Neural Insensitivity to the Effects of Hunger in Women Remitted From Anorexia Nervosa
Abstract
Objective:
Anorexia nervosa has the highest mortality rate of any psychiatric condition, yet the pathophysiology of this disorder and its primary symptom, extreme dietary restriction, remains poorly understood. In states of hunger relative to satiety, the rewarding value of food stimuli normally increases to promote eating, yet individuals with anorexia nervosa avoid food despite emaciation. This study’s aim was to examine potential neural insensitivity to these effects of hunger in anorexia nervosa.
Methods:
At two scanning sessions scheduled 24 hours apart, one after a 16-hour fast and one after a standardized meal, 26 women who were in remission from anorexia nervosa (to avoid the confounding effects of malnutrition) and 22 matched control women received tastes of sucrose solution or ionic water while functional MRI data were acquired. Within a network of interest responsible for food valuation and transforming taste signals into motivation to eat, the authors compared groups across conditions on blood-oxygen-level-dependent (BOLD) signal and task-based functional connectivity.
Results:
Participants in the two groups had similar BOLD responses to sucrose and water tastants. A group-by-condition interaction in the ventral caudal putamen indicated that hunger had opposite effects on tastant response in the control group and the remitted anorexia nervosa group, with an increase and a decrease, respectively, in BOLD response when hungry. Hunger had a similar opposite effect on insula-to-ventral caudal putamen functional connectivity in the remitted anorexia nervosa group compared with the control group. Exploratory analyses indicated that lower caudate response to tastants when hungry was associated with higher scores on harm avoidance among participants in the remitted anorexia nervosa group.
Conclusions:
Reduced recruitment of neural circuitry that translates taste stimulation to motivated eating behavior when hungry may facilitate food avoidance and prolonged periods of extremely restricted food intake in anorexia nervosa.
Individuals with anorexia nervosa are able to severely restrict food consumption and maintain an extremely low weight (1). Because there are no proven treatments that normalize core symptoms in adults with anorexia nervosa, this is often a chronic disorder resulting in high morbidity and mortality. In healthy individuals, hunger is dysphoric and increases reward salience and subjective value of food to drive consumption (2). In contrast, individuals with anorexia nervosa often describe eating as anxiogenic, and food refusal may reduce dysphoric mood (3). Do individuals with anorexia nervosa have an altered response to the motivating signals of hunger? While individuals with anorexia nervosa have been shown to have alterations in neural mechanisms coding motivation, salience, and valuation of food when ill and after weight restoration (4), few studies (5) have systematically examined whether there is altered functioning in this circuitry in individuals with anorexia nervosa when hungry that might persist after recovery. Understanding the mechanisms contributing to the neurobiology of self-starvation in anorexia nervosa could identify new treatment targets.
One method of identifying food-related neural mechanisms coding motivation, salience, and valuation is to measure brain response to palatable tastants, such as sucrose, using neuroimaging (6). This approach has identified a neural circuit in humans (7) that extends beyond pure gustatory chemosensory processing to include regions involved in motivation and reward processing, and which maps onto analogous circuits in rodents (8) and nonhuman primates (9). Specifically, tastes of sucrose signals are transmitted from sweet taste receptors in the tongue through the brainstem and thalamus to the primary gustatory cortex in the insula. In humans, the anterior insula receives chemosensory taste input from the mid-insula (10). Recent studies suggest that the insula taste cortex identifies sweetness, and the amygdala, which is involved in processing emotions, codes the valence, specifies its hedonic value, and elicits execution of selective behaviors (8). The hypothalamus, a homeostatic center, regulates metabolic processes, including hunger and food intake, with motivation-reward systems associated with the hedonic drive to eat (11). In primates, the orbitofrontal cortex processes the reward value of taste (7) and the ventromedial prefrontal cortex acts as a visceromotor area and governs the hypothalamus, amygdala, and insula. Ultimately, taste information is integrated with reward value and homeostatic drives (7, 11) via projections from the anterior insula and amygdala to the striatum (9) to guide motivated eating behavior. In healthy individuals, hunger, compared with satiety, increases brain response in these reward, attention, and motivation regions in response to palatable foods (6, 7, 12, 13).
In this study, we used a palatable taste task with functional MRI (fMRI) to investigate the response to tastants in the hungry and fed states in the food reward-motivation circuit. We studied women in remission from anorexia nervosa to avoid the confounding effects of malnutrition on this circuitry, in comparison to healthy control women. Our previous studies (14, 15) supported our hypothesis that women in remission from anorexia nervosa relative to control women would show a diminished response to taste of sucrose in the insula and striatum when in a hungry compared with a fed state, suggesting that hunger may not generate signals to motivate eating (7) in anorexia nervosa. We also considered whether anorexia nervosa might entail an exaggerated response to being fed (enhanced satiety) and a normal response to hunger. Thus, we performed a group-by-condition-by-tastant interaction analysis in the insula, striatum, amygdala, and medial orbitofrontal cortex to test these hypotheses in regions that integrate sensory/hedonic aspects of taste and interoceptive awareness in the service of homeostasis.
Methods
Subjects
Twenty-six women with remitted anorexia nervosa with no history of binge-eating behavior (18 restricting only, eight with purging behaviors) were compared with 22 age- and weight-matched healthy control women. Participants in the remitted anorexia nervosa group met DSM-IV-TR criteria for anorexia nervosa in the past (1), but for at least 1 year before participating in this study, they did not endorse pathological eating behavior or cognitions and they maintained above 85% of average body weight, maintained weight stability (within 3 kg), and had regular menstrual cycles (14). Individuals were excluded from the study if they had a history of alcohol or drug abuse or dependence during the past 3 months; currently met diagnostic criteria for major depressive disorder, any anxiety disorder, or obsessive-compulsive disorder (lifetime diagnosis was not exclusionary); had a medical or neurologic diagnosis; were taking any psychoactive medication during the past 3 months; or had any MRI contraindications. The study was approved by the Institutional Review Board of the University of California, San Diego (UCSD), and all participants provided written informed consent and received compensation. (See the online supplement for details regarding participants and assessment tools.)
Experimental Design
Participants were housed and meals were provided by the UCSD Clinical and Translational Research Institute for 72 hours to ensure dietary compliance. On day 1, participants were evaluated and consumed the same standardized meals (calculated as 30 kcal/kg per day). On days 2 and 3, participants performed a taste task (14, 15) during fMRI on two visits scheduled 24 hours apart (see Table S1 in the online supplement). For the “hungry” condition, participants fasted for 16 hours prior to scanning, with ad libitum water permitted. During the “fed” condition, participants consumed standardized meals (calculated as 30 kcal/kg per day) the day before scanning, as well as a weight-adjusted standardized breakfast (30% of overall daily caloric needs, approximating 450–500 kcal; 53% carbohydrates, 32% fat, and 15% protein) 2 hours before the 9:00 a.m. scan. Study visit order on days 2 and 3 was randomized across participants and scheduled in the early follicular menstrual phase. Participants underwent scanning on one of two 3-T scanners, and each participant underwent scanning on the same scanner for both visits (16). During the taste task, participants received pseudo-random delivery of 1.0 cc of 10% sucrose solution or ionic water over 80 trials (see the online supplement). These solutions were chosen to allow comparison of the findings with those of our previous work, in which control of food intake prior to scanning was limited (14, 15). For each imaging session, participants provided self-report ratings of hunger and thirst both before and after scanning, and of tastant pleasantness before scanning (Figure 1).
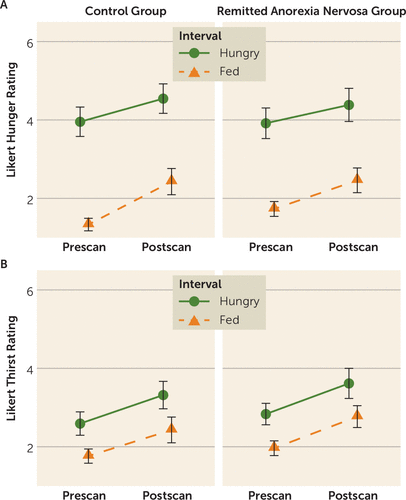
FIGURE 1. Self-report Likert visual analogue scale values for pre- and postscan measures of hunger and thirst in control women and women in remission from anorexia nervosaa
a Groups were compared on hunger and thirst ratings across conditions and time points using a group by condition (hungry, fed) by interval (prescan, postscan) linear mixed-effects model with subject as a random effect. For hunger, there was a main effect of condition (F=181.37, df=1,134, p<0.001), with post hoc analyses suggesting that all participants reported greater hunger during the hungry condition relative to the fed condition (t=13.47, df=134, p<0.001). There was a main effect of interval (F=17.59, df=1,134, p<0.001), with post hoc analyses suggesting that all participants reported greater hunger at the postscan assessment relative to the prescan assessment (t=4.26, df=134, p<0.001). However, there was no main effect of group, and interactions were not statistically significant. Similar effects were observed for thirst; there was a main effect of condition (F=40.55, df=1,134, p<0.001), with all participants reporting greater thirst in the hungry relative to the fed condition (t=6.27, df=134, p<0.001), and a main effect of interval (F=26.93, df=1,134, p<0.001), with all participants reporting greater thirst at the postscan assessment relative to the prescan assessment (t=5.15, df=134, p<0.001). However, there was no main effect of group, and interactions were not statistically significant.
Image Analysis
After preprocessing (see the online supplement), statistical analyses were performed using a generalized linear model, with individual events modeled using the SPMG3 function in AFNI (http://afni.nimh.nih.gov/afni/). Six motion parameters (three rotations and three translations) were used as nuisance regressors to account for motion artifact.
Task-based activation.
To examine whether women in the remitted anorexia nervosa and control groups differed in brain response to palatable tastants when hungry and when fed, we employed a linear mixed-effects analysis of group by condition (hungry, fed) by tastant (sucrose, water). Group, condition, and tastant were treated as fixed effects. We designed this study to match our previous comparisons of sucrose and water (14), since we reasoned that, when hungry, women with remitted anorexia nervosa would have less response to a primary reward like sucrose. However, our initial analyses unexpectedly demonstrated no significant interactions for tastant (i.e., no group-by-condition-by-tastant, group-by-tastant, or condition-by-tastant interactions). Therefore, we tested a simplified model that included the group-by-condition interaction (collapsed across tastants) and the main effect of tastant. Subject was treated as a random effect and nested within scanner, as recommended when multiple scanners are employed for data collection (17).
To improve statistical power and reduce an inflated false discovery rate, primary analyses were restricted to a single mask (see Figure S1 in the online supplement) comprising a well-defined taste and motivation neurocircuit (7, 15): left and right insula, amygdala, ventral striatum (nucleus accumbens and the most ventral parts of both the putamen and the caudate [18]), dorsal caudate, and putamen. The orbitofrontal cortex was excluded because of substantial susceptibility artifact. Intrinsic smoothness was estimated using the spatial autocorrelation function (acf) option in AFNI’s 3dFWHMx. Minimum cluster sizes were calculated with AFNI’s 3dClustSim to guard against false positives. For both region-of-interest and whole-brain analyses, minimum cluster sizes corresponded to a voxel-wise probability of p<0.001 and a cluster-wise alpha of 0.05 (two-sided) to correct for multiple comparisons. Exploratory voxel-wise analyses were also performed (see Table S2 in the online supplement).
Task-based functional connectivity.
To follow up on results from the analyses described above, we conducted a generalized psychophysiological interaction analysis (19) to assess the influence of hungry and fed states on group differences in functional connectivity within gustatory-reward circuitry during taste processing. Functionally relevant seed regions were identified across all participants for the main effect of condition. This data-driven approach avoids circularity by accounting for the main effects of task condition but isolating effects distinct from the condition itself (19, 20) (see the online supplement for details).
Relationship to clinical variables.
Within-group, within-visit exploratory voxel-wise Huber robust regressions (21) conducted in R were used to examine associations of BOLD response for tastants (sucrose and water combined) with current body mass index (BMI), age, harm avoidance (as assessed by the harm avoidance subscale of the Temperament and Character Inventory), and trait anxiety. Additional analyses in the remitted anorexia nervosa group included lowest lifetime postpubertal BMI, illness duration, and duration of remission. Significant clusters within our search region mask were identified using AFNI’s 3dClustSim for small-volume correction, with a peak voxel threshold of p<0.01. Results were Bonferroni-corrected for four experimental conditions and four clinical measures for women in the control group (p<0.003) and six clinical measures for women in the remitted anorexia nervosa group (p<0.002).
Results
Women in the control and remitted anorexia nervosa groups had similar BMI, age, years of education, IQ, and scores on the Beck Depression Inventory (Table 1). The remitted anorexia nervosa group endorsed higher levels of anxiety and harm avoidance. Women in both groups reported significantly greater hunger during the hungry condition relative to the fed condition, and the groups did not differ on ratings of hunger or thirst (Figure 1) or tastant pleasantness (see Figure S2 in the online supplement). Participants rated water as slightly more pleasant than the sucrose solution (see the online supplement).
| Characteristic | Control Group (N=22) | Remitted Anorexia Nervosa Group (N=26) | |||
|---|---|---|---|---|---|
| N | % | N | % | p | |
| Scanner | n.s. | ||||
| GE Signa Excite | 10 | 45.5 | 14 | 53.8 | |
| GE MR750 | 12 | 54.5 | 12 | 46.2 | |
| Mean | SD | Mean | SD | p | |
| Age (years) | 25.7 | 6.3 | 26.2 | 6.6 | n.s. |
| Current BMI | 22.0 | 2.1 | 21.9 | 1.7 | n.s. |
| Lowest BMI | 20.4 | 1.5 | 14.7 | 1.5 | <0.01 |
| Duration of illness (months) | 70.8 | 61.3 | |||
| Duration of recovery (months) | 67.0 | 60.5 | |||
| Education (years) | 15.7 | 1.3 | 16.6 | 2.8 | n.s. |
| IQa | 111.5 | 10.9 | 112.9 | 11.8 | n.s. |
| Estradiol level (pg/mL) | 12.8 | 7.7 | 12.5 | 5.9 | n.s. |
| N | % | N | % | ||
| Lifetime diagnoses | |||||
| Major depressive disorder | 0 | 0.0 | 15 | 57.7 | |
| Any anxiety disorder | 1 | 4.5 | 10 | 38.5 | |
| Obsessive-compulsive disorder | 0 | 0.0 | 4 | 15.4 | |
| Past substance abuse or dependence | |||||
| Alcohol | 0 | 0.0 | 3 | 11.5 | |
| Cannabis | 0 | 0.0 | 1 | 3.8 | |
| Mean | SD | Mean | SD | p | |
| Clinical assessmentsb | |||||
| State-Trait Anxiety Inventory | |||||
| State anxiety | 24.6 | 5.9 | 29.7 | 9.2 | 0.03 |
| Trait anxiety | 23.9 | 1.0 | 28.7 | 1.4 | 0.01 |
| Harm avoidance score from the Temperament and Character Inventory | 7.2 | 5.4 | 11.2 | 5.95 | 0.02 |
| Beck Depression Inventory | 1.0 | 3.4 | 2.0 | 2.4 | 0.25 |
TABLE 1. Characteristics of participants in a functional MRI study on neural insensitivity to effects of hunger in anorexia nervosa
Region-of-Interest Analysis
At a voxel-wise p<0.001 (corrected alpha=0.05), a main effect of condition (hungry > fed), seemingly driven by a greater response to hunger in the control group, was found in the left ventral striatum (see Figure S3 and Table S3 in the online supplement). No main effect of tastant was observed. A group-by-condition interaction was detected in two clusters within the left ventral caudal putamen (Figure 2A; see also Table S3). Within-group post hoc analyses revealed that in both clusters, women in the control group were significantly more responsive to tastants when hungry compared with when fed. In contrast, women in the remitted anorexia nervosa group were significantly less responsive to tastants when hungry compared with when fed. Between-group post hoc analyses indicated that when hungry, women in the remitted anorexia nervosa group showed lower response to tastants than control women. The remitted anorexia nervosa and control groups’ responses did not differ when participants were in the fed state. Using a less stringent threshold (voxel-wise p<0.01, corrected alpha=0.05), a similar group-by-condition interaction driven by reduced tastant response in the remitted anorexia nervosa group when hungry was detected in the left anterior insula (see Table S3 and Figure S4 in the online supplement). A post hoc analysis within the remitted anorexia nervosa group determined that anorexia nervosa subtype and lifetime history of depression or anxiety did not significantly contribute to our findings (see the online supplement).
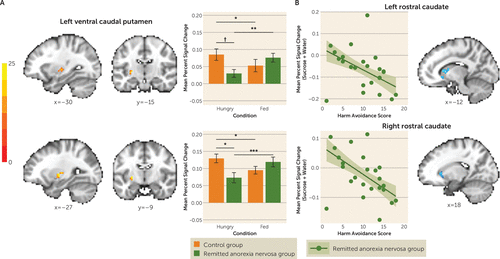
FIGURE 2. Response to sucrose and water tastants when hungry and fed, in control women and women in remission from anorexia nervosaa
a In panel A, linear mixed-effect results show two separate clusters demonstrating an interaction of group by condition (hungry, fed) in response to tastants (data for sucrose and water combined) within the left ventral caudal putamen (top peak coordinates: x=−30, y=−15, z=0; bottom peak coordinates: x=−27, y=−9, z=0). Control women were significantly more responsive to tastants when hungry compared with when fed (p values, <0.037). In contrast, women in the remitted anorexia nervosa group were significantly less responsive to tastants when hungry compared with when fed (p values, <0.001). When hungry, women with remitted anorexia nervosa showed lower response to tastants than control women in both clusters, but this finding was statistically significant within only one cluster (shown in the bottom panel, p=0.035). Intrinsic smoothness was estimated using the spatial autocorrelation function (acf) option in AFNI’s 3dFWHMx. Minimum cluster sizes were calculated with AFNI’s 3dClustSim to guard against false positives (voxel-wise p<0.001, alpha=0.05). In panel B, the plots demonstrate statistically significant relationships between the blood-oxygen-level-dependent percent signal change response to tastants when hungry and harm avoidance within the left and right rostral caudate (left peak coordinates: x=−12, y=18, z=6; right peak coordinates: x=18, y=24, z=3) for remitted anorexia nervosa using Huber robust regression (left: t=−3.46, p=0.017; right: t=−3.94, p=0.009).
†p<0.1. *p<0.05. **p<0.01. ***p<0.001.
Task-Based Functional Connectivity Analysis
Seed regions were derived from the main effect (across all subjects) of condition and corresponded to the peak coordinates of two clusters: the right ventral caudal putamen (x=33, y=−9, z=0) and the right dorsal mid-insula (x=36, y=6, z=0).
Group-by-condition interactions indicated that metabolic state (hungry, fed) modulated group differences in functional connectivity between 1) the right dorsal mid-insula seed and left ventral caudal putamen, right dorsal rostral putamen, and left anterior insula targets (Figure 3A; see also Table S4 in the online supplement), and 2) the right ventral caudal putamen seed and left ventral caudal putamen, right dorsal rostral putamen, and right amygdala targets (Figure 3B; see also Table S4). Post hoc analyses indicated that in the control group, functional connectivity to tastants was greater when hungry than when fed, whereas in the remitted anorexia nervosa group, functional connectivity to tastants was lower when hungry than when fed (see Table S4).
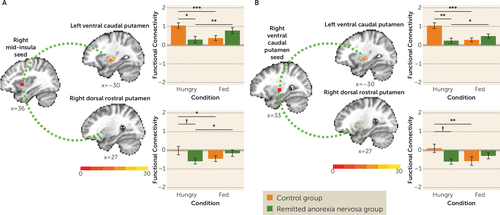
FIGURE 3. Modulation of functional connectivity by metabolic state (hungry, fed) in control women and women in remission from anorexia nervosaa
a Panel A summarizes the functional connectivity analyses for the right caudal mid-insula seed. Linear mixed-effect results demonstrated an interaction of group by condition (hungry, fed) between the right insula seed and clusters within the left ventral caudal putamen (peak coordinates: x=−30, y=−15, z=0) and the right dorsal rostral putamen (peak coordinates: x=27, y=9, z=6) in response to tastants (data for sucrose and water combined). Panel B summarizes the functional connectivity analyses for the right ventral caudal putamen seed. Linear mixed-effect results demonstrated an interaction of group by condition (hungry, fed) between the right putamen seed and clusters within the left ventral caudal putamen (peak coordinates: x=−30, y=−15, z=0) and the right dorsal rostral putamen (peak coordinates: x=27, y=6, z=6) in response to tastants (data for sucrose and water combined).
†p<0.1. *p<0.05. **p<0.01. ***p<0.001.
Relationship to Clinical Variables
Women in the remitted anorexia nervosa group with the highest harm avoidance scores showed the lowest response to tastants when hungry in the left and right rostral caudate (t=−3.46, p<0.001, and t=−3.94, p<0.001, respectively) (Figure 2B). There were no relationships between response to tastants and other clinical variables tested in control women or women with remitted anorexia nervosa, such as duration of illness or remission.
Discussion
The relentless ability to restrict eating and become severely emaciated has been one of the most puzzling symptoms of anorexia nervosa. In this study we found that in women in remission from anorexia nervosa, striatal and, at a lower statistical threshold, insular activation was abnormally unresponsive to taste stimulation in the hungry state. As expected, this circuitry, which guides motivated behavior, such as eating (7), was activated in control women when hungry compared with being fed (6, 12). Specifically, we identified a region of the ventral caudal putamen in which 1) the control group showed an increase but the remitted anorexia nervosa group showed a decrease in BOLD response to tastants when hungry compared with when fed, and 2) the remitted anorexia nervosa group showed a lower BOLD response to tastants compared with the control group only when hungry. Functionally, this region of the putamen is connected to the anterior and mid-insula (22), and, at a lower statistical threshold, the remitted anorexia nervosa group showed a similar response pattern that was opposite that of the control group in the left anterior insula. Moreover, the control group showed increased mid-insula-to-ventral caudal putamen functional connectivity to tastants when hungry compared with when fed, whereas the remitted anorexia nervosa group showed decreased functional connectivity between these regions when hungry compared with when fed, and compared with control women when hungry. Overall, the interaction suggests that in women with remitted anorexia nervosa, the response to taste after eating may be “normal,” but the response to taste during hunger is abnormal. Research is needed in symptomatic groups using paradigms that measure food-specific reward value. We speculate, however, that our results are compatible with two possible main processes: the afferent metabolic signals that are translated into motivational behavior (food seeking) are attenuated and/or the afferent signals are excessively suppressed by top-down modulatory brain regions. Future investigation will need to disambiguate these possibilities.
We postulated that when hungry, women with remitted anorexia nervosa would show less response to sucrose (a food and primary reward) compared with water. If there was a deficit specifically related to sucrose consumption, it might be possible to test this, because water and sucrose have been shown to activate different groups of neurons in the primary taste cortex in nonhuman primates (23). Counter to our hypothesis, we did not detect differences in response to water and sucrose. However, fMRI studies may not have the resolution to distinguish between response to water and sucrose in subregions of the primary taste cortex or related regions (12, 24). Furthermore, water and sucrose similarly activate taste circuitry and regions associated with emotion and motivation (7, 12). Because we did not detect response differences between water and sucrose, we collapsed responses across tastants to examine group and condition effects.
Reduced activation to tastants when hungry in the ventral caudal putamen and reduced functional connectivity between the right and left ventral caudal putamen are consistent with other studies showing disruption in limbic striatal circuitry in individuals with current or remitted anorexia nervosa (16, 25, 26). Nonhuman primate studies have shown that the ventral caudal putamen receives sensory-limbic inputs from the insula, as well as from the amygdala, orbitofrontal cortex, and temporal lobe, and is distinct from the sensorimotor dorsal putamen, which is afferently regulated by the motor cortex (9). Human neuroimaging studies have shown that it is functionally (22) and structurally (27) connected to prefrontal limbic and premotor cortex regions (Figure 4B). This circuitry is integral to reward processing and the preparation for and control of actions triggered by external stimuli (28). Reduced recruitment of the striatal aspects of this circuitry in response to tastants when hungry may impair the translation of taste reward value to motivated eating behavior in anorexia nervosa. However, the molecular mechanisms contributing to the selective sensitivity of this region to hunger signaling in anorexia nervosa require further study.
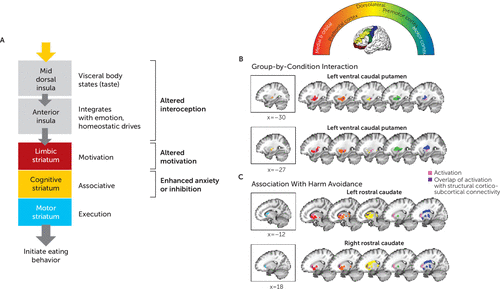
FIGURE 4. Association of BOLD findings with known frontostriatal structural connectivitya
a Panel A is a schematic diagram of insula-striatal pathways. The mid-insula relays somato- and viscerosensory signals to the anterior insula and striatum. The anterior insula projects along the ventromedial axis of the striatum, including the rostral ventral striatum and ventral caudal putamen. Through feed-forward connections, dopamine-mediated information progresses from the limbic to cognitive to sensorimotor areas of the striatum (57) to generate action selection related to the mediation and regulation of goal-directed behavior, such as the consumption of palatable food. The semicircular diagram above panels B and C illustrates the rostro-caudal gradient of striatum-to-frontal-cortex structural connectivity as identified in healthy adults (27). Areas of the striatum are color-coded by their ultimate cortical projection targets, as indicated within the semicircle. In panels B and C, the brain images depict blood-oxygen-level-dependent activation from the present study in the context of the above-identified structural circuits in healthy adults. Reference slices depicting significant clusters identified for each analysis in this study (from Figure 2) are shown in boxes on the left. Colored areas shown on brain slices on the right reflect the gradients from the top semicircular panel. Overlap between clusters from our main analyses and these structural projection areas is shown in dark purple. Ventral caudal putamen clusters showing group-by-condition (hungry, fed) interactions overlap primarily with areas of the putamen that have been shown previously in healthy adults to project to limbic (red, orange) and premotor (green) associative cortices. Rostral caudate clusters showing associations with harm avoidance in the remitted anorexia nervosa group overlap with areas of the caudate that have been shown previously to project to limbic (red, orange) and cognitive control–related dorsolateral prefrontal cortices (yellow). BOLD=blood-oxygen-level-dependent. Structural connectivity maps are adapted from a figure by Draganski et al. (27) published under a Creative Commons license.
At a lower threshold, the remitted anorexia nervosa group showed reduced activation to tastants when hungry in the anterior insula (left anterior short gyrus and middle short gyrus) and reduced functional connectivity between the right anterior (middle short gyrus and posterior short gyrus) and mid-dorsal insula and ventral caudal putamen. The insula is a hub for interoception (the awareness and integration of internal body signals to regulate behavior) (10), and accumulating evidence suggests disturbances in anterior and mid-dorsal insular function in individuals with current or remitted anorexia nervosa (14, 15, 29–31). The human gustatory cortex maps to the anterior and mid-insula (7). Moreover, the mid-insula is a chemosensory region involved in relaying various somato- and viscerosensory signals to the anterior insula and striatum (7, 29). Specifically, the mid-dorsal insula integrates gustatory information with information about the body’s homeostatic needs (hunger and satiety signaling) to modulate feeding behavior. The anterior insula projects along the ventromedial axis of the striatum, including the rostral ventral striatum and ventral caudal putamen (9), to mediate and regulate goal-directed behavior, such as the consumption of palatable food (7). Together, these findings raise the question of whether there is a disconnect between the insula and striatum in anorexia nervosa, resulting in a failure to integrate taste information with motivational and homeostatic drives (10).
Our findings here are, to our knowledge, the first to suggest that individuals with remitted anorexia nervosa have an altered insular and striatal response to taste that is moderated by metabolic state. Our earlier studies of tastant responses in remitted anorexia nervosa similarly showed hypoactive response in the anterior insula, ventral putamen, and other striatal regions (14, 15). There was less supervision of prestudy eating in these early studies, raising the possibility of reduced food intake prior to scanning. Comparison of the present findings with those of our earlier studies is also confounded by updates in neuroimaging statistical methods (32) and circuit identification (our earlier studies averaged activation across an anatomically defined region of interest rather than performing voxel-wise analyses within an anatomical search region).
Few studies have compared hungry and fed states in anorexia nervosa. Using a different design, Cowdrey et al. (33) also found altered insular and striatal function during taste processing in remitted anorexia nervosa. Holsen et al. (5) showed similar hungry-state hypoactivation in the anterior insula and limbic regions in response to pictures of food compared with objects in anorexia nervosa independent of illness state. Holsen et al. (5) also noted that earlier studies showed less consistent results, possibly because of methodological differences. Our study only assessed neural response to taste, but other studies suggest altered mid-insular and striatal responses to other interoceptive stimuli (30, 31), reward prediction error (25, 34), and food images (35). We previously showed reduced ventral striatal response to monetary rewards when hungry (16), suggesting that a dysfunction of homeostatic influences on neural processing of salient stimuli or reward is not restricted to food in anorexia nervosa but may generalize to secondary reinforcers (e.g., money) as well. Evidence that nutritional state strongly affects reward and interoceptive processing in anorexia nervosa has significant implications for assessing these constructs and may explain the mixed findings in previous studies that did not manipulate or control for metabolic state.
Participants in the remitted anorexia nervosa group who were more harm avoidant showed less activation in response to taste when hungry in regions of the rostral caudate known to receive input from orbitofrontal and medial prefrontal cortices as well as the dorsolateral prefrontal cortex (Figures 2B and 4C). This raises the possibility that high harm avoidance is associated with reduced engagement of the striatal limbic and associative control circuits that translate food reward to motivated eating, and could be related to altered dopaminergic function. Harm avoidance—a construct of anxiety, inhibition, and inflexibility (36)—and anxiety alone have been associated with caudate dopamine availability in healthy human subjects (37) and rodents (38). Altered dopamine metabolism (39, 40) is found in remitted anorexia nervosa, and dorsal striatum function measured by BOLD response or dopamine metabolism has been linked to elevated anxiety, harm avoidance, and sensitivity to punishment in anorexia nervosa (25, 40, 41). Dorsal striatum dopamine signaling also plays a role in feeding (11). Dopamine-depleted mice, which do not initiate feeding behavior, will resume normal eating after restoration of dopamine selectively in the dorsal striatum, but not the ventral striatum (42). Thus, dorsal striatum dopamine signaling may serve as a permissive “action initiation” signal, promoting nutritive food retrieval and consumption in response to metabolic demand (42). In the context of these previous findings, we speculate that a lower dorsal striatum response to taste when hungry among participants with the most pronounced behavioral inhibition in the remitted anorexia nervosa group may reflect a reduced eating action initiation signal. More research using tasks that include behavioral responses is needed to test this hypothesis. Other potential explanations of dorsal caudate dysfunction in anorexia nervosa include efficient cognitive control (43), development of habitual eating behavior (44), or anxiety associated with eating (41).
Taken together, these findings highlight circuitry that may play a key role in pathological eating in anorexia nervosa (Figure 4A). Of note, this circuitry has also been implicated in appetitive changes associated with major depression: increased appetite is associated with greater response to food stimuli in limbic reward circuitry, whereas appetite loss is associated with hypoactivation within the anterior and mid-insula (45). The pathophysiology driving these disturbances in anorexia nervosa remains to be determined. It is possible that top-down processes inhibit these signals or that there is altered homeostatic system modulation (4). Interestingly, our participants with remitted anorexia nervosa reported hunger and fullness levels similar to those of the control participants (Figure 1), raising the possibility that bottom-up hunger signaling is intact but not accurately translated within the insula-striatal network that motivates action. We propose (Figure 4A) that disturbance in the mid or anterior insula may result in a distorted signal about hunger or feedback about energy balance, the disturbance in the ventral caudal putamen may result in diminished motivation, and a lower caudate response may inhibit initiation among the most harm-avoidant individuals, each serving to maintain food avoidance. Whether developing anorexia nervosa requires disturbances in all three of these processes (interoception, motivation, inhibition) or whether having any one disturbance is sufficient to impair the signal through this network remains uncertain. Factors such as a distorted body image may initially drive restricted eating. If individuals are also vulnerable to failing to respond to homeostatic and reward signals that stimulate eating when starved, they thus may be able to maintain food avoidance.
Limitations and Future Directions
This study has several methodological strengths. We included only participants who had been weight stable (<3 kg weight change) and physiologically and cognitively remitted from anorexia nervosa for at least 1 year, and we systematically monitored and manipulated prescan nutritional status. The study also has some limitations. The passive task was designed specifically to characterize differences in hard-wired circuitry underlying neural sensitivity to gustatory processing, limiting our ability to assess top-down cognitive control, decision making, or learning (46). Unexpectedly, all participants rated the water as more pleasant-tasting than the sucrose solution and both the water and sucrose solutions as moderately pleasant-tasting. This likely limited our ability to isolate activation associated with sweet taste reward. Additionally, these ratings were made only after the task was completed; future studies should include pre- and postscan pleasantness ratings.
Although signal dropout precluded inclusion of the orbitofrontal cortex and hypothalamus in our gustatory-reward circuit mask, exploratory voxel-wise whole brain analyses (see the online supplement) were consistent with the region-of-interest results, with extended findings in thalamic, medial prefrontal, and parietal regions. Although we balanced diagnosis across scanners (χ2=0.37, p=0.60) and participants underwent scanning on the same scanner for both scanning sessions, differences in the magnet hardware may have influenced findings between participants. Since individuals with anorexia nervosa have dysfunctional eating (unlike our participants with remitted anorexia nervosa), it is not clear from our cross-sectional study whether dysfunction within this insular and striatal network in remitted anorexia nervosa is a trait-level alteration or a scar of being underweight. Reward- and anxiety-related alterations associated with anorexia nervosa persist after symptom remission and may even be present in childhood (4, 47); we therefore hypothesized that our study would detect trait-like alterations in neural activation. Changes in other circuits not studied here (e.g., executive control circuits [48]) may be required to compensate for these persistent reward- or anxiety-related alterations to promote normalized behavior. In our sample, BOLD response was not associated with duration of illness or remission, providing further support for trait-like alterations; however, longitudinal research among individuals at risk for developing anorexia nervosa is needed to understand alterations that may predispose individuals to anorexia nervosa and persist after remission. Moreover, further research using paradigms that directly assess food reward valuation and approach in currently symptomatic individuals with anorexia nervosa will be necessary to more rigorously test our hypothesized association between reduced hungry-state activation and reduced motivation to eat.
Finally, our results indicate hungry-state differences in the neural response to predictable receipt of uncertain tastants (sucrose or water). These results cannot be generalized to all food stimuli. For example, other studies suggest different response to aversive taste processing (33, 49). It remains unknown why differences appear specific to hunger and not satiety, and further research is needed to understand the effects of hunger-state differences on unpredictable tastants.
Implications
Treatments for anorexia nervosa are only marginally effective because we lack an understanding of the underlying neural mechanisms of the disorder. If reduced recruitment of the neural circuitry implicated in translating reward signals to motivated behaviors when hungry interferes with food approach and eating initiation after restriction in anorexia nervosa, particularly among individuals with high anxiety, pharmacological and psychotherapeutic strategies that directly target these processes may be beneficial. For example, this circuitry involves dopaminergic projections. Recent findings for olanzapine and aripiprazole (50–53) suggest that investigation of medications targeting the dopamine system may improve eating behavior and reduce anxiety in anorexia nervosa. In addition, these findings support investigating behavioral strategies for enhancing initiation to eat or compensating for altered homeostatic drives (54, 55). Finally, a registry aimed at understanding how individuals with anorexia nervosa recover could be of much benefit in developing new treatment strategies. Such registries have been of use for developing insights into successful weight loss in obesity (56).
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5). Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 2009; 30:1625–1635Crossref, Medline, Google Scholar
3 : Pre-meal anxiety and food intake in anorexia nervosa. Appetite 2010; 55:214–218Crossref, Medline, Google Scholar
4 : Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80(Pt B):132–142Crossref, Medline, Google Scholar
5 : Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci 2012; 37:322–332Crossref, Medline, Google Scholar
6 : Central gustatory processing in humans. Adv Otorhinolaryngol 2006; 63:191–220Medline, Google Scholar
7 : Reward systems in the brain and nutrition. Annu Rev Nutr 2016; 36:435–470Crossref, Medline, Google Scholar
8 : The coding of valence and identity in the mammalian taste system. Nature 2018; 558:127–131Crossref, Medline, Google Scholar
9 : Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 2005; 490:101–118Crossref, Medline, Google Scholar
10 : How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Medline, Google Scholar
11 : Homeostasis meets motivation in the battle to control food intake. J Neurosci 2016; 36:11469–11481Crossref, Medline, Google Scholar
12 : Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009; 44:1008–1021Crossref, Medline, Google Scholar
13 : Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage 2013; 67:322–330Crossref, Medline, Google Scholar
14 : Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology 2008; 33:513–523Crossref, Medline, Google Scholar
15 : Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 2013; 170:1143–1151Link, Google Scholar
16 : Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry 2015; 77:642–652Crossref, Medline, Google Scholar
17 : Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging 2012; 36:39–54Crossref, Medline, Google Scholar
18 : Rostrocaudal gradients of dopamine D2/3 receptor binding in striatal subregions measured with [(11)C]raclopride and high-resolution positron emission tomography. Neuroimage 2013; 82:252–259Crossref, Medline, Google Scholar
19 : A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012; 61:1277–1286Crossref, Medline, Google Scholar
20 : Generalized psychophysiological interaction (PPI) analysis of memory related connectivity in individuals at genetic risk for Alzheimer’s disease. J Vis Exp 2017; 14:129Google Scholar
21 : Robust estimation of location parameter. Ann Math Stat 1964; 35:73–101Crossref, Google Scholar
22 : The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 2012; 108:2242–2263Crossref, Medline, Google Scholar
23 : Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol 1990; 63:689–700Crossref, Medline, Google Scholar
24 : Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol 2003; 90:1865–1876Crossref, Medline, Google Scholar
25 : Association of elevated reward prediction error response with weight gain in adolescent anorexia nervosa. Am J Psychiatry 2017; 174:557–565Link, Google Scholar
26 : Association of brain reward learning response with harm avoidance, weight gain, and hypothalamic effective connectivity in adolescent anorexia nervosa. JAMA Psychiatry 2018; 75:1071–1080Crossref, Medline, Google Scholar
27 : Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 2008; 28:7143–7152Crossref, Medline, Google Scholar
28 : Neuroscience: the sources of human volition. Science 2009; 324:731–733Crossref, Medline, Google Scholar
29 : Convergent gustatory and viscerosensory processing in the human dorsal mid-insula. Hum Brain Mapp 2017; 38:2150–2164Crossref, Medline, Google Scholar
30 : Neural hypersensitivity to pleasant touch in women remitted from anorexia nervosa. Transl Psychiatry 2018; 8:161Crossref, Medline, Google Scholar
31 : Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharm 2016; 41:521–528Crossref, Medline, Google Scholar
32 : Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113:7900–7905Crossref, Medline, Google Scholar
33 : Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry 2011; 70:736–743Crossref, Medline, Google Scholar
34 : Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. J Psychiatry Neurosci 2016; 41:304–311Crossref, Medline, Google Scholar
35 : What can food-image tasks teach us about anorexia nervosa? a systematic review. J Eat Disord 2018; 6:31Crossref, Medline, Google Scholar
36 : Measurement of temperament and character in mood disorders: a model of fundamental states as personality types. J Affect Disord 1998; 51:21–32Crossref, Medline, Google Scholar
37 : Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol 2011; 87:164–167Crossref, Medline, Google Scholar
38 : Dopaminergic modulation of risky decision-making. J Neurosci 2011; 31:17460–17470Crossref, Medline, Google Scholar
39 : Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology 1999; 21:503–506Crossref, Medline, Google Scholar
40 : Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biol Psychiatry 2005; 58:908–912Crossref, Medline, Google Scholar
41 : Dopaminergic activity and altered reward modulation in anorexia nervosa: insight from multimodal imaging. Int J Eat Disord 2017; 50:593–596Crossref, Medline, Google Scholar
42 : Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci 2008; 1129:35–46Crossref, Medline, Google Scholar
43 : Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J Psychiatry Neurosci 2015; 40:307–315Crossref, Medline, Google Scholar
44 : Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat Neurosci 2015; 18:1571–1573Crossref, Medline, Google Scholar
45 : Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry 2016; 173:418–428Link, Google Scholar
46 : Reward system abnormalities in anorexia nervosa: navigating a path forward. JAMA Psychiatry 2018; 75:993–994Crossref, Medline, Google Scholar
47 : Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry 2004; 161:2215–2221Link, Google Scholar
48 : Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatry 2003; 54:934–942Crossref, Medline, Google Scholar
49 : Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: an fMRI study. J Psychiatr Res 2017; 90:94–101Crossref, Medline, Google Scholar
50 : Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry 2019; 176:449–456Link, Google Scholar
51 : The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int J Eat Disord 2017; 50:447–450Crossref, Medline, Google Scholar
52 : Atypical antipsychotics as augmentation therapy in anorexia nervosa. PLoS One 2015; 10:
53 : Aripiprazole in anorexia nervosa and low-weight bulimia nervosa: case reports. Int J Eat Disord 2011; 44:269–275Crossref, Medline, Google Scholar
54 : Temperament-based treatment for anorexia nervosa. Eur Eat Disord Rev 2015; 23:12–18Crossref, Medline, Google Scholar
55 : The acceptability, feasibility, and possible benefits of a neurobiologically informed 5-day multifamily treatment for adults with anorexia nervosa. Int J Eat Disord 2018; 51:863–869Crossref, Medline, Google Scholar
56 : Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes 2016; 9:37–46Medline, Google Scholar
57 : Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23:285–300Crossref, Medline, Google Scholar



