A Single Ketamine Infusion Combined With Motivational Enhancement Therapy for Alcohol Use Disorder: A Randomized Midazolam-Controlled Pilot Trial
Abstract
Objective:
Pharmacotherapy and behavioral treatments for alcohol use disorder are limited in their effectiveness, and new treatments with innovative mechanisms would be valuable. In this pilot study, the authors tested whether a single subanesthetic infusion of ketamine administered to adults with alcohol dependence and engaged in motivational enhancement therapy affects drinking outcomes.
Methods:
Participants were randomly assigned to a 52-minute intravenous administration of ketamine (0.71 mg/kg, N=17) or the active control midazolam (0.025 mg/kg, N=23), provided during the second week of a 5-week outpatient regimen of motivational enhancement therapy. Alcohol use following the infusion was assessed with timeline followback method, with abstinence confirmed by urine ethyl glucuronide testing. A longitudinal logistic mixed-effects model was used to model daily abstinence from alcohol over the 21 days after ketamine infusion.
Results:
Participants (N=40) were mostly middle-aged (mean age=53 years [SD=9.8]), predominantly white (70.3%), and largely employed (71.8%) and consumed an average of five drinks per day prior to entering the study. Ketamine significantly increased the likelihood of abstinence, delayed the time to relapse, and reduced the likelihood of heavy drinking days compared with midazolam. Infusions were well tolerated, with no participants removed from the study as a result of adverse events.
Conclusions:
A single ketamine infusion was found to improve measures of drinking in persons with alcohol dependence engaged in motivational enhancement therapy. These preliminary data suggest new directions in integrated pharmacotherapy-behavioral treatments for alcohol use disorder. Further research is needed to replicate these promising results in a larger sample.
Pathological alcohol use leads to an estimated 3.8% of all deaths globally (1, 2). Although alcohol-related costs exceed 1% of the gross national product in developed countries, most affected individuals are not in treatment (3). Of those in treatment, a large number do not respond to available medications or behavioral treatments (4, 5). More effective pharmacotherapy options are needed, as well as methods to enhance efforts aimed at behavioral modification.
Maladaptive patterns of alcohol use may stem from preexisting vulnerabilities and may also be driven by neural adaptations resulting from problematic alcohol use itself (6). This may manifest clinically as deficits that jeopardize efforts aimed at initiating and maintaining abstinence, including heightened reactivity, dampened interest in non-alcohol-related pursuits, and stress sensitivity (6, 7). Our preliminary research with cocaine users suggests that ketamine, a high-affinity N-methyl-d-aspartate receptor antagonist with putative neurotrophic and modulatory effects, may represent an effective treatment for problematic substance use by exerting rapid benefits on such vulnerabilities (8, 9). We also found that study subjects with cocaine dependence who received a single subanesthetic infusion of ketamine as they initiated mindfulness-based relapse prevention had a significantly greater likelihood of end-of-study abstinence compared with those who received midazolam (10).
Given that addiction to alcohol, cocaine, and other drugs implicate comparable pathophysiology and clinical challenges (6, 7), it is possible that ketamine could demonstrate similar benefits in persons with alcohol dependence. The primary purpose of the present trial was to investigate whether a single ketamine infusion, compared with midazolam, promotes self-reported abstinence from drinking in adults with alcohol dependence who are engaged in motivational enhancement therapy, a psychotherapy platform of modest efficacy aimed at facilitating changes in behavior (11). The outpatient infusion was provided on a designated quit day during the second week of this 5-week trial and at least 24 hours after the last occasion of alcohol use. Other aims of the study were to evaluate the effect of ketamine on heavy drinking days (more than four drinks per day for men and more than three drinks per day for women) and on time to relapse or study dropout.
Methods
Treatment-seeking adults with alcohol dependence were randomly assigned to receive a 52-minute intravenous infusion of ketamine (0.71 mg/kg, N=17) or an active control of midazolam (0.025 mg/kg, N=23) in the second week of this 5-week outpatient trial, which started in September 2014 and concluded in September 2017. Consenting participants underwent alcohol use monitoring and measures (including urine toxicology), physician visits, and motivational enhancement therapy. Infusions occurred on a quit day during week 2. This day was designated in advance during the week-1 motivational enhancement therapy session, and participants were required to abstain from alcohol for at least 24 hours before the infusion. In the absence of preliminary data to inform our hypotheses of efficacy, we aimed to enroll up to 60 participants, with enough power to detect a large difference between the two treatment groups in proportion of daily abstinence. Fifty participants were enrolled, and 40 were randomly assigned over the course of the study period. No interim analyses were conducted before completion of the trial.
Participants
During screening, participants were assessed with the Mini-International Neuropsychiatric Interview for DSM-IV and underwent medical and psychiatric evaluation, including serum collection (electrolytes, CBC, and liver function tests) and other diagnostic tests, such as ECG and vital sign assessment. Study applicants were considered eligible if they were <70 years old, had no medical illness or psychiatric comorbidity, and met DSM-IV criteria for alcohol dependence and minimum daily (at least four heavy drinking days over the past 7 days) or weekly (35 drinks per week for men and 28 drinks per week for women) use while not using other substances. Alcohol and other drug use were determined by self-report and urine toxicology. Individuals with a history of severe withdrawal symptoms (e.g., seizures, cardiac instability, and delirium) were excluded, as were those with a history of psychotic or dissociative symptoms and with current depressive symptoms indicative of a DSM-IV disorder. The study was approved by the institutional review board at New York State Psychiatric Institute, and written informed consent was obtained from all participants.
Study Setting
All procedures and outpatient visits took place at New York State Psychiatric Institute on the Columbia University Medical Center campus. All staff involved in this trial were blinded to treatment condition.
Motivational Enhancement Therapy and Outpatient Visits
Participants were engaged in motivational enhancement therapy delivered by staff trained in the manual used in Project MATCH. Motivational enhancement therapy is a behavioral treatment for various substance use disorders (11) that involves a variety of strategies to promote motivation and self-directed change (12). In previous trials (13, 14), this therapy demonstrated only modest efficacy; the authors therefore considered it unlikely to obscure medication efficacy.
Six motivational enhancement therapy sessions were provided over 5 weeks. In week 1, participants engaged in an initial session, during which goals were explored and motivational statements elicited. Weekly subsequent sessions during weeks 2–5 were provided to achieve these goals. An additional session was provided during week 2, 24 hours after infusion, in order to capitalize on the hypothesized motivation-enhancing effects of ketamine, which may be most pronounced within the 24- to 48-hour postinfusion period. Sessions were audiotaped and supervised by a psychiatrist (E.D.) for fidelity to the manual.
Leading up to the infusion, participants were counseled to reduce the number of drinks per day in preparation for abstinence initiation and infusion administration during week 2. Participants came to the clinic twice weekly for motivational enhancement therapy and to meet with a psychiatrist, with visits spaced by 3–4 days, except during week 2, which involved 3 consecutive days. The timeline followback assessment was administered at each visit to quantify the number of drinks for each calendar day since the last visit. A positive urine ethyl glucuronide test among individuals who reported abstinence for this period led to the days being marked as drinking days. Measures related to alcohol-related vulnerabilities, such as craving and arousal (assessed with the visual analogue scale), withdrawal (assessed with the Clinical Institute Withdrawal Assessment), self-efficacy (assessed with the Alcohol Abstinence Self-Efficacy Scale and the Drug-Taking Confidence Questionnaire) (15, 16), perceived stress (assessed with the modified Perceived Stress Scale) (17), mindfulness (assessed with the Five Facet Mindfulness Questionnaire) (18), and impulsivity (assessed with the Barrett Impulsiveness Scale) (19), were collected at each visit, along with urine samples for toxicology (six-panel dipstick, ethyl glucuronide). Participants were provided with referrals at the end of the trial. Telephone follow-up was conducted 6 months after the trial.
Participants were compensated with $10 on screening and appointment days to defray the costs of travel, as well as $25 for the screening itself.
Infusion Procedures
Participants were asked to abstain from alcohol for ≥24 hours before the infusion, as well as to fast after midnight. They were informed that they might receive any of several medications, in addition to ketamine or midazolam. This blinding procedure was intended to disguise what drug was specifically given so as to minimize expectancy effects.
Participants were randomly assigned (1:1) by a statistician using randomly sized blocks to receive an intravenous infusion of ketamine or midazolam. Medication assignment and preparation were performed at the New York State Psychiatric Institute pharmacy.
Active control (a 2-minute saline bolus followed by a 50-minute slow-drip intravenous infusion of midazolam, 0.025 mg/kg) or ketamine hydrochloride (a 2-minute 0.11-mg/kg bolus in saline followed by a 50-minute slow-drip intravenous infusion of 0.6 mg/kg) was administered between 10:00 a.m. and 12:00 p.m. on the second appointment day of week 2. This dose of ketamine was selected because it was the highest dose tolerated by participants in preliminary studies (8, 9) and may be more efficacious than lower doses for alcohol dependence given evidence that chronic alcohol use blunts glutamate receptor sensitivity (6). A bolus was provided before the infusion in order to obtain a potent subanesthetic serum level of ketamine at the start of medication administration. Midazolam was chosen as the active control because it alters consciousness without any known persistent (>8 hours) effect on alcohol dependence. Blood pressure, heart rate, and blood oxygen saturation were continuously monitored. Medical coverage was provided for up to 3 hours postinfusion, and a brief psychiatric evaluation was conducted before discharge.
As in our previous studies (8–10), we provided relaxation and mindfulness-based exercises before and during infusions. After the infusion, participants completed a subjective-effects assessment battery.
Statistical Analysis
The study participants’ baseline demographic characteristics are summarized in Table 1.
| Characteristic | Treatment Arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Sample (N=40) | Midazolam (N=23) | Ketamine (N=17) | |||||||
| Demographic characteristics | |||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age (years) | 40 | 53.0 | 9.8 | 23 | 55 | 8.3 | 17 | 50.4 | 11.3 |
| N | % | N | % | N | % | ||||
| Gender | |||||||||
| Female | 21 | 52.5 | 14 | 60.9 | 7 | 41.2 | |||
| Male | 19 | 47.5 | 9 | 39.1 | 10 | 58.8 | |||
| Race | |||||||||
| Asian | 2 | 5.4 | 2 | 9.1 | 0 | 0.0 | |||
| Black or African American | 5 | 13.5 | 1 | 4.5 | 4 | 26.7 | |||
| White | 26 | 70.3 | 15 | 68.2 | 11 | 73.3 | |||
| Multiracial | 4 | 10.8 | 4 | 18.2 | 0 | 0.0 | |||
| Ethnicity | |||||||||
| Not Hispanic or Latino | 33 | 82.5 | 20 | 87.0 | 13 | 76.5 | |||
| Hispanic or Latino | 7 | 17.5 | 3 | 13.0 | 4 | 23.5 | |||
| Employment | |||||||||
| Unemployed | 11 | 28.2 | 5 | 22.7 | 6 | 35.3 | |||
| Employed | 28 | 71.8 | 17 | 77.3 | 11 | 64.7 | |||
| Education | |||||||||
| Completed grades 7–12 | 2 | 5.0 | 1 | 4.3 | 1 | 5.9 | |||
| Graduated from high school or received General Educational Development certification | 3 | 7.5 | 2 | 8.7 | 1 | 5.9 | |||
| College degree or associate’s degree | 31 | 77.5 | 17 | 73.9 | 14 | 82.4 | |||
| Master’s degree | 4 | 10.0 | 3 | 13.0 | 1 | 5.9 | |||
| First-degree family history of alcoholism | 36 | 90.0 | 19 | 82.6 | 17 | 100.0 | |||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Clinical measures | |||||||||
| Hamilton Depression Rating Scale | 38 | 2.9 | 2.5 | 22 | 2.9 | 2.5 | 16 | 2.9 | 2.7 |
| Five Facet Mindfulness Questionnaire | 40 | 137.3 | 20.9 | 23 | 140.3 | 21.4 | 17 | 133.2 | 20.2 |
| Clinical Institute Withdrawal Assessment | 38 | 3.5 | 4.3 | 23 | 3.4 | 4.2 | 15 | 3.6 | 4.6 |
| Perceived Stress Scale (modified) | 40 | 25.2 | 7.3 | 23 | 24.9 | 7.7 | 17 | 25.6 | 6.8 |
| Behavioral Inhibition System | 40 | 60.3 | 11.3 | 23 | 58.2 | 11.4 | 17 | 63.1 | 10.9 |
| Alcohol Abstinence Self-Efficacy Scale | 40 | 54.1 | 17.7 | 23 | 53.6 | 17.0 | 17 | 54.7 | 19.1 |
| Average number of drinks per day 7 days before consent (baseline) | 40 | 6.6 | 4.1 | 23 | 6.5 | 4.3 | 17 | 6.8 | 3.9 |
| Average number of drinks per day 7 days before infusion | 40 | 2.9 | 2.1 | 23 | 3.4 | 2.1 | 17 | 2.1 | 2.0 |
| Number of heavy drinking days 7 days before consent (baseline) | 37 | 5.1 | 1.4 | 20 | 5.4 | 1.4 | 17 | 4.7 | 1.4 |
| Number of heavy drinking days 7 days before infusion | 40 | 1.9 | 2.1 | 23 | 2.1 | 2.1 | 17 | 1.7 | 2.0 |
TABLE 1. Baseline demographic and clinical characteristics of study participants receiving ketamine or midazolam
The primary outcome of self-reported drinking days by timeline followback after the infusion was dichotomized so that each day was defined as abstinent (0 drinks) or nonabstinent (at least one drink). Days with missing data were treated as nonabstinent days. A longitudinal logistic mixed-effects model with a logit link and a random intercept was used to account for between-subject variances. The fixed effect of time (days postinfusion), treatment, and time-by-treatment interaction, adjusted by baseline total drinks, was used to analyze the longitudinal primary outcome: abstinent days during the 21 days postinfusion. Additionally, because the observed data do not follow a linear trend, time was also tested as a quadratic effect by testing whether the effect of time squared was significant. The Akaike information criterion and Bayesian information criterion summary fit statistics were used to identify which model best fit the observed data.
Reduction in heavy drinking days and other secondary measures were analyzed with longitudinal mixed-effects models, with the effect of study week, treatment, and study week-by-treatment two-way interaction adjusted by the corresponding outcome measures at baseline. A random intercept was used to account for between-subject variances, and a generalized estimating equation structure was included to account for within-subject correlations over time as an autoregressive (AR[1]) process.
We used the logit link to model binary outcomes (i.e., heavy drinking day) and the log link function to model outcomes (i.e., craving, arousal, and withdrawal) with right-skewed distributions. All other secondary outcomes were approximately normally distributed and modeled with identity-link functions.
The secondary outcome, time to relapse, was defined as time to the first heavy drinking day or time to dropout, whichever came first, and was analyzed by using Kaplan-Meier survival curves and the log-rank test. Time to first alcohol use and time to first heavy drinking day were similarly analyzed.
All analyses were performed in SAS, version 9.4, with all tests two-sided at a 5% level of significance.
Results
Participants
Ninety-five individuals were screened for participation in the study, 50 were enrolled, and 40 were randomly assigned in an intent-to-treat analysis (Figure 1). Participants were heavy drinkers, with minimal psychiatric comorbidity (Table 1). Infusions were well tolerated, with the most common adverse effects being sedation (midazolam, N=12; ketamine, N=8) and headache (midazolam, N=4; ketamine, N=6), persisting about 12 hours postinfusion. Scores on the Clinician-Administered Dissociative States Scale were significantly higher in the ketamine group immediately following infusions (median=19, interquartile range, 9–30.75) compared with the midazolam group (median=2, interquartile range, 0.25–9.25; χ2=7.87, p=0.005). Two participants in the ketamine group experienced mild agitation lasting 1 hour postinfusion. There were no incidents of persistent psychoactive effects or initiation of drug misuse (benzodiazepines, opioids, or ketamine) in either study group.
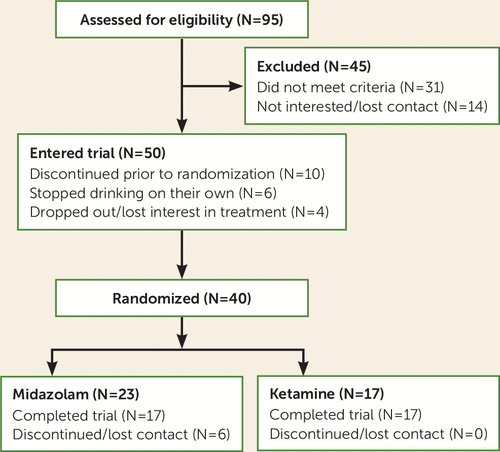
FIGURE 1. CONSORT flow diagram for a study of a single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder
Across the 21 days after infusion, 47.1% (N=8/17) of participants in the ketamine group used alcohol, and 17.6% (N=3/17) had a heavy drinking day. Among participants in the midazolam group, 59.1% (N=13/22) used alcohol, 40.9% (N=9/22) had a heavy drinking day, and 52.2% (N=12/23) relapsed. Throughout the entire study period, six participants dropped out of the midazolam group. Of these, one participant did not return after the infusion, and five dropped out after the first week. Four participants had heavy drinking days before dropping out, and one dropped out while still abstinent (as assessed during the participant’s final visit). No participants dropped out of the ketamine group.
Primary Outcome: Alcohol Abstinence
The longitudinal logistic mixed-effects model for alcohol-abstinent days with time treated as a quadratic term yielded the best-fit model (Akaike information criterion=569.04, Bayesian information criterion=580.87) compared with the initial model with time treated as a linear term (Akaike information criterion=576.18, Bayesian information criterion=586.31). The quadratic effect of time was significant (F=8.21, df=1, 797, p=0.004), as well as the time-by-treatment interaction (F=25.1, df=1, 797, p<0.001), adjusted for total baseline drinks (F=1.09, df=1, 797, p=0.297). The observed and model-estimated proportions of alcohol abstinence across the 21 days postinfusion are shown in Figure 2. The modeled proportion of abstinence in the ketamine group remained stable while decreasing significantly in the control (midazolam) group. The observed number needed to treat was 6, and the modeled number needed to treat was 4. The score on the Clinician-Administered Dissociative States Scale was not significantly associated with end-of-study abstinence (odds ratio=1.11, p=0.068).
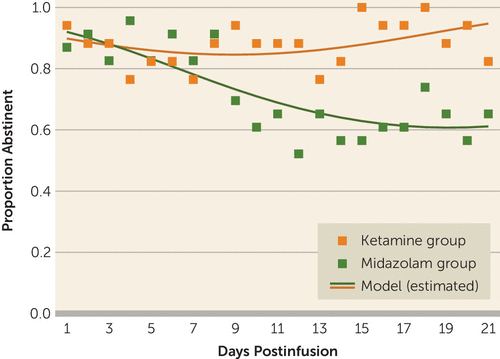
FIGURE 2. Observed and model-estimated proportions and standard errors of abstinence across the 21-day postinfusion period among study participants receiving ketamine or midazolam (N=40)
A sensitivity analysis without the assumption that missing days were nonabstinent days was performed for the primary outcome (abstinent days during the 21 days postinfusion). When treating missing days as missing, we found results similar to those reported above, suggesting that the assumption about missing days did not affect the obtained results. An analysis without adjustment for baseline total drinks also led to similar results.
Telephone interviews were conducted 6 months after the trial. Data were collected for 19 of the 40 participants (47.5%); 21 participants (52.5%) could not be reached by telephone. Of those participants who could be reached, eight were in the ketamine group and 11 in the midazolam group. Seventy-five percent of participants (N=6) in the ketamine group reported abstinence, compared with 27% (N=3) in the midazolam group.
Secondary Outcomes
When modeling heavy drinking days, the interaction between treatment group and time was significant (F=12.34, df=1, 798, p<0.001) (Figure 3). In the midazolam group, the probability of a heavy drinking day increased with each postinfusion day (odds ratio=1.19, 95% CI=1.14–1.25, p<0.001), while the odds of a heavy drinking day did not change significantly for the ketamine group (odds ratio=0.98, 95% CI=0.89–1.08, p=0.74).
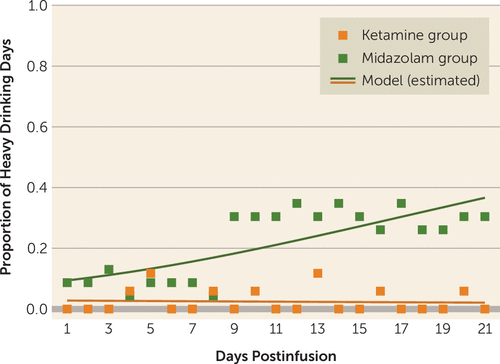
FIGURE 3. Observed and model-estimated proportions of heavy drinking days across the 21-day postinfusion period among study participants receiving ketamine or midazolam (N=40)
When modeling the secondary outcomes (craving, withdrawal, mindfulness, impulsivity, stress sensitivity, and self-efficacy), the two-way interaction of study week-by-treatment group was not significant in any of the models.
Time to Relapse, First Use, and First Heavy Drinking Day
On the basis of the log-rank test, participants in the ketamine group had significantly longer time to relapse (defined as the first heavy drinking day or dropout) (χ2=4.2, p=0.04), compared with participants in the midazolam group (Figure 4). However, there were no significant differences between groups in time to first use or to first heavy drinking day.
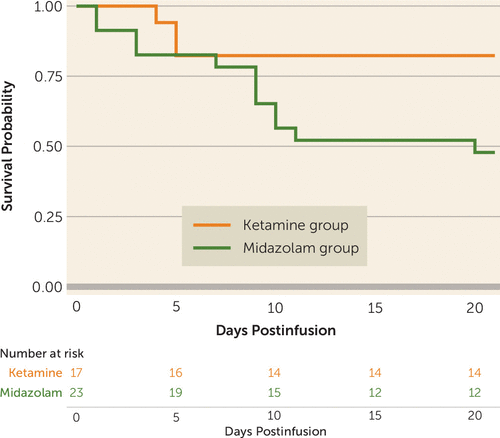
FIGURE 4. Time to relapse among study participants receiving ketamine or midazolam
Discussion
To our knowledge, this is the first trial to investigate the efficacy of a single ketamine infusion for alcohol use disorder. These preliminary data indicate that, compared with midazolam, ketamine led to a lower likelihood of alcohol use over a 21-day period after infusion, as well as a lower likelihood of heavy alcohol use and longer time to relapse. This suggests that ketamine in combination with motivational enhancement therapy may be an effective pharmacotherapy for initiating and sustaining abstinence from alcohol.
These data expand on previous findings on ketamine infusions in individuals with cocaine dependence. In the laboratory setting, any improvements (i.e., reductions in use and craving) that were observed from a single infusion were typically transient, subsiding after several days (8, 9). A subsequent trial embedding a single infusion into a mindfulness-based framework demonstrated more persistent effects, suggesting that an integrated model might work to promote synergy between the behavioral treatment and ketamine (10). Early trials evaluating so-called ketamine psychedelic therapy also employed an integrated approach, with intramuscular ketamine combined with existentially oriented psychotherapy to treat alcohol and opioid use disorders (20, 21).
Building on these previous findings, we paired motivational enhancement therapy and ketamine in this trial with the assumption that they might work together to marshal motivation toward initiating and sustaining abstinence. In previous studies, psychological mechanisms have been implicated in the impact of ketamine on the motivation to quit and other dependence-related vulnerabilities, perhaps through shifts in perspective (22). A psychotherapy framework aimed at further enhancing motivation, such as motivational enhancement therapy, may serve to focus these perspectival shifts toward deepening commitment and readiness for change. This dovetails with early research examining comparable compounds integrated into addiction-oriented psychotherapy, such as psilocybin and LSD (lysergic acid diethylamide), whereby certain psychoactive effects were intended to facilitate a reappraisal of personal values and existential meaning, as well as a deliberate restructuring of commitments and behavior.
In this trial, no participants dropped out of the ketamine group, but six dropped out of the control (midazolam) group, with four resuming heavy alcohol use before dropping out. Although the sample size was small, this suggests that ketamine may be helpful in promoting engagement with behavioral treatment, as hypothesized (10). In turn, motivational enhancement therapy may be helpful in carrying forward the apparent effects of ketamine on reduction in substance use beyond what has been observed in previous laboratory-based research without a supportive or behavioral framework during follow-up (8, 9). It is not possible, however, to conclude from this trial whether motivational enhancement therapy was necessary for these persistent effects in the absence of a two-by-two factorial design (e.g., participants receiving infusions without motivational enhancement therapy).
Surprising findings were the high rates of abstinence initiation irrespective of medication assignment, with most participants in both treatment groups able to stop drinking for a brief period after the designated quit day. Similarly, there was no significant difference between groups in time to first use or first heavy use. Yet, there were significant differences between groups over time in the proportion of both drinking days and heavy drinking days, with participants assigned to the control (midazolam) group significantly more likely both to drink and to drink heavily. Taken together, these findings suggest that ketamine provided protection against a lapse evolving into continued use (relapse) or into dropout from treatment. This may stem from ketamine minimizing the abstinence violation response (i.e., individuals losing hope after using and consequently relapsing or disengaging from treatment). These findings suggest a new usefulness for ketamine in facilitating addiction treatment and reducing the risk of relapse, namely, by maintaining motivation for sobriety even in the face of stressors, challenges, and lapses.
An issue in studying treatment with ketamine (similar to those emerging in studies of psychedelics, such as psilocybin, or in studies of MDMA [3,4-methylenedioxymethamphetamine]) is the problem of blinding participants and investigators, given the distinctive psychoactive effects of these agents. The substantial dissociative effects of ketamine may lead patients to feel that they received the active medication and engender placebo effects. We aimed to address this issue by using an active control (midazolam) and employing a minor deception whereby participants were informed that they may receive any of a variety of psychoactive compounds (as opposed to a binary randomization between midazolam and ketamine). However, we did not test the integrity of the blind by ascertaining what participants or staff believed was received during infusions. Additionally, even though a history of ketamine or benzodiazepine misuse was exclusionary, we did not determine the extent to which previous exposure to ketamine or midazolam may have enabled participants to identify what they received.
The findings that participants in the control group sustained abstinence for the first 7–10 days after the infusion, with overall abstinence rates higher than the modest response observed in previous trials that tested motivational enhancement therapy alone (e.g., 28% in Project MATCH) (13, 14), are of interest. These data suggest that midazolam may indeed have functioned as a good placebo or that there may be some transient benefit to a low-dose midazolam infusion. Heavy drinkers making a quit attempt may experience some degree of alcohol withdrawal or craving that could be relieved with midazolam. It is possible, therefore, that the impact of ketamine observed in this trial might have been more pronounced if an inactive control without beneficial effects (such as saline) had been used as the comparator.
Greater clarification of the therapeutic mechanisms of ketamine, as well as of its possible neurotoxicity and bladder complications with repeated administrations (23), can aid clinicians in understanding how to best harness its clinical potential while minimizing potential risks. As in a previous trial with cocaine users (10), these data suggest that a single dose may have enduring benefits, especially when integrated into a behavioral treatment. The persistence of these effects, well after ketamine and its metabolites are expected to have cleared, indicates that its therapeutic activity extends beyond direct neural activation (as in an agonist model of addiction treatment) to include sustained effects on decision making and behavior.
Neurotrophic, modulatory, and even psychological mechanisms have all been proposed to account for the sustained antidepressant effect of a single dose of ketamine (22–27). These downstream effects on diverse neural circuits may have relevance to addiction treatment as well. Preclinical research suggests that neurotrophic mechanisms involving mTOR (mammalian target of rapamycin) may be involved in the antidipsotropic effects of ketamine in rodents (28) and that ketamine modulates prefrontal functional connectivity to disrupt drug reinstatement in monkeys (29).
Several studies indicate that individuals with a first-degree family history of alcoholism may have a heightened and more prolonged antidepressant response to ketamine, perhaps as a result of epigenetic changes, glutamate subreceptor variations, and environmental factors (30, 31). The neural changes associated with a predisposition to alcohol use disorder, and perhaps with disordered use itself, may therefore work to optimize the efficacy of ketamine. It is possible that the activity of ketamine may have been similarly enhanced by such factors in our sample, with the majority of participants endorsing a first-degree family history of alcoholism (Table 1), even though this was not an antidepressant response because individuals with depressive symptoms were excluded.
The inherited and acquired neural vulnerabilities associated with problematic substance use are shared for different substance use disorders, including cocaine, alcohol, and opioids. As suggested by findings from this trial and from previous research with both cocaine and opioid users (8–10, 20, 21), dependence-related vulnerabilities across different substance use disorders may be equally susceptible to the therapeutic effect of ketamine, which may include changes in functional connectivity as well as alterations in glutamatergic, opioid, and dopaminergic signaling (13, 22). For example, a recent small study suggested that there may be opioid-related mechanisms behind the antidepressant response (32), although this finding is preliminary, and other data suggest otherwise (33). Given emerging data indicating the effect of certain ketamine enantiomers and metabolites, such as (R)-ketamine and (2R,6R)-hydroxynorketamine, on glutamate and dopamine neurotransmission (23), future studies examining serum levels of ketamine and of its metabolites may be helpful in clarifying ketamine’s mechanism of action.
Limitations
These results are preliminary, and little is known about the utility and safety of ketamine infusions in the treatment of substance use disorders outside of specialized research settings. Caution and restraint in clinical settings are warranted. Several other limitations underscore the preliminary nature of these findings. First, our sample was both small and homogeneous, with inadequate power for evaluating secondary outcomes and with minor discrepancies between treatment arms. The hazards of inferring treatment effects from small samples are well known (34). Second, the duration of treatment was short (5 weeks), with only 21 days of alcohol use monitoring by using the timeline followback method after infusions. Study follow-up occurred several months after the final study visit, and some participants could not be reached, resulting in a responder rate lower than that of other clinical trials involving more closely spaced follow-up visits. A longer follow-up with more frequent assessments would have allowed for better assessment of the duration of ketamine’s efficacy. Third, more than a quarter of participants in the control group dropped out. This is consistent with our hypothesis that ketamine promotes engagement with behavioral modification. Although the approach we used for missing observations is standard in clinical trials for substance use disorders, namely, to treat dropout as relapse, this assumption may not be entirely accurate, and with a small sample size, the findings could be sensitive to just a few unobserved violations of this assumption. Hence, missing data for the primary outcome were analyzed as both “using” and “missing,” with no difference in results. Fourth, fidelity to the motivational enhancement therapy manual was monitored through supervision and review of audiotaped sessions only. Lastly, the sample was selected for minimal psychiatric comorbidity, which limits generalizability.
The minor deception that we employed to further protect the blind and to minimize expectancy and placebo effects constitutes another limitation; informing individuals that they might receive any of a variety of medications would not pertain to clinical settings, and it is not clear whether there would have been additional risks stemming from participants knowing that they received ketamine. As in other clinical procedures involving substance abuse liability (e.g., provision of postoperative opioids), various safeguards were employed that work to minimize the risk of illicit use, such as careful patient selection, preparation, monitoring, and postprocedure support. With these safeguards in place, there has been no incidence of ketamine misuse, to our knowledge, in any of the research to date (although follow-up was short in these studies), suggesting that its propensity to be approached problematically can be effectively managed even when administered to substance users (8–10, 35).
Conclusions
Despite several limitations, the study findings represent a first step in understanding a potential clinical role for ketamine in the treatment of alcohol use disorder. Ketamine was effective in providing individuals already engaged in motivational enhancement therapy with significantly greater odds of alcohol abstinence in the initial weeks after a quit attempt. The question remains whether a single ketamine infusion would promote abstinence in the long term and whether there is indeed synergy with behavioral treatments. Further research can build on these promising preliminary findings by replicating this study in a larger sample and with longer follow-up, as well as by more rigorously examining the hypothesis of synergy between ketamine and behavioral treatment.
1 : Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009; 373:2223–2233Crossref, Medline, Google Scholar
2 : Alcohol consumption and non-communicable diseases: epidemiology and policy implications. Addiction 2011; 106:1718–1724.Crossref, Medline, Google Scholar
3 : Global status report on alcohol and health, 2018. Geneva, World Health Organization, 2018. https://www.who.int/substance_abuse/publications/global_alcohol_report/enGoogle Scholar
4 : Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry 2015; 72:757–766Crossref, Medline, Google Scholar
5 Substance Abuse and Mental Health Services Administration: National Survey on Drug Use and Health 2015. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2015. https://www.datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2015-nsduh-2015-ds0001-nid16894Google Scholar
6 : Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 2008; 33:166–180Crossref, Medline, Google Scholar
7 : The brain on drugs: from reward to addiction. Cell 2015; 162:712–725Crossref, Medline, Google Scholar
8 : The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry 2014; 76:40–46Crossref, Medline, Google Scholar
9 : Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry 2017; 22:76–81Crossref, Medline, Google Scholar
10 : A single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: a randomized clinical trial. Am J Psychiatry 2019; 176:923–930Link, Google Scholar
11 : Motivational interviewing for substance abuse. Cochrane Database Syst Rev 2011; (5):
12 : Motivational interviewing. Annu Rev Clin Psychol 2005; 1:91–111Crossref, Medline, Google Scholar
13 : Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. J Stud Alcohol 1998; 59:631–639Crossref, Medline, Google Scholar
14 : Motivational interviewing, enhancement, and brief interventions over the last decade: a review of reviews of efficacy and effectiveness. Psychol Addict Behav 2017; 31:862–887Crossref, Medline, Google Scholar
15 Alcohol Abstinence Self-Efficacy Scale (AASE) https://pubs.niaaa.nih.gov/publications/AssessingAlcohol/InstrumentPDFs/08_AASE.pdfGoogle Scholar
16 : Development and validation of the Drug-Taking Confidence Questionnaire: A Measure of Coping Self-Efficacy. Addict Behav 1997; 22:655–670Crossref, Medline, Google Scholar
17 : A global measure of perceived stress. J Health Soc Behav 1983; 24:385–396Crossref, Medline, Google Scholar
18 : Using self-report assessment methods to explore facets of mindfulness. Assessment 2006; 13:27–45Crossref, Medline, Google Scholar
19 : Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 1995; 51:768–774Crossref, Medline, Google Scholar
20 : Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs 1997; 29:165–183Crossref, Medline, Google Scholar
21 : Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abuse Treat 2002; 23:273–283Crossref, Medline, Google Scholar
22 : Therapeutic infusions of ketamine: do the psychoactive effects matter? Drug Alcohol Depend 2014; 136:153–157Crossref, Medline, Google Scholar
23 : Beyond ketamine: new approaches to the development of safer antidepressants. Curr Neuropharmacol 2017; 15:963–976Crossref, Medline, Google Scholar
24 : Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc Cogn Affect Neurosci 2016; 11:1227–1235Crossref, Medline, Google Scholar
25 : Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 2017; 22:562–569Crossref, Medline, Google Scholar
26 : Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord 2014; 159:56–61Crossref, Medline, Google Scholar
27 : Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 2015; 40:1307Crossref, Medline, Google Scholar
28 : Ketamine’s antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol 2014; 18:1–7Google Scholar
29 : mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res 2013; 247:9–16Crossref, Medline, Google Scholar
30 : Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord 2012; 14:880–887Crossref, Medline, Google Scholar
31 : Effects of ketamine treatment on cocaine-induced reinstatement and disruption of functional connectivity in unanesthetized rhesus monkeys. Psychopharmacology (Berl) 2019; 236:2105–2118Crossref, Medline, Google Scholar
32 : Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 2018; 175:1205–1215Link, Google Scholar
33 : Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 2019; 76:337–338Crossref, Medline, Google Scholar
34 : Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry 2006; 63:484–489Crossref, Medline, Google Scholar
35 : Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 2018; 5:65–78Crossref, Medline, Google Scholar



