards and principles to ensure consistency across the two sites. Psychiatric evaluators at both sites were extensively trained in the use of the SCID and the MADRS by gold-standard videos; prior assessments of interrater reliability in these measures yielded kappa coefficients greater than 0.75 and intraclass correlation coefficients greater than 0.8.
| Time Point | ||||||
|---|---|---|---|---|---|---|
| Measure or Step | Screening | Baseline | Week 2 | Week 4 | Week 6 | Week 8 |
| Diagnostic screening | ||||||
| SCID-IV and MGH ATRQ | x | |||||
| Depression symptom severity | ||||||
| MADRS | x | x | x | x | x | x |
| CGI-S | x | x | x | x | x | |
| IDS-SR | x | x | x | x | x | |
| Fatigue and sleepiness | ||||||
| BFI | x | x | x | x | x | |
| ESS | x | x | x | x | x | |
| FSS | x | x | x | x | x | |
| Sexual function | ||||||
| DISF | x | x | x | x | x | |
| Quality of life | ||||||
| SF-36 | x | x | x | x | x | |
| Safety measures | ||||||
| CHRT | x | x | x | x | x | |
| SAFTEE-SI | x | x | x | x | x | |
| Hormone assessments | x | x | x | x | x | x |
| Drug dosingb | ||||||
| Dosage titrationsc | x | x | x | |||
| Drug discontinuation | x | |||||
TABLE 1. Study schema and drug dosing schedule in a study of low-dose testosterone augmentation for antidepressant-resistant major depression in womena
Hormone Assessment
Samples were collected before 10 a.m., stored at −80°C, and batched for analysis. Serum testosterone, free testosterone, and cortisol concentrations were assayed by Mayo Medical Laboratories (Rochester, Minn.). Serum testosterone was measured using liquid chromatography with tandem mass spectrometry, and free testosterone by equilibrium dialysis.
Statistical Analysis
The primary efficacy endpoint was change in MADRS score. Chi-square tests or t tests were used, as appropriate, to compare the distributions of baseline variables and evaluate baseline group equivalence. Outcome variables were measured at five time points: at baseline (before treatment) and at weeks 2, 4, 6, and 8. The efficacy analysis used a repeated-measures analysis of variance, with both treatments set to placebo at baseline, and to their respective groups at follow-up. The treatment effect using this model measures the average difference between the treatments at weeks 2 through 8, corrected for baseline values. The variance-covariance matrix was left unspecified. With 50 participants in each treatment group, we predicted greater than 80% power to detect a 5-point difference in the change in MADRS scores (baseline to 8 weeks) between the testosterone and placebo groups (22, 23).
The same procedure was used for analysis of change in hormones, except that for all hormone levels other than testosterone and free testosterone, only two measurements were available, at baseline and at week 8. We tested for an interaction between baseline free testosterone level and treatment by introducing an interaction term and a main effect of testosterone into this model. Interaction testing between menopausal status and treatment was performed using the same methods. Additionally, within-group analyses were performed using paired t tests. Response and remission rates were compared across the two groups using Fisher’s exact test. Data are presented as means and standard deviations.
The Systematic Assessment for Treatment Emergent Events–Systematic Inquiry (SAFTEE-SI) questionnaire categorizes adverse event severity as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). Treatment-emergent side effects were defined as an increase by 2 or more levels of severity from pretreatment baseline assessment. The proportion of participants in each of the two treatment groups who reported threshold side effects at any time during the treatment period were compared using Fisher’s exact test.
fMRI Substudy
Thirty-one participants at the Massachusetts General Hospital site were evaluated to enroll 20 participants in the functional MRI (fMRI) substudy, and 11 of them either declined (N=8) or were not eligible (N=3). fMRI scans were performed at baseline and at week 8. In premenopausal women, all testing was performed during the follicular phase of the menstrual cycle.
fMRI data were acquired using a 3-T Siemens Skyra whole-body scanner equipped for echo planar imaging (Siemens Medical Systems, Iselin, N.J.), with a three-axis gradient head coil. Images were projected using a rear projection system and the E-Prime 2.0 stimulus presentation software program. After automated scout and shimming procedures and two high-resolution three-dimensional magnetization-prepared rapid gradient echo sequences, fMRI images (i.e., blood-oxygen-level-dependent signal) were acquired using a T2*-weighted sequence (39 horizontal slices aligned perpendicular, 3.1-mm thickness, TE=28 ms, TR=2.0 seconds, flip angle=90°).
Participants completed a rapid event-related emotional conflict paradigm (24, 25) in which faces with fearful and happy expressions were presented with the words “happy” or “fear” written across them; the participants’ task was to identify the emotional expression of the faces while ignoring the words, which were either congruent or incongruent with the facial expression.
fMRI data were processed using SPM8 (Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). fMRI images were motion corrected and normalized to Montreal Neurological Institute space. Condition effects were modeled with regressors representing the occurrence of each trial type (incongruent or congruent). For each participant (first-level analysis), condition effects were estimated at each voxel, and statistical parametric maps (SPMs; i.e., contrast images) were produced for each condition (incongruent or congruent). To estimate conditions at the group level (second-level analysis), individual participants’ SPM contrast images were entered into a second-level random-effects analysis, using a flexible factorial model with subject as the first factor and condition (incongruent or congruent) as the second factor. The a priori specified region of interest was the ACC, and the posterior cingulate cortex (PCC) was a secondary prespecified region of interest. For each, we adopted a statistical significance threshold of p<0.05 uncorrected. The ACC and PCC were defined with masks provided by the Anatomical Automatic Labeling tool (26) implemented in the WFU PickAtlas (http://www.ansir.wfubmc.edu) (27, 28).
Results
Sample Characteristics
The baseline clinical characteristics of the 101 participants who underwent randomization to a treatment group are summarized in Table 2. Sixty-six percent had one, 25% had two, and 9% had three failed antidepressant trials at baseline. The mean age was 47 years (SD=14, range=21–70), and the mean MADRS score was 26.6 (SD=5.9), with no significant differences between the testosterone and placebo groups. The proportions of premenopausal women did not differ significantly between the testosterone (59%) and placebo (48%) groups (p=0.32). Eighty-seven (86%) participants completed the 8-week study. Five participants in the testosterone group (8%) and nine in the placebo group (18%) dropped out before the week-8 visit (p=0.26). Reasons for discontinuation in the testosterone group included lack of efficacy (N=2), physician decision (N=1), acne (N=1), and personal reasons (N=1). Reasons for discontinuation in the placebo group included lack of efficacy (N=3), lost to follow-up (N=4), protocol violation (N=1), and personal reasons (N=1). Two of these participants returned for end-of-study visits (one in each treatment group); their data were included in the week-8 (completers) data set. Mean final testosterone dosage in the treatment group was 12.2 mg/day (SD=5.6, median=10 mg/day, range=2.5–25 mg/day).
| Characteristic | Testosterone Group (N=51) | Placebo Group (N=50) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Premenopausal | 30 | 59 | 24 | 48 |
| Mean | SD | Mean | SD | |
| Age (years) | 46 | 14 | 48 | 14 |
| Body mass index | 28.2 | 7.6 | 30.3 | 7.9 |
| Number of failed antidepressant trials | 1.5 | 0.7 | 1.4 | 0.6 |
| Total testosterone (ng/dL) | 19 | 11 | 18 | 9 |
| Free testosterone (ng/dL) | 0.3 | 0.2 | 0.3 | 0.2 |
| MADRS score | 26.8 | 6.3 | 26.3 | 5.4 |
TABLE 2. Baseline clinical characteristics of participants in a study of low-dose testosterone augmentation for antidepressant-resistant major depression in womena
Hormone Levels
Mean total testosterone and free testosterone levels increased significantly over time within the group of women receiving testosterone and also compared with the group receiving placebo. Mean total testosterone levels were 19 ng/dL (SD=11) at baseline and 105 ng/dL (SD=70) at 8 weeks in the testosterone group, and 18 ng/dL (SD=9) at baseline and 18 ng/dL (SD=10) at 8 weeks in the placebo group (p<0.0001, within and between groups). Free testosterone levels also significantly increased within the testosterone group and between groups, from 0.3 ng/dL (SD=0.2) to 1.9 ng/dL (SD=1.1) in the testosterone group and 0.3 ng/dL (SD=0.2) to 0.4 ng/dL (0.2) in the placebo group (both p<0.001). Morning serum cortisol levels decreased within the testosterone group (p<0.05) and did not change in the placebo group; there was no significant difference between groups.
Depression Symptom Severity
MADRS scores decreased from baseline to week 8 in both groups (testosterone group: from 26.8 [SD=6.3] to 15.3 [SD=9.6]; placebo group: from 26.3 [SD=5.4] to 14.4 [SD=9.3]), with no statistical difference between groups (p=0.91) (Table 3, Figure 1). Remission status (a MADRS score ≤10) was achieved at study end by 36% in the testosterone group and 44% in the placebo group (p=0.52). Categorical response (improvement ≥50% in MADRS score) was experienced by 47% and 49% (p=1.00) of the testosterone and the placebo group, respectively. Response was not moderated by baseline free testosterone level or menopausal status (i.e., there was no interaction between treatment effect and baseline free testosterone level or menopausal status). Additionally, Clinician Global Impressions severity ratings of “much improved” or “very much improved” characterized 31% of the testosterone group and 41% of the placebo group (p=0.37). All other measures, including measures of fatigue and sexual function, improved from baseline to 8 weeks in both the testosterone and placebo groups, with no significant difference between groups (Table 3). There was no effect of depression symptom severity or study site in post hoc analyses (data not shown).
| Testosterone Group (N=51) | Placebo Group (N=50) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks | Baseline | 8 Weeks | ||||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p |
| Depression symptom severity | |||||||||
| MADRS score | 26.8 | 6.3 | 15.3 | 9.6 | 26.3 | 5.4 | 14.4 | 9.3 | 0.91 |
| CGI-S score | 4.3 | 0.6 | 3.1 | 1.4 | 4.3 | 0.6 | 2.8 | 1.2 | 0.78 |
| IDS-SR | 35.9 | 10.5 | 20.1 | 11.2 | 34.9 | 10.0 | 19.1 | 10.1 | 0.81 |
| Fatigue and sleepiness | |||||||||
| BFI | 6.2 | 1.9 | 4.0 | 2.3 | 5.6 | 2.0 | 3.7 | 2.8 | 0.92 |
| ESS | 8.0 | 4.7 | 6.0 | 4.2 | 9.4 | 4.7 | 6.8 | 3.6 | 0.80 |
| FSS | 45.0 | 12.5 | 38.6 | 13.8 | 42.4 | 12.3 | 35.9 | 13.9 | 0.81 |
| Sexual function | |||||||||
| DISF | 32.8 | 27.3 | 45.1 | 31.3 | 38.7 | 30.1 | 47.5 | 34.0 | 0.37 |
| Quality of life | |||||||||
| SF-36 PCS | 66.7 | 20.3 | 72.6 | 19.6 | 66.7 | 20.6 | 74.3 | 18.3 | 0.43 |
| SF-36 MCS | 29.5 | 13 | 54.5 | 22.4 | 33.1 | 14.5 | 54.5 | 19.7 | 0.27 |
| Hormone levels | |||||||||
| Total testosterone (ng/dL) | 19 | 11 | 105 | 70 | 18 | 9 | 18 | 10 | <0.0001 |
| Free testosterone (ng/dL) | 0.3 | 0.2 | 1.9 | 1.1 | 0.3 | 0.2 | 0.4 | 0.2 | <0.001 |
TABLE 3. Psychiatric assessments and hormone levels in a study of low-dose testosterone augmentation for antidepressant-resistant major depression in womena
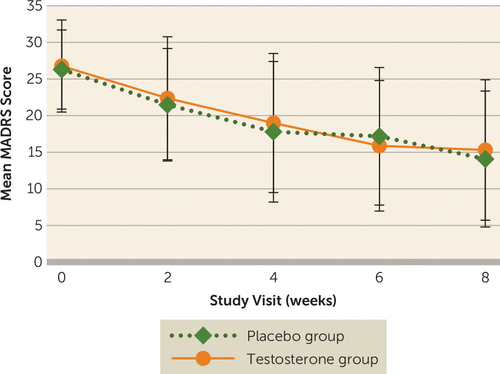
FIGURE 1. Depression severity over time, as assessed by mean MADRS score, in a study of low-dose testosterone augmentation for antidepressant-resistant major depression in womena
a MADRS=Montgomery-Åsberg Depression Rating Scale. There was no significant difference between the testosterone and placebo groups. Error bars indicate standard deviation.
Safety and Tolerability
Changes in total SAFTEE-SI scores, which measure treatment-emergent symptoms, did not differ between the groups at any time point. Acne, hot flashes, and headache were each reported in >5% of participants. All side effects are reported in Table 4. No reported side effect differed significantly in frequency between the testosterone and placebo groups, and there were no serious adverse events.
| Testosterone Group (N=51) | Placebo Group (N=50) | |||
|---|---|---|---|---|
| Reported Event | N | % | N | % |
| Hot flashes | 2 | 4 | 6 | 12 |
| Acne | 7 | 14 | 3 | 6 |
| Headache | 6 | 12 | 5 | 10 |
| Increase in facial or body hair | 1 | 2 | 4 | 8 |
| Skin irritation at site of cream application | 1 | 2 | 3 | 6 |
| Delayed menses | 0 | 0 | 2 | 4 |
| Breast tenderness | 2 | 4 | 1 | 2 |
| Scalp hair loss | 1 | 2 | 0 | 0 |
TABLE 4. Participant-reported adverse events in a study of low-dose testosterone augmentation for antidepressant-resistant major depression in womena
fMRI Substudy Findings
Of posttreatment scan completers (N=18), eight were in the testosterone group and 10 in the placebo group. Baseline free testosterone levels in all fMRI study subjects (N=20) were inversely associated with baseline dorsal ACC activation (x=0, y=8, z=40; number of voxels [Ke]=43; Z=2.64, p=0.004 uncorrected) and PCC activation (x=6, y=−42, z=50; Ke=24; Z=2.65, p=0.004 uncorrected) in response to the emotional conflict task. Pre- to posttreatment change in ACC and PCC activation in response to the emotional conflict task (incongruent versus congruent stimuli) did not differ significantly between the testosterone and placebo groups. Only these prespecified regions of interest were analyzed.
Discussion
Augmentation with transdermal testosterone, administered at low dosages designed to raise free testosterone levels to the high-normal female reference range, did not improve depression symptom severity, fatigue, or sexual dysfunction to a greater degree than placebo. Additionally, fMRI data from a subset of trial participants showed no difference in activation of the dorsal and pregenual ACC after testosterone treatment compared with placebo. We found that low-dose adjunctive testosterone had an excellent safety profile in this 8-week trial. The placebo response rate in this trial was high (49%), which may have accounted in part for the lack of observed treatment effect.
Major depression disproportionately affects women, and inadequate treatment response in depression is highly prevalent. For example, the multicenter Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial demonstrated that approximately two-thirds of patients with major depression do not achieve remission after 8–12 weeks of adequate antidepressant therapy (29). Although there are effective augmentation therapies available, such as lithium and atypical antipsychotics, many patients do not respond to or are intolerant of these interventions. Even when adequate responses are obtained, these drugs may cause significant side effects, which limit their long-term use (30–36). Previous trials and our pilot data supported the evaluation of transdermal testosterone in a properly scaled prospective randomized trial. Testosterone was an excellent candidate for study in women with antidepressant-resistant depression because of its potential antidepressant effects, its ability to improve fatigue and sexual function, and its favorable side effect profile (13, 15, 37–42). Nevertheless, in this rigorously designed placebo-controlled study, we did not find a significant improvement in mood with testosterone compared with placebo, although, as noted above, the high placebo response rate may have accounted for the lack of significant differences. As these were antidepressant-resistant patients, who by definition are a more challenging population to treat, the results of this study may not be generalizable to other depressed patients.
It should also be noted that the response and remission rates we observed for testosterone administration in this study are in line with those that the literature typically suggests for antidepressants (43). However, the placebo response rate was also high, and it was similar to that for testosterone. Placebo response rates in depression studies are often high (44), and it has been shown that studies with greater than a 40% placebo response rate are unlikely to demonstrate a statistically significant effect of the antidepressant (45). Additionally, studies demonstrate a higher placebo response rate when patients with a lower severity of depression are included (46, 47), as was the case in this study. High placebo response rates may challenge underlying assumptions when designing trials using current methods, and several initiatives have focused on novel strategies to reduce placebo response in depression studies (48–51). Studies implementing these strategies may produce better quality data, with greater separation rates between active treatment and placebo in cases where the drug is truly effective. Therefore, further studies employing these strategies may be warranted.
To our knowledge, this is the first randomized placebo-controlled study of adjunctive low-dose testosterone in women with antidepressant-resistant major depression. Fooladi et al. (52) assessed effects on libido and sexual function in a randomized trial of low-dose testosterone in women (N=44) with treatment-emergent loss of libido who were on stable SSRI or SNRI therapy. The authors observed no improvements in their primary endpoint, reported level of libido, but they did find an increase in frequency of sexual activity. Consistent with our findings in women with antidepressant-resistant major depression, they reported no group difference in change in depression severity as a secondary endpoint. However, women with severe depression (Beck Depression Inventory–II scores >28) were excluded from that study, resulting in recruitment of a sample with mild depression (a mean baseline Beck Depression Inventory–II score of 8.0). Although these results may not be generalizable to a more severely ill or antidepressant-resistant population, they are consistent with the results of our study.
The anterior cingulate cortex (ACC) has been implicated in the pathophysiology of major depressive disorder (53, 54). In a previous fluorodeoxyglucose positron emission tomography study examining the effects of low-dose testosterone in women with anorexia nervosa and relative androgen deficiency (55), we found lower cerebral metabolism in women with anorexia nervosa than in control subjects, with increases in subgenual ACC activation after testosterone administration. Consistent with these prior findings, here we report an inverse association between baseline free testosterone levels and both ACC and PCC activity (as measured by fMRI), suggesting that further study of the possible role of gonadal steroids, including androgens, in the etiopathology and/or as treatment targets in antidepressant-resistant depression is warranted. However, we did not find a difference in activation of the dorsal and pregenual ACC or PCC after testosterone administration compared with placebo in this study.
Limitations of this study were those inherent in all blinded clinical trials of a disorder characterized by symptom heterogeneity and response vulnerable to placebo effect, in this case major depression. It is possible that a more homogeneous population of postmenopausal women with lower levels of testosterone at baseline would allow detection of differences in adjunctive testosterone compared with placebo. Moreover, we cannot rule out type II error in the context of a higher than expected placebo response.
Conclusions
This rigorously designed double-blind clinical trial did not find significant group differences between adjunctive low-dose transdermal testosterone and placebo for antidepressant augmentation in women with treatment-resistant major depression, and had a high placebo response rate. Low-dose testosterone was well tolerated but failed to differentially affect overall depression symptom severity, fatigue, or sexual dysfunction in women treated for 8 weeks with dosages titrated to achieve blood levels near the upper end of the normal reference range. Additionally, testosterone did not result in greater activity compared with placebo in a brain region (ACC and PCC) implicated in major depression etiopathology. Based on our findings and the results of several other recent clinical trials, we conclude that the addition of low-dose testosterone to ongoing, ineffective antidepressant medications should not be recommended for women with major depression. These negative results are important given the number of women who use off-label male-branded or compounded testosterone. Further studies of adjunctive testosterone in antidepressant-resistant major depression using strategies designed to reduce placebo effects may be warranted.
1 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
2 : The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol 1992; 13:163–215Medline, Google Scholar
3 : Neurotropic action of androgens: principles, mechanisms, and novel targets. Exp Gerontol 2004; 39:1651–1660Crossref, Medline, Google Scholar
4 : Androgens, brain, and behavior. Am J Psychiatry 1996; 153:974–984Link, Google Scholar
5 : Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res Bull 1997; 44:365–375Crossref, Medline, Google Scholar
6 : Gender-specific steroid metabolism in neural differentiation. Cell Mol Neurobiol 1997; 17:603–626Crossref, Medline, Google Scholar
7 : 5 alpha-reductase isozymes in the central nervous system. Steroids 1998; 63:246–251Crossref, Medline, Google Scholar
8 : Androgen-activating enzymes in the central nervous system. J Steroid Biochem Mol Biol 1999; 69:117–122Crossref, Medline, Google Scholar
9 : Characterization of aromatase cytochrome P450 activity in the human temporal lobe. J Clin Endocrinol Metab 1999; 84:2795–2801Medline, Google Scholar
10 : Brain aromatase and 5alpha-reductase, regulatory behaviors and testosterone levels in adult rats on phytoestrogen diets. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York), 1999; 221(2):131–135Google Scholar
11 : Androgen actions on central serotonin neurotransmission: relevance for mood, mental state, and memory. Behav Brain Res 1999; 105:53–68Crossref, Medline, Google Scholar
12 : Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003; 160:105–111Link, Google Scholar
13 : Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 2000; 343:682–688Crossref, Medline, Google Scholar
14 : Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2006; 91:1683–1690Crossref, Medline, Google Scholar
15 : Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause 2006; 13:770–779Crossref, Medline, Google Scholar
16 : Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr 2009; 14:688–694Crossref, Medline, Google Scholar
17 : Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol 2019; 7:754–766Crossref, Medline, Google Scholar
18 : The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 2003; 28(suppl 3):1–23Google Scholar
19 First M, Spitzer R, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
20 : Research: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther 2010; 16:322–325Crossref, Medline, Google Scholar
21 Lawley Pharmaceuticals. AndroFeme 1 Product Information. West Leederville, Australia, 2012Google Scholar
22 : A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 2012; 81:87–97Crossref, Medline, Google Scholar
23 : A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry 2005; 66:85–93Crossref, Medline, Google Scholar
24 : Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51:871–882Crossref, Medline, Google Scholar
25 : Neuroticism and individual differences in neural function in unmedicated major depression: findings from the EMBARC study. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2:138–148Crossref, Medline, Google Scholar
26 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
27 : Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 2004; 21:450–455Crossref, Medline, Google Scholar
28 : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
29 : Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163:28–40Link, Google Scholar
30 : Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354:1243–1252Crossref, Medline, Google Scholar
31 : Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry 2007; 68:826–831Crossref, Medline, Google Scholar
32 : Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 2009; 166:980–991Link, Google Scholar
33 : Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand 2008; 117:253–259Crossref, Medline, Google Scholar
34 : Augmentation strategies in the treatment of major depressive disorder. CNS Spectr 2007; 12(suppl 22):1–18Medline, Google Scholar
35 : The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2007; 68:843–853Crossref, Medline, Google Scholar
36 : The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2008; 28:156–165Crossref, Medline, Google Scholar
37 : Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab 1998; 83:2717–2725Medline, Google Scholar
38 : Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab 2005; 90:1428–1433Crossref, Medline, Google Scholar
39 : Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med 2005; 165:1582–1589Crossref, Medline, Google Scholar
40 : Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol 2005; 105:944–952Crossref, Medline, Google Scholar
41 : Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab 2005; 90:5226–5233Crossref, Medline, Google Scholar
42 : Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause 2006; 13:387–396Crossref, Medline, Google Scholar
43 : The STAR*D trial: revealing the need for better treatments. Psychiatr Serv 2009; 60:1466–1467Link, Google Scholar
44 : Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002; 287:1840–1847Crossref, Medline, Google Scholar
45 : Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry 2012; 73:1300–1306Crossref, Medline, Google Scholar
46 : Placebo response in depression. Dialogues Clin Neurosci 2002; 4:105–113Medline, Google Scholar
47 : A model of placebo response in antidepressant clinical trials. Am J Psychiatry 2013; 170:723–733Link, Google Scholar
48 : Massachusetts General Hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry 2013; 21:269–274Crossref, Medline, Google Scholar
49 : Guarding the gate: remote structured assessments to enhance enrollment precision in depression trials. J Clin Psychopharmacol 2017; 37:176–181Crossref, Medline, Google Scholar
50 : The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom 2003; 72:115–127Crossref, Medline, Google Scholar
51 : The nature of placebo response in clinical studies of major depressive disorder. J Clin Psychiatry 2015; 76:456–466Crossref, Medline, Google Scholar
52 : Testosterone improves antidepressant-emergent loss of libido in women: findings from a randomized, double-blind, placebo-controlled trial. J Sex Med 2014; 11:831–839Crossref, Medline, Google Scholar
53 : The dynamic characteristics of the anterior cingulate cortex in resting-state fMRI of patients with depression. J Affect Disord 2018; 227:391–397Crossref, Medline, Google Scholar
54 : Distinctive neuroanatomical substrates for depression in bipolar disorder versus major depressive disorder. Cereb Cortex 2019; 29:202–2014Crossref, Medline, Google Scholar
55 : Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res 2004; 132:197–207Crossref, Medline, Google Scholar



