Association of Prefrontal-Striatal Functional Pathology With Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking After Treatment Initiation
Abstract
Objective:
Alcohol use disorder (AUD) is associated with neuroadaptations in brain stress and reward circuits. It is not known whether such neuroadaptations are affected by number of days of alcohol abstinence and whether they influence heavy drinking during the early treatment phase. The authors used a novel functional MRI (fMRI) approach to assess brain responses during sustained exposure to standardized visual stimuli of stressful, alcohol cue, and neutral control images combined with prospective assessment of drinking outcomes during early outpatient treatment, in two related studies.
Methods:
In study 1, 44 treatment-entering patients with AUD and 43 demographically matched healthy control subjects participated in the fMRI experiment to identify dysfunctional responses associated with chronic alcohol abuse. In study 2, 69 treatment-entering patients with AUD were assessed for whether fMRI responses at treatment initiation were influenced by alcohol abstinence and were prospectively predictive of early heavy drinking outcomes.
Results:
Relative to control subjects, patients with AUD showed significant hyperreactivity in the ventromedial prefrontal cortex (vmPFC) in response to neutral images, but significant hypoactivation in the vmPFC and ventral striatum in response to stress images and to alcohol cues relative to response to neutral images. In study 2, this specific prefrontal-ventral striatal dysfunction was associated with fewer days of alcohol abstinence and also predicted greater number heavy drinking days during the subsequent 2 weeks of treatment engagement.
Conclusions:
Number of days of alcohol abstinence at treatment initiation significantly affected functional disruption of the prefrontal-striatal responses to stress images and to alcohol cues in patients with AUD, and the severity of this disruption in turn predicted greater heavy drinking during early treatment. Treatments that target this functional prefrontal-striatal pathology could improve early treatment outcomes in AUD.
Individuals in recovery from alcohol use disorder (AUD) struggle with poor treatment adherence, early dropout, and high risk of relapse (1, 2). Furthermore, patients with AUD enter outpatient treatment with varying lengths of abstinence, from less than 24 hours up to a few weeks (3). Little is known about the neurobiological state during early abstinence, a critical time for outpatient treatment initiation. Current research on brain recovery with abstinence from alcohol includes evidence from animal models (4–6) and data from a select group of AUD patients who can abstain for months or years at a time (7–10). However, over 35% of AUD patients entering outpatient treatment relapse within 30 days, and over 65% relapse within 90 days (1, 11, 12). Furthermore, the effects of each day of alcohol abstinence on functional brain recovery and the impact of such recovery on lapses to heavy drinking during early treatment are not known.
Previous research indicates that chronic alcohol intake is associated with neuroadaptations in functional brain responses in stress and reward circuits (for reviews, see references 13, 14). These neuroadaptations interfere with adaptive emotion processing, stress regulation, and cognition (15) and are associated with high alcohol craving, thereby increasing susceptibility to continued heavy drinking and high relapse risk after treatment (11). Specifically, we have shown (16) that in AUD patients who have been abstinent for 4 weeks, disrupted activity of the ventromedial prefrontal cortex (vmPFC), encompassing the orbitofrontal cortex (OFC) and the rostral anterior cingulate cortex (rACC), and ventral striatal responses to stress, alcohol cue, and neutral-relaxed conditions was associated with higher stress- and alcohol-cue-induced craving and increased relapse risk after inpatient treatment. While the vmPFC and ventral striatal regions make up the salience network and are identified as a key circuit in reward prediction (17, 18) in addiction models (19), the vmPFC and ventral striatum have been shown to be responsive to stress and negative mood cues (20) and predictive of resilient and adaptive coping under stress (21). The effect of days of alcohol abstinence on the integrity of brain functional responses of this key prefrontal-striatal circuit known to predict alcohol relapse has not been systematically investigated thus far.
To address this gap, we conducted two related studies utilizing a unique multimethod functional MRI (fMRI) experimental approach combined with a prospective clinical outcome design. A novel sustained stress and reward provocation task employed a series of standardized and matched visual cues for alcohol, stress, or neutral stimuli over a brief period in a block design (22). Study 1 assessed the brain functional responses to stress images and to alcohol cues relative to an active neutral-control cue block in 44 treatment-entering AUD patients and 43 demographically matched control subjects to specifically examine possible functional disruption of the prefrontal-striatal circuit. Study 2 utilized an expanded group of the treatment-entering AUD patients (N=69) to assess whether the fMRI responses to stress images and to alcohol cues in AUD patients are influenced by number of days of abstinence at treatment entry, and whether such abstinence-related neurobiological functioning is predictive of subsequent heavy drinking during the 2-week period after treatment initiation. Based on previous research (16, 23), we hypothesized in study 1 that the treatment-entering AUD patients would show altered vmPFC and ventral striatal response to the alcohol, stress, and neutral conditions relative to demographically matched, social-drinking healthy control subjects. In study 2, we hypothesized that the extent of functional brain alterations in the vmPFC and ventral striatal regions to stress images and to alcohol cues would be significantly associated with days of alcohol abstinence and that brain changes related to days of abstinence would prospectively predict heavy drinking days during the first 2 weeks of treatment engagement.
Methods
Participants
Participants were recruited from the greater New Haven, Conn., area through advertising on social media and flyers in community locations and treatment facilities. In study 1, treatment-seeking men and women (N=44) 21−60 years of age who met criteria for moderate to severe AUD, as determined by the Structured Clinical Interview for DSM-5 (24), were studied during acute abstinence (1–12 days), along with 43 demographically matched healthy, non-bingeing, social-drinking men and women (healthy control subjects). The AUD and control participants in study 1 were matched for age, sex, race, IQ, and education, but not for drinking measures and nicotine use (Table 1). In study 2, 42 of the AUD sample from study 1 were included, and an additional 27 AUD patients were enrolled, comprising a sample of 69 treatment-seeking men and women whose pre-scan length of alcohol abstinence varied between 24 hours and 35 days. In both study 1 and study 2, all AUD patients were scanned during the intake phase, before initiation of outpatient treatment, and all participants were scanned only once for this research (Figure 1A). Across both studies, participants were required to have a negative alcohol breath test on scan day. Exclusion criteria included a history of psychotic disorders, pregnancy, any nonremovable metal in their bodies, a history of loss of consciousness for longer than 30 minutes, and a current other medical, psychiatric, or substance use disorder (excluding nicotine) and taking medications for such illnesses. We screened 398 individuals, including 176 patients with AUD. Of the 176 AUD patients, 10.8% (19/176) were on psychiatric medications and an additional 4% (7/176) were on medications for medical conditions and were excluded. Individuals requiring medical detoxification from alcohol were also excluded.
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Healthy Control Subjects (N=43) | AUD Patients (N=44) | AUD Patients (N=69) | |||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 32 | 10.0 | 33 | 11.0 | 36 | 11.0 |
| Education (years) | 16.4 | 2.3 | 14.7 | 1.9 | 13.8 | 2.1 |
| Shipley IQ estimate | 113 | 7.0 | 112 | 7.0 | 106 | 7.0 |
| Years of alcohol use | 12.5 | 2.2 | 13.3 | 1.7 | 14.2 | 1.3 |
| Days of alcohol use/monthb | 6.0 | 0.9 | 19.1 | 1.2 | 23.6 | 4.7 |
| Amount of alcohol use/monthb | 11.5 | 2.0 | 119.3 | 15.1 | 139.0 | 26.2 |
| Days abstinent at scan | 11.75 | 10.7 | 5.5 | 1.2 | 4.7 | 0.9 |
| N | % | N | % | N | % | |
| Male | 23 | 53.5 | 28 | 63.6 | 42 | 61.0 |
| Caucasian | 25 | 58.1 | 26 | 59.0 | 28 | 41.0 |
| Smokerb | 0 | 0.0 | 21 | 47.7 | 36 | 52.0 |
| Lifetime mood disorderb | 6 | 13.9 | 16 | 36.4 | 16 | 23.2 |
| Lifetime anxiety disorderb | 1 | 2.3 | 28 | 65.1 | 26 | 37.7 |
| Lifetime PTSD | 1 | 2.3 | 1 | 2.3 | 1 | 1.5 |
| Lifetime other substance use disorderb | 3 | 6.9 | 12 | 27.2 | 12 | 17.4 |
TABLE 1. Demographic and clinical characteristics of participants in a study of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiationa
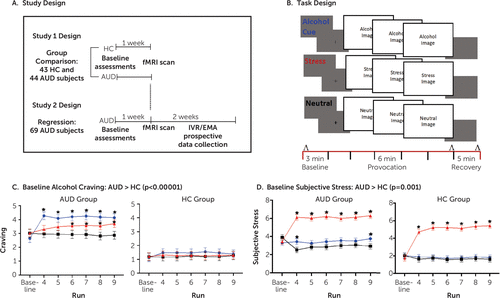
FIGURE 1. Study and task designs, with craving and subjective stress ratings by group and conditiona
a As illustrated in panel A, in study 1, healthy control (HC) subjects and treatment-seeking alcohol use disorder (AUD) patients were recruited for a single functional MRI (fMRI) scan. In study 2, treatment-entering AUD patients with varying days of abstinence underwent a single initial fMRI scan and were then followed using intensive longitudinal measurement for 2 weeks. IVR/EMA=interactive voice response/ecological momentary assessment. In panel B, the study 1 and study 2 fMRI paradigm for provocation of stress and alcohol cue relative to neutral reactivity uses sustained exposures to six 1-minute consecutive runs of visual images for alcohol, stress, or neutral conditions presented in a block design. Plasma cortisol levels were measured before, immediately after, and at 5-minute recovery time points after provocation runs (indicated by ʌ). Subjective stress and craving were measured after all baseline and provocation runs (indicated by black lines) in each condition block. Heart rate was measured continuously (indicated by red line). In panel C, for study 1, alcohol craving at baseline and in response to alcohol cue, neutral, and stressful visual images is plotted for the AUD and healthy control groups, showing a baseline difference between groups (AUD group > healthy control group, p<0.000001). After correction for baseline levels of craving, the AUD group also showed significantly higher craving responses to alcohol cues compared with the healthy control group (F=286.2, df=1, 595, p<0.0001) and to stress images (F=209.3, df=1, 595, p<0.0001) but not to neutral stimuli. Within the AUD group, craving was significantly higher in response to alcohol cues than the neutral condition at each time point (p values <0.05) and during later runs in the stress condition relative to the neutral condition (p values <0.05). Asterisks denote a significant difference from the neutral condition. In panel D, the subjective stress responses to alcohol cue, neutral, and stressful visual images are plotted for the AUD and healthy control groups. The AUD group shows greater baseline subjective stress relative to the healthy control group (F=41.92, df=1, 255, p<0.0001). Both the AUD and healthy control groups showed significantly greater subjective response to stress images than to neutral images (F=160.1, df=2, 903, p<0.0001) and to alcohol cues (F=206.8, df=2, 882, p<0.0001). In the AUD group, at the beginning and end of the alcohol cue runs, subjective stress was higher than in the neutral condition (p values <0.05). Asterisks denote a significant difference from the neutral condition.
Weekly Behavioral Counseling in Study 2
After the fMRI scan at treatment entry, all AUD patients started weekly behavioral counseling with a training master’s-level alcohol and drug abuse counselor who utilized the standardized 12-step facilitation therapy from the National Institute on Alcohol Abuse and Alcoholism (25) as part of their standard of care, which was not compared with any other behavioral treatment.
The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
fMRI Provocation Procedure
The novel fMRI paradigm involved brief successive exposure to three conditions: 1) a block of highly appetitive images of alcoholic beverages and people drinking alcoholic beverages alone or in social contexts; 2) a block of highly threatening, aversive, and stressful images; and 3) a no-cue control block involving neutral images (Figure 1B) (22). Pictures included images from the International Affective Picture System (26) and those developed at the Yale Stress Center; selected alcohol cue images from the web were downloaded and rated using the same rating method as the International Affective Picture System images. The alcohol cues included images of wine, beer, cocktails, champagne, and hard liquor. The stress condition involved highly aversive images of terror, violence, mutilation, fear, disgust, and desperation. The neutral condition consisted of neutral images of mountains, grass, trees, streams, stones, and trails.
Each image within a block was presented for 5 seconds, with a 1-second interstimulus interval, over six successive runs of 66 seconds each (11 images per run; 396 images per 6-minute block). Each alcohol cue, stress, and neutral condition was counterbalanced across participants and presented in randomized order to prevent order effects in the responses. Baseline brain responses were collected within each condition block where provocation stimuli runs were preceded by three 66-second runs of gray fixation baseline blocks for comparison with the respective alcohol cue, stress, or neutral provocation runs (Figure 1B). There was a 5-minute postprovocation recovery period after each condition, during which participants were provided audio-recorded instructions on progressive relaxation. Visual analogue scales (ratings ranged from 1 to 9) were used for assessing alcohol craving and subjective stress after each 66-second run within each block.
fMRI Methods and Procedures
Study participants arrived at the Yale Magnetic Resonance Research Center at 7:00 a.m. in a fasting state. Transportation was provided if it was otherwise unavailable. Breath alcohol and urine toxicology screens were used to confirm drug and alcohol abstinence for each intake session and the scanning session. Participants were settled in a hospital bed in a preparation/recovery room, where an intravenous line was inserted in the nondominant forearm for repeated blood drawing for cortisol data collection during the scan. Participants then completed a practice task consisting of 10 trials using stimuli that were not used for the in-scan fMRI task. After a 45-minute adaptation period, subjects underwent fMRI scanning, with repeated blood draws, and a pulse oximeter was placed on the nondominant forefinger to obtain heart rate during the scan.
Physiological measurements.
Heart rate was obtained from the pulse oximeter during the baseline and provocation runs in each condition. Pulse was estimated and averaged for baseline and each provocation run, and change from baseline within each condition was computed for each condition. Blood sampling for the assessment of cortisol levels was performed for each condition block at baseline, after the six provocation runs, and after a 5-minute recovery period for each condition. Blood samples were obtained from an indwelling catheter on the subjects’ forearm, which was accessible without moving the subject before and after each condition (22). Blood was collected in EDTA-coated tubes (BD Biosciences, Franklin Lakes, N.J.). Blood samples were immediately stored on ice, and plasma was separated by centrifugation at 4°C for 10 minutes at 1000×g. Aliquots of plasma were stored in polypropylene tubes at −80°C until assayed. Cortisol levels were assessed using a radioimmunoassay procedure at the Yale Center for Clinical Investigation Core Laboratory. Within-person change in cortisol responses were computed by subtracting cortisol values measured at recovery-provocation for each condition and then further assessing change in stress and alcohol cue responses relative to the neutral condition (see the Supplemental Methods section in the online supplement).
fMRI acquisition.
Scanning was performed in a 3-T multiband Siemens Trio or Prisma MRI system equipped with a standard quadrature head coil, using the T1 magnetization-prepared rapid gradient-echo (MPRAGE) sequence for structural scanning and T2*-sensitive gradient-recalled single shot echo planar pulse sequence for functional scans. Blood-oxygen-level-dependent signals were acquired with a 64-channel head coil with a multiband accelerated echo planar imaging sequence. Seventy-five axial slices parallel to the anterior commissure–posterior commissure line covering the whole brain were acquired (TR=1000 ms, TE=30 ms, bandwidth=1,894 Hz/pixel, flip angle=55°, field of view=220×220 mm, slice thickness=2 mm, no gap).
fMRI data preprocessing.
Data were converted from Digital Imaging and Communication in Medicine format to Analyze format using XMedCon. General linear models were used for individual-level analysis on each voxel in the entire brain volume with a regressor (time during picture viewing) for each run per condition using BioImage Suite. Trials with linear motion in excess of 1.5 mm or rotation greater than 2° were discarded. The regressor was each visual image run relative to the baseline gray fixation period, resulting in a relative stress images-stress baseline contrast, an alcohol cue images-alcohol cue baseline contrast, and a neutral images-neutral baseline contrast for each run. Temporal filtering was carried out by including drift correction in the general linear model. Each trial was spatially smoothed using a 6-mm Gaussian kernel and individually normalized to generate beta maps (3.44×3.44×4 mm). To account for individual anatomical differences, three sequential registrations were performed using BioImage Suite: linear registration of raw data into two-dimensional anatomical images, the two-dimensional to three-dimensional (1×1×1 mm) linear registration, and a nonlinear registration to a reference three-dimensional image, the Colin27 Brain. The overall preprocessing approach did not utilize any prewhitening correction to address potential serial autocorrelations of time-series data, as additional preprocessing with prewhitening correction using the autoregressive moving average (ARMA) model from AFNI yielded remarkably similar second-level results (see Figure S2 in the online supplement).
fMRI statistical parameters.
In both study 1 and study 2, to correct for multiple comparisons, we used family-wise error correction determined by Monte Carlo simulation using AFNI’s 3dClustSim, version 16.3.05. A p value of 0.001 was considered statistically significant for whole brain family-wise error correction, and a cluster correction of p=0.05 was used. The cluster-forming threshold was 320 mm3.
Study 1: group analyses.
Second-level group differences in terms of age, sex, IQ, and race were examined using chi-square tests, and continuous measures were examined with independent-samples t tests. Linear mixed-effect models were used to assess subjective stress, alcohol craving, heart rate, and cortisol responses during each condition to ensure the validity of the stress and emotion provocation manipulation. For brain responses, voxelwise whole brain data were analyzed using linear mixed-effect models with subjects as a random effect. Condition was treated as a within-subject fixed-effect factor and group as the between-subject factor. Covariates included sex, age, and days abstinent from alcohol. Condition effects represent activation to the active cue relative to baseline in each condition. The neutral condition served as an active control comparison condition for the stress and alcohol cue challenge conditions, because it does not increase alcohol craving and it controls for the nonspecific effects of the experimental manipulation. Thus, group differences are presented in the form of alcohol cue-neutral and stress-neutral contrasts and also responses in the neutral control condition alone.
Study 2: abstinence and fMRI.
Second-level voxelwise whole brain linear mixed-effect analysis was used, with number of abstinence days as the predictor variable of functional brain responses to cues. Condition was treated as a within-subject fixed-effect factor and subject as a random factor. Covariates included sex and age. Significant interactions between condition and abstinence days were further assessed with stress and alcohol cue relative to neutral contrasts. Beta values were extracted from the significant regions of interest for illustration purposes only.
Study 2: Prospective Daily Assessment of Early Treatment Outcome
At the first intake, AUD patients completed an orientation to the daily diary assessments via a smartphone application (MetricWire). The daily surveys assessed how many glasses of beer, wine, and mixed drinks were consumed per day during the first 14 days of treatment engagement. The quantity of each type of drink was summed for each day to create an index of total drinks consumed per day. Measurements were collected during an evening survey that was available from 8:00 p.m. to 2:00 a.m. In addition, a morning survey was available from 8:00 a.m. to 2:00 p.m., which was used to estimate drinking if the participants missed the previous day’s evening survey.
Compliance With Daily Diaries
Participants completed a total of 1,026 logs, of which 37.5% were of drinking days. On average, participants drank on 5.20 days (SD=4.36, range=0–14) during the 2-week period. The average number of drinks consumed on drinking days was 4.58 (SD=4.52). Consistent with previous patient treatment-based daily diary research (27), adherence to daily reporting was 71.2%. Information about daily alcohol consumption was supplemented with a weekly substance use calendar, based on the timeline follow-back assessment method (28), for any patient who missed daily drinking diary entries on a particular day.
Study 2: Craving, Cortisol Responses, and fMRI Prospective Early Treatment Outcome Analyses
First, we used Cox proportional hazards regression to prospectively evaluate the effects of abstinence days at treatment entry for impact on heavy drinking days during early treatment. The Wald test was performed to determine whether the number of days of abstinence at treatment initiation predicted a swifter lapse to heavy drinking during early treatment. Second, we used multiple regression models to assess whether abstinence days predicted subjective alcohol craving, stress, and cortisol responses. Finally, we used linear mixed-effect models covarying for sex, age, and abstinence days to assess the effects of alcohol craving, stress, and cortisol response on number of heavy drinking days during early treatment and on the interaction of functional brain response (voxelwise, whole brain) with condition, and drinking outcome during early treatment. A priori hypothesized regions of interest that significantly predicted heavy drinking days during treatment were extracted to create scatterplots of abstinence-related brain responses and heavy drinking days during early treatment.
Results
Participants’ Demographic Characteristics
As shown in Table 1, in study 1, the AUD and healthy control groups were matched for age, sex, race, IQ, years of education, and years of regular alcohol consumption, but, as expected, they differed substantially and significantly in frequency of drinking alcohol (p<0.0001) and usual amount of alcohol consumed. The AUD samples in study 1 and study 2 included significantly more smokers than the study 1 healthy control group but did not differ significantly from each other (χ2=0.074, N=113, p=0.79). The AUD sample in study 1 also had significantly more lifetime diagnoses of mood disorders, anxiety disorders, and other substance use disorders than the study 1 healthy control group. The healthy control group did not differ significantly in lifetime diagnoses of posttraumatic stress disorder (PTSD) from the AUD samples.
The AUD participants in study 2 did not differ significantly from the AUD participants in study 1 in age, years of education, IQ, race, alcohol use variables, proportion of smokers, or lifetime diagnoses of mood disorders, PTSD, or other substance use disorders. The AUD sample in study 1 had significantly higher rates of lifetime anxiety disorder diagnoses than the sample in study 2. Among both the healthy control and AUD groups for study 1 and study 2, participants with and without a lifetime history of comorbid diagnoses did not differ significantly in abstinence days, craving, stress, or cortisol responses.
Study 1
Manipulation check for stress and alcohol cue provocation.
Alcohol craving
The AUD group showed greater baseline alcohol craving than the healthy control group (F=56.54, df=1, 255, p<0.0001) across conditions. After correction for baseline levels of craving in each group, there was a group-by-condition interaction (F=3.36, df=6, 595, p=0.003), such that the AUD group also showed significantly higher craving responses to alcohol cues (F=286.2, df=1, 595, p<0.0001) and stress images (F=209.3, df=1, 595, p<0.0001) than the healthy control group (Figure 1C). There was no significant group difference in craving increases elicited by the neutral stimuli.
Subjective stress
The AUD group showed greater baseline subjective stress relative to the healthy control group (F=41.92, df=1, 255, p<0.0001). After correcting for baseline differences, there was a condition main effect such that both the AUD and healthy control groups showed significantly greater subjective response to stress images (F=160.1, df=2, 903, p<0.0001) and to alcohol cues (F=206.8, df=2, 882, p<0.0001) relative to the neutral condition (Figure 1D). Within the AUD group, there was an interaction between condition and time point, such that relative to the neutral condition, subjective stress was greater at the beginning and end of the alcohol cue block (p values <0.05) (see Figure 1D).
Cortisol response
Within-person cortisol responses (recovery − provocation) to conditions were significantly different in the AUD group compared with the healthy control group. In the neutral control condition, cortisol was higher in the AUD group relative to the healthy control group (t=2.29, p<0.03). Assessing stress and alcohol cue responses relative to neutral responses, we found significant group (F=6.40, df=1, 58, p<0.01) and condition (F=4.13, df=1, 58, p<0.05) main effects but no interactions. The group effect resulted from the healthy control group showing an increased cortisol response to stress and alcohol cue relative to neutral response, whereas the AUD group did not (t=2.1, df=85, p=0.04) (see Figure S1A in the online supplement). The condition effect was due to greater overall increases in cortisol levels in response to alcohol cue, relative to neutral cue, than with stress relative to neutral cue across both groups (t=2.03, df=85, p<0.05). These data suggest significantly altered cortisol responses in the AUD group relative to the healthy control group.
Heart rate
The AUD group showed elevated basal heart rate relative to the healthy control group across conditions (t=6.68, df=85, p<0.0001). The AUD group also showed blunted heart rate response to alcohol cues relative to neutral images, compared with the healthy control group (F=5.013, df=1, 170, p=0.03), but similar increases in heart rate responses to stress relative to neutral images were observed across both groups (F=3.329, df=1, 170, p=0.07) (see Figure S1B in the online supplement).
Whole Brain Voxel-Based Analyses
Highly significant differences between the AUD and healthy control groups were found in cortico-striatal-limbic regions in response to the stress, alcohol, and neutral conditions in whole brain analyses (p<0.001, AlphaSim correction at p<0.05) (see Table S1 in the online supplement). As hypothesized, and as shown in Figure 2, the AUD group showed hyperactivation of the vmPFC, including the rACC, in the neutral condition, but hypoactivation in the vmPFC/rACC in the stress-neutral contrast and the alcohol cue-neutral contrast relative to the healthy control group. Additionally, the healthy control group showed increased ventral striatal activation in response to alcohol cues, which was absent in the AUD group (alcohol-neutral contrast). Finally, the AUD group showed vmPFC hypoactivation and both ventral striatal and dorsal striatal hyperactivation in the stress-alcohol cue contrast, whereas the healthy control group did not.
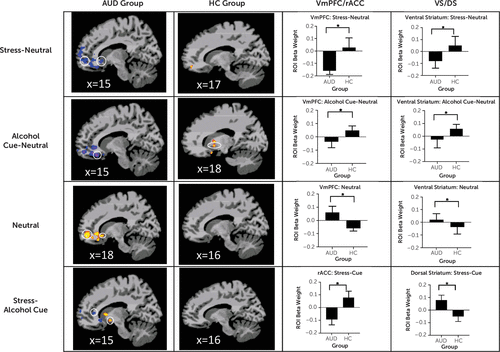
FIGURE 2. Study 1: Whole brain voxel-based analyses of fMRI contrasts for the AUD and healthy control groups, focused on hypothesized region-of-interest functional cue reactivity, and average change in brain response for the regions of interesta
a The first two columns present sagittal slices showing average activation for the alcohol use disorder (AUD) group and the healthy control (HC) group. The third and fourth columns show the average change in region-of-interest (ROI) beta weights for the ventromedial prefrontal cortex/rostral anterior cingulate (vmPFC/rACC) and ventral striatal/dorsal striatal (VS/DS) regions of interest for the AUD and HC groups. All differences were significant after whole brain correction at p<0.001, with an AlphaSim cluster correction at 0.05. In row 1, in the stress-neutral contrast, the AUD group shows vmPFC and ventral striatal hypoactivation (shown in blue) relative to the healthy control group (peak differences, MNI coordinates: 17, 52, −15 and 15, 14, −9, respectively). In row 2, in the alcohol cue-neutral contrast, the AUD group shows vmPFC and ventral striatal hypoactivation (blue-purple) relative to the healthy control group (peak differences, MNI coordinates: 15, 24, −21 and 18, 8, −12, respectively). In row 3, in the neutral-baseline contrast, the AUD group shows vmPFC and ventral striatal hyperactivation (yellow/red) relative to the healthy control group (peak differences, MNI coordinates: 18, 51, −12 and 18, 17, −9, respectively). In row 4, in the stress-alcohol cue contrast, the AUD group shows rostral anterior cingulate hypoactivation (blue) and dorsal striatal hyperactivation (yellow/red) relative to the healthy control group (peak differences, MNI coordinates: 15, 37, 10 and 15, 7, −11, respectively). (A full list of group differences is provided in Table S1 in the online supplement.)
Study 2
Alcohol abstinence days and craving, stress, and cortisol responses.
A lower number of abstinence days was significantly associated with higher mean alcohol craving during stress provocation (r=−0.28, p<0.02) and higher maximum alcohol craving during the neutral condition (r=−0.22, p<0.05), but not with alcohol cue-provoked craving. Number of abstinence days was not significantly associated with subjective stress ratings. Cortisol responses to stress and alcohol cues relative to neutral and in the neutral condition were also not significantly associated with number of abstinence days.
Alcohol abstinence days and brain functional responses to cues.
Number of abstinence days was significantly associated with brain responses in several cortico-striatal-limbic regions during stress and alcohol cues relative to neutral images (see Table S1 in the online supplement). More importantly, as hypothesized, number of abstinence days significantly influenced the extent of disrupted functional responses in the vmPFC/rACC and ventral striatum across the stress, alcohol, and neutral conditions. Specifically, lower number of abstinence days was associated with greater vmPFC/rACC hypoactivation in response to stress images and greater ventral striatal hypoactivation in response to alcohol cues, but greater vmPFC/rACC and ventral striatal hyperactivation in the neutral condition (Figure 3).
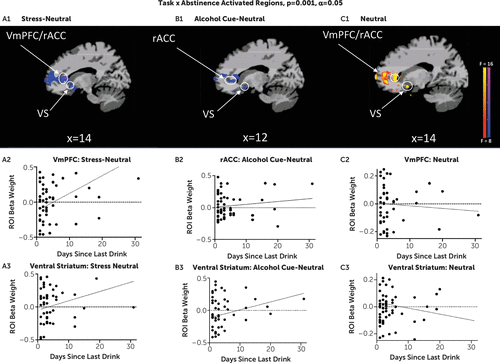
FIGURE 3. Study 2: Prefrontal and ventral striatal associations with number of days of pretreatment abstinence, from whole brain voxel-based analyses, in patients with AUDa
a In panels A1–A3, in the stress condition (stress-neutral contrast), lower number of days abstinent from alcohol in the alcohol use disorder (AUD) group on scan day and at treatment entry was associated with greater hypoactivation (blue) of the ventromedial prefrontal cortex/rostral anterior cingulate cortex (vmPFC/rACC) and ventral striatal regions in response to stress relative to the neutral contrast (peak activation association, MNI coordinates: 2, 36, 12 and 9, 19, −2, respectively). ROI=region of interest. In panels B1–B3, in the alcohol cue condition (alcohol cue-neutral contrast), lower number of days abstinent from alcohol in the AUD group on scan day and at treatment entry was associated with greater hypoactivation (blue) of the vmPFC/rACC and ventral striatal regions in response to alcohol cues relative to neutral contrast (peak activation association, MNI coordinates: 2, 34, −3 and 11, 8, −11, respectively). Activation of rACC in panels A1 and B1 extends into the lateral ventricle. In panels C1–C3, in the neutral condition (neutral-baseline contrast), lower number of days abstinent from alcohol in the AUD group on scan day and at treatment entry was associated with greater hyperactivation (yellow/red) of the vmPFC/rACC and ventral striatal regions in the neutral condition (peak activation association, MNI coordinates: 14, 41, 7 and 14, 9, −10, respectively). All differences were significant after whole brain correction at p<0.001, with an alpha of 0.05. (A full list of associations is provided in Table S1 in the online supplement.)
Relationship between abstinence days and early treatment outcome.
The Cox proportional hazards regression analysis indicated that number of abstinence days significantly predicted subsequent heavy drinking days outcome (Wald test=6.1, p<0.01), with lower number of abstinence days associated with greater likelihood of quicker lapse to heavy drinking during early treatment. For each additional day of abstinence at treatment initiation, the risk of lapse to heavy drinking in the first 2 weeks decreased by 14%, at a hazard ratio of 0.873 (Figure 4A).
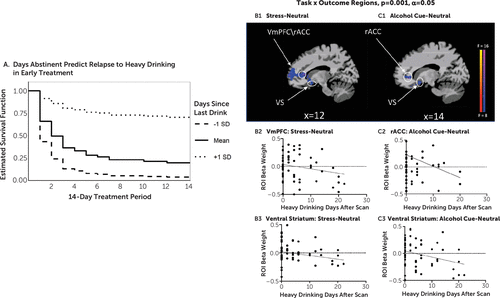
FIGURE 4. Study 2: Whole brain relationship between abstinence days-covaried task (stress, alcohol cue, neutral condition) and heavy drinking days outcome during early AUD treatmenta
a Panel A is an estimated survival function for time to heavy drinking day lapse among treatment-entering patients with alcohol use disorder (AUD), shown by length of abstinence in the first 2 weeks of treatment initiation. Participants with a longer abstinence (+1 SD) were less likely overall to lapse to heavy drinking and they lapsed to heavy drinking at a slower rate than those with a shorter abstinence duration (−1 SD). In panels B1–B3, participants with AUD who showed greater ventromedial prefrontal cortex (vmPFC) and ventral striatal hypoactivation (blue) in response to stress images (stress-neutral contrast; peak activation association, MNI coordinates: 14, 38, 3 and 12, 8, –12, respectively) relapsed to heavy drinking faster than those with increased activation of these regions in response to stress. rACC=rostral anterior cingulate cortex; ROI=region of interest. In panels C1–C3, participants with AUD who showed greater hypoactivation in the vmPFC and ventral striatum (blue) in response to alcohol cues (alcohol cues-neutral contrast; peak activation association, MNI coordinates: 12, 38, 2 and 14, 10, 12, respectively) relapsed to heavy drinking faster than those with increased activation of these regions in response to alcohol cues. All differences were significant after whole brain correction at p<0.001, with an alpha of 0.05. (A full list of associations is provided in Table S1 in the online supplement.)
Relationship between craving, subjective stress, and cortisol response and heavy drinking days during early treatment.
While high alcohol craving in response to alcohol cues (short of statistical significance, p<0.05), high subjective stress during the neutral condition (p<0.03), and high cortisol response during the neutral condition (p<0.05) were each associated with the heavy drinking days outcome independently, these responses were not significantly predictive of the heavy drinking days outcome when number of abstinence days was added to a multivariate regression model (see Table S2 in the online supplement). Therefore, only number of abstinence days was used as a predictor variable in the following fMRI and outcome analyses.
Disrupted brain responses to stress and alcohol cues and heavy drinking outcome.
Disrupted functional responses related to number of abstinence days in the hypothesized vmPFC/rACC and ventral striatal regions, and additional cortico-striatal-limbic regions, during stress, alcohol cue relative to neutral, and neutral condition alone were significantly predictive of heavy drinking days during early treatment (see Table S1 in the online supplement). Regions of interest for the hypothesized regions of the vmPFC/rACC and ventral striatum were extracted from the significant voxel-based interaction effects of condition by outcome (heavy drinking days) analyses to illustrate the prediction of heavy drinking days during early treatment. The results indicate that both lower vmPFC/rACC hypoactivation to stress images (stress-neutral contrast) and greater vmPFC/rACC prefrontal hyperactivity in the neutral state (neutral-baseline contrast) predicted increased heavy drinking days during early treatment. Furthermore, lower ventral striatal hypoactivation in response to alcohol cues (alcohol cues-neutral contrast) was predictive of more heavy drinking days during early treatment (see Figure 4B,C).
Discussion
In two related studies, using a novel brief sustained-exposure stress, alcohol cue, and neutral control visual stimuli paradigm, we provide previously unreported evidence of 1) disrupted prefrontal-striatal functional responses to stress and alcohol cue provocation and to the neutral control condition in participants with AUD entering treatment relative to healthy social drinkers, and 2) a significant influence of number of days of alcohol abstinence on extent of disrupted prefrontal-striatal function in response to stress, alcohol cue, and neutral provocation, which in turn had a significant prospective effect on drinking outcomes in early treatment. In study 1, AUD patients, on treatment entry, showed highly significant dysfunctional blunted vmPFC and ventral striatal responses to stress images and to alcohol cues relative to neutral images as compared with demographically matched control subjects but vmPFC/rACC and ventral striatal hyperactivation to the neutral condition. These results supported our hypothesis of significant prefrontal-striatal functional pathology during early abstinence in treatment-entering AUD patients. In study 2, we found that number of days of alcohol abstinence at treatment initiation significantly affected the altered vmPFC/rACC and ventral striatal functional response, such that lower number of days of abstinence prior to the scan was associated with greater disruption of the vmPFC/rACC and ventral striatal activity in response to the stress, alcohol, and neutral conditions. Additionally, after controlling for the effects of abstinence days on brain responses to cues, we found that greater dysfunctional prefrontal and striatal responses to stress and to alcohol cues prospectively predicted more heavy drinking days in the first 2 weeks of outpatient treatment for AUD.
It is notable that dysfunctional prefrontal-ventral striatal functional responses were found in the AUD group relative to the control group in study 1, and that such dysfunction was also related to important clinical indicators, such as number of days of abstinence at treatment entry and drinking outcome during early treatment engagement. We used a whole brain analytic approach to specifically assess the hypothesized prefrontal-ventral striatal circuit across the two studies. The similarity of brain findings across the two related studies speaks to the strength and robustness of the results. Furthermore, while craving, subjective stress, and cortisol response were each correlated with heavy drinking day outcome, only number of abstinence days and abstinence-related dysfunctional brain responses predicted drinking outcome in early treatment. These findings suggest that extent of brain functional pathology along with days of abstinence may be important markers of early treatment outcome and recovery.
Because relapse to drinking and treatment dropout during early outpatient treatment are common, identifying predictors of heavy drinking and relapse during early recovery is critical. Stress and alcohol cues in the environment are two main reasons patients cite for relapse (29, 30). These results are consistent with our current understanding of the underlying neurobiology of AUD, in which chronic alcohol-related neuroadaptations in prefrontal-ventral striatal circuits may hamper a person’s ability to recruit prefrontal executive control functions in response to stress cues, while also showing hypoactivation in the ventral striatal, appetitive brain regions to respond appropriately to alcohol rewarding stimuli, and this combined dysfunction may increase risk of having heavy drinking days and risk of relapse (31). This is also consistent with growing evidence that AUD patients are unable to appropriately regulate responses to stress and alcohol cues in the environment during recovery initiation (29). Additionally, a number of previous studies have reported down-regulation of dopamine and blunted ventral striatal responses to alcohol and to alcohol cues in AUD patients and in binge drinkers or heavy drinkers (32–34). Furthermore, we have previously shown ventral striatal hyperactivation to neutral cues in AUD patients (16), suggesting a heightened tonic salience of alcohol, and probability of approach behavior in the presence of alcohol cues (35, 36), consistent with previous work in AUD patients in fMRI and PET studies (13, 37). Dysfunctional ventral striatal responses predicting future alcohol use outcomes are also consistent with previous data on prediction of drinking outcomes in the laboratory (38) as well as in real-world settings (39).
Findings from study 2 corroborate those of study 1 in that disruption of prefrontal-striatal functioning in AUD patients was significantly associated with fewer days of abstinence—that is, greater prefrontal hypoactivation during stress, but greater hyperactivity during neutral states, and greater ventral striatal hypoactivity to alcohol cues were associated with lower number of days of abstinence prior to treatment entry. These findings support animal studies that suggest that the brain structural and functional state is highly dynamic during early recovery (5, 40). Indeed, it appears that with each day of abstinence, there is improvement in functioning of prefrontal-striatal circuits known to be critical for executive control, resilient coping, and regulation of craving and reward responses (22, 41–43). Although these findings and others (44) suggest that longer abstinence periods may be required to reverse disrupted vmPFC and ventral striatal functioning, a critical clinical issue is that such chronic alcohol-related disruptions have a significant impact on heavy drinking lapses and thus may jeopardize initiation of early recovery by increasing risk of relapse.
Our findings suggest the need for development of targeted therapeutics to reverse such prefrontal-striatal dysfunction early in treatment—that is, treatments that aim to reverse vmPFC/rACC and ventral striatal functional disruptions in order to support successful initiation of recovery. Noradrenergic compounds, such as prazosin and guanfacine, which are known to rescue prefrontal stress pathways (45, 46), have the potential to restore vmPFC/rACC functioning and executive control as well as to reduce stress and drug craving in patients with substance use disorders (47, 48). Additionally, intensive outpatient or partial hospitalization programs that provide higher levels of support and counseling to maintain alcohol abstinence may also further improve vmPFC functioning to counter high-risk stress situations and alcohol-related contexts during early recovery.
This study has several strengths, including careful assessment of the individual variation in length of abstinence and daily mobile health assessment of drinking behavior during initial treatment engagement to obtain daily data on drinking outcome without introducing bias from poor recall (49). Another important strength was the validation of a novel standardized visual stress and alcohol cue provocation paradigm in a patient sample, which can be easily implemented and replicated by other laboratories and in different patient samples.
These novel findings must be considered in light of the study limitations. First, our sample size limited the ability to systematically assess sex differences in the sample. Second, it is important to replicate this research in nonsmoking patients with AUD, as different patterns of co-occurring drug use are common among individuals with AUD and may limit the generalizability of our findings. Third, because AUD is associated with higher levels of chronic stress and traumatic experiences, samples with varying levels of trauma and stress exposure may elucidate differential functional dysfunction in AUD patients with and without trauma. Finally, given the challenges of achieving and maintaining alcohol abstinence, future studies also need to consider the effects of significant reductions in alcohol use compared with complete abstinence on early brain recovery.
Despite these limitations, our findings in this study are the first to show a direct relationship between lower number of alcohol abstinence days and greater disruption in prefrontal-striatal function in response to stress, alcohol cue, and neutral conditions, which in turn are predictive of subsequent heavy drinking during early treatment. These findings support the critical need for developing targeted medications and interventions that will facilitate rescue of disrupted prefrontal-striatal functioning to initiate early brain recovery from AUD.
1 : Relapse rates in addiction programs. J Clin Psychol 1971; 27:455–456Crossref, Medline, Google Scholar
2 : Predictors of mental health treatment seeking and engagement in a community mental health center. Community Ment Health J 2017; 53:510–514Crossref, Medline, Google Scholar
3 : “I already stopped”: abstinence prior to treatment. Addiction 2000; 95:65–76Crossref, Medline, Google Scholar
4 : Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol Res Health 2008; 31:377–388Medline, Google Scholar
5 : Neuroimaging in alcohol use disorder: from mouse to man. J Neurosci Res (Online ahead of print, April 22, 2019)Google Scholar
6 : Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res 2017; 38:183–206Medline, Google Scholar
7 : Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 1995; 19:1177–1191Crossref, Medline, Google Scholar
8 : Influence of improved drinking habits on brain atrophy and cognitive performance in alcoholic patients: a 5-year follow-up study. Alcohol Clin Exp Res 1989; 13:137–141Crossref, Medline, Google Scholar
9 : Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 2012; 69:842–852Crossref, Medline, Google Scholar
10 : Neuroplasticity in human alcoholism: studies of extended abstinence with potential treatment implications. Alcohol Res 2015; 37:125–141Medline, Google Scholar
11 : New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 2011; 13:398–405Crossref, Medline, Google Scholar
12 : Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci 2017; 37:10867–10876Crossref, Medline, Google Scholar
13 : Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 2013; 18:121–133Crossref, Medline, Google Scholar
14 : Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 2012; 37:2267–2276Crossref, Medline, Google Scholar
15 : Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Res 2013; 213:202–209Crossref, Medline, Google Scholar
16 : Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 2013; 70:727–739Crossref, Medline, Google Scholar
17 : Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8:1481–1489Crossref, Medline, Google Scholar
18 : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356Crossref, Medline, Google Scholar
19 : Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35:217–238Crossref, Medline, Google Scholar
20 : Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol 1997; 106:243–250Crossref, Medline, Google Scholar
21 : Imaging resilience and recovery in alcohol dependence. Addiction 2018; 113:1933–1950Crossref, Medline, Google Scholar
22 : Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci USA 2016; 113:8837–8842Crossref, Medline, Google Scholar
23 : Central and peripheral biomarkers of stress response for addiction risk and relapse vulnerability. Trends Mol Med 2018; 24:173–186Crossref, Medline, Google Scholar
24 First MB, Williams JBW, Karg RS, et al: Structured Clinical Interview for DSM-5–Research Version (SCID-5-RV). Washington, DC, American Psychiatric Association, 2015Google Scholar
25 : Twelve Step Facilitation Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence, vol. 1. Collingdale, Pa, Diane Publishing Company, 1992Google Scholar
26 : International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, University of Florida, Center for Research in Psychophysiology, 1999Google Scholar
27 : Compliance with ecological momentary assessment protocols in substance users: a meta-analysis. Addiction 2019; 114:609–619Crossref, Medline, Google Scholar
28 : Timeline follow-back: a technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods. Edited by Allen L, Litten R. Totowa, NJ, Humana Press, 1992, pp 41–72Crossref, Google Scholar
29 : Stress, cognitive factors, and coping resources as predictors of relapse in alcoholics. Addict Behav 1999; 24:687–693Crossref, Medline, Google Scholar
30 : Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011; 218:69–82Crossref, Medline, Google Scholar
31 : Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017; 122:136–147Crossref, Medline, Google Scholar
32 : Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry 2016; 21:348–356Crossref, Medline, Google Scholar
33 : Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 2005; 58:779–786Crossref, Medline, Google Scholar
34 : Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend 2016; 160:163–169Crossref, Medline, Google Scholar
35 : Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 2004; 29:393–402Crossref, Medline, Google Scholar
36 : Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001; 157:67–74Crossref, Medline, Google Scholar
37 : Molecular imaging in alcohol dependence, in Handbook of Clinical Neurology, vol 125, Alcohol and the Nervous System. Amsterdam, Elsevier, 2014, pp 293–311Google Scholar
38 : Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [¹¹C]raclopride. Biol Psychiatry 2010; 68:689–696Crossref, Medline, Google Scholar
39 : Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry 2013; 70:661–663Crossref, Medline, Google Scholar
40 : Transient CNS responses to repeated binge ethanol treatment. Addict Biol 2016; 21:1199–1216Crossref, Medline, Google Scholar
41 : Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 2001; 39:376–389Crossref, Medline, Google Scholar
42 : Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15:85–93Crossref, Medline, Google Scholar
43 : Role of the medial prefrontal cortex in coping and resilience. Brain Res 2010; 1355:52–60Crossref, Medline, Google Scholar
44 : Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 2012; 125:27–36Crossref, Medline, Google Scholar
45 : The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother 2010; 10:1595–1605Crossref, Medline, Google Scholar
46 : A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol 2015; 29:300–311Crossref, Medline, Google Scholar
47 : Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res 2012; 36:351–360Crossref, Medline, Google Scholar
48 : Guanfacine effects on stress, drug craving, and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 2012; 26:958–972Crossref, Medline, Google Scholar
49 : Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess 2009; 21:486–497Crossref, Medline, Google Scholar



