Hyperactivity and Reduced Activation of Anterior Hippocampus in Early Psychosis
Abstract
Objective:
In schizophrenia, the anterior hippocampus is hyperactive and shows reduced task-related recruitment, but the relationship between these two findings is unclear. The authors tested the hypothesis that hyperactivity impairs recruitment of the anterior hippocampus during scene processing.
Methods:
Functional MRI data from 45 early-psychosis patients and 35 demographically matched healthy control subjects were analyzed using a block-design 1-back scene-processing task. Hippocampal activation in response to scenes and faces compared with scrambled images was measured. In a subset of 20 early-psychosis patients and 31 healthy control subjects, baseline hippocampal activity using cerebral blood volume (CBV) mapping was measured. Correlation analyses were used to examine the association between baseline hippocampal activity and task-related hippocampal activation.
Results:
Activation of the anterior hippocampus was significantly reduced and CBV in the anterior hippocampus was significantly increased in the early stages of psychosis. Increased CBV in early-psychosis patients was inversely correlated with task-related activation during scene processing in the anterior hippocampus.
Conclusions:
Anterior hippocampal hyperactivity in early-psychosis patients appears to limit effective recruitment of this region during task performance. These findings provide novel support for the anterior hippocampus as a therapeutic target in the treatment of cognitive deficits in psychosis.
Recent neural models have proposed that hippocampal excitation-inhibition imbalance leads to the development of schizophrenia (1–3). Normal hippocampal function requires a balance of excitation and inhibition, governed by glutamatergic principal cells and GABAergic interneurons. Abnormalities in neurotransmitter systems (glutamate [4, 5] and GABA [6]) and inhibitory interneurons (3, 7) have been identified as contributors to hippocampal dysfunction in schizophrenia. There is now converging evidence that such changes in hippocampal microcircuitry lead to hyperactivity, which may serve as both a critical regulator of neural circuits altered in schizophrenia (8) and a candidate biomarker for psychosis (9). Volume deficits emerge in the anterior hippocampus during the early stages of psychosis (10) and in people at clinical high risk for developing a psychotic disorder (11). Hyperactivity resulting from an excitation-inhibition imbalance is hypothesized to lead to this hippocampal volume deficit (3, 4), but reliable, noninvasive neuroimaging markers of anterior hippocampal function in psychosis are lacking. Identifying and targeting functional changes in the anterior hippocampus before they progress to structural deficits during the critical period following illness onset is a crucial step in improving outcomes (12).
Neuroimaging studies are ideally suited to testing the hypothesis of an excitation-inhibition imbalance in the anterior hippocampus because they can measure the net activity of principal cells and interneurons (13). Anterior hippocampal hyperactivity has been consistently observed using cerebral blood volume (CBV) and cerebral blood flow in people with chronic schizophrenia (14, 15) and high-risk individuals who progress to a psychotic disorder (16, 17). In contrast, there is evidence for posterior hippocampal hyperactivity only in patients with chronic schizophrenia (18, 19). CBV is regarded as the gold standard in vivo measure of baseline hippocampal activity (20), but only three CBV studies of persons with psychosis have been published, with sample sizes of 18, 15, and 10 (14–16).
In this study, we set out to establish a robust, noninvasive, and feasible neuroimaging method to study hippocampal hyperactivity in psychosis. Meta-analyses of hippocampal resting-state connectivity (21) and memory function (22) indicate the presence of functional disturbances in chronic schizophrenia. The few task-based studies that have examined hippocampal function in early psychosis have produced mixed evidence for increased anterior hippocampal activation (23) or decreased activation of anterior and posterior regions (24, 25) during memory tasks. Critically, we do not know whether abnormal baseline hippocampal activity impairs task-related hippocampal function in the early stages of psychosis.
Here, we combined a scene-processing functional MRI (fMRI) task that reliably activates the hippocampus in healthy individuals with CBV imaging to test the hypothesis that anterior hippocampal hyperactivity in psychosis limits functional recruitment during task performance. Recent work has demonstrated that the anterior hippocampus has a role in both perceptual scene processing and construction of mental scenes (reviewed in reference 26). Anterior hippocampal response to scenes is reliably measurable at the single subject level (27) and is not related merely to memory-related functions (28). We hypothesized that in early-psychosis patients, decreased activation of the anterior hippocampus during scene processing stems from increased baseline activity that limits effective recruitment of this region during task performance. These data may help resolve existing discrepancies regarding the nature of hippocampal dysfunction in early psychosis.
Methods
Participants
Participants (N=100) were 61 patients with a nonaffective psychotic disorder and 39 healthy control individuals from an ongoing longitudinal study of brain structure and function in the early stages of psychosis (Table 1). Some participants in this cohort have been included in previous studies of hippocampal volume (10) and relational memory (29), but the CBV and fMRI data presented here are novel. To specifically target early pathology (30), the majority of patients were recruited during the initial months of illness; the average duration of psychosis was approximately 7 months, ranging from less than 1 month to 24 months. More than 80% of the patients were still in the first episode of an emerging psychotic disorder, and more than half of the sample were studied after a single hospitalization for psychosis. On average, patients reported having prodromal symptoms for approximately 1.6 years. Patients were recruited from the inpatient and outpatient clinics of the Vanderbilt University Medical Center Psychiatric Hospital, and healthy control subjects were recruited from the surrounding community through advertisements. Psychiatric diagnoses were assessed with the Structured Clinical Interview for DSM-IV-TR (31). Inclusion and exclusion criteria are described in the online supplement. The Vanderbilt University Institutional Review Board approved the study, and all participants provided written informed consent and received monetary compensation for their time.
| fMRI | CBV | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Healthy Control Group (N=35) | Early-Psychosis Group (N=45) | Healthy Control > Early Psychosis | Healthy Control Group (N=31) | Early-Psychosis Group (N=20) | Healthy Control > Early Psychosis | ||||||||
| Mean | SD | Mean | SD | t | df | p | Mean | SD | Mean | SD | t | df | p | |
| Age (years) | 22.06 | 2.68 | 21.62 | 4.15 | 0.57 | 76 | 0.57 | 22.10 | 2.66 | 20.80 | 2.26 | 1.86 | 45 | 0.07 |
| Participant education (years) | 14.93 | 2.09 | 13.64 | 2.59 | 2.45 | 78 | 0.02 | 15.04 | 2.06 | 13.08 | 1.71 | 3.71 | 46 | <0.001 |
| Parental education (years) | 14.82 | 1.91 | 15.86 | 2.81 | –1.96 | 75 | 0.05 | 14.80 | 1.86 | 16.10 | 2.73 | –1.87 | 30 | 0.07 |
| WTAR score | 113.12 | 12.01 | 104.70 | 15.40 | 2.71 | 76 | 0.008 | 113.07 | 12.53 | 105.95 | 16.13 | 1.67 | 34 | 0.10 |
| PANSS scores | ||||||||||||||
| Positive scale | 17.76 | 7.14 | 18.85 | 7.47 | ||||||||||
| Negative scale | 18.53 | 7.70 | 18.70 | 8.07 | ||||||||||
| General psychopathology scale | 34.18 | 9.90 | 35.00 | 10.30 | ||||||||||
| Duration of psychosis (months) | 7.58 | 6.45 | 7.55 | 6.16 | ||||||||||
| Duration of prodrome (months) | 18.68 | 20.46 | 20.96 | 21.72 | ||||||||||
| Antipsychotic dosage in chlorpromazine equivalents (mg/day) | 286.49 | 211.59 | 286.84 | 244.86 | ||||||||||
| N | % | N | % | χ2 | df | p | N | % | N | % | χ2 | df | p | |
| Male | 28 | 80 | 36 | 80 | 0.0 | 1 | 1.0 | 25 | 81 | 15 | 75 | 0.23 | 1 | 0.63 |
| Race | 0.04 | 2 | 0.98 | 1.60 | 2 | 0.45 | ||||||||
| Caucasian | 28 | 80 | 36 | 80 | 25 | 81 | 15 | 75 | ||||||
| Black | 6 | 17 | 8 | 18 | 6 | 19 | 4 | 20 | ||||||
| Right-handed | 31 | 89 | 43 | 96 | 1.38 | 1 | 0.24 | 27 | 87 | 20 | 100 | 2.80 | 1 | 0.09 |
| Tobacco user | 0 | 0 | 12 | 27 | 10.98 | 1 | <0.001 | 0 | 0 | 6 | 30 | 10.54 | 1 | 0.001 |
| Diagnosis | ||||||||||||||
| Schizophreniform disorder | 33 | 73 | 14 | 70 | ||||||||||
| Schizophrenia | 10 | 22 | 6 | 30 | ||||||||||
| Schizoaffective disorder | 2 | 4 | 0 | 0 | ||||||||||
| In first episode of psychosis at enrollment | 36 | 80 | 17 | 85 | ||||||||||
| First hospitalization at enrollment | ||||||||||||||
| Yes | 23 | 51 | 10 | 50 | ||||||||||
| No | 17 | 38 | 8 | 40 | ||||||||||
| Never hospitalized | 5 | 11 | 2 | 10 | ||||||||||
| Current antipsychotic treatment | 37 | 82 | 15 | 75 | ||||||||||
TABLE 1. Demographic and clinical characteristics of healthy control subjects and early-psychosis patients in a functional MRI (fMRI) and cerebral blood volume (CBV) study of hippocampal activationa
Figure 1 provides details of subject attrition during the study. Of the 100 individuals who participated in the fMRI study, 20 (four healthy control subjects, 16 patients) were excluded from the analysis because of poor data quality, technical problems, or inability to tolerate the scanner. Of the 80 individuals included in the fMRI analysis, 29 (four healthy control subjects, 25 patients) did not complete the CBV mapping study, mainly because of lack of consent or medical reasons. The study design was efficient for the healthy control group, with an overall attrition rate of 21%, but inefficient for the patient group, with attrition rates of 26% and 56% for the fMRI and CBV studies, respectively. Patients with fMRI and CBV data compared with patients with only fMRI data were matched on all demographic and clinical factors with the exception that patients with only fMRI data had slightly lower positive symptom ratings than those with both fMRI and CBV data (Positive and Negative Syndrome Scale positive subscale, t=−2.12, p=0.04) (see Table S1 in the online supplement). The primary details regarding task fMRI and CBV data analyses are presented below; additional details regarding data acquisition, quality control, and analysis are provided in the online supplement.
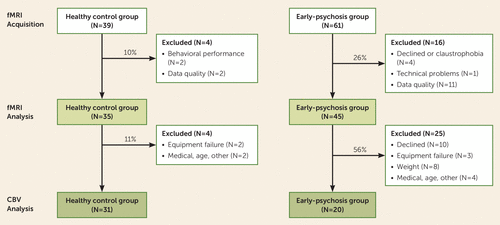
FIGURE 1. Flow diagram showing attrition for participants recruited into a study of hippocampal activation in early-psychosis patients and healthy control subjectsa
a The rate of attrition of patients for cerebral blood volume (CBV) studies was approximately two times higher than the rate for functional MRI (fMRI) studies.
Task fMRI
Participants completed a single run of a block-design 1-back task (Figure 2A) during fMRI scanning. Stimuli were black-and-white images that consisted of indoor or outdoor scenes, scenes featuring male or female faces, and scrambled versions of scene images. Echo-planar functional imaging data and T1-weighted structural images were acquired on a 3-T Philips Intera Achieva scanner at the Vanderbilt University Institute of Imaging Science. We analyzed structural and functional data with SPM12 (http://www.fil.ion.ucl.ac.uk/spm) in MATLAB 2018a (MathWorks, Natick, Mass.) using standard parameters. The first-level analysis included separate regressors for the scene, face, and scramble conditions. Our primary analyses used the contrast estimating the difference in the average response to scene and face compared with scrambled conditions to increase statistical power. For brevity, in the main text we refer to the average response to the scene and face conditions relative to the scrambled image condition as “scene.”
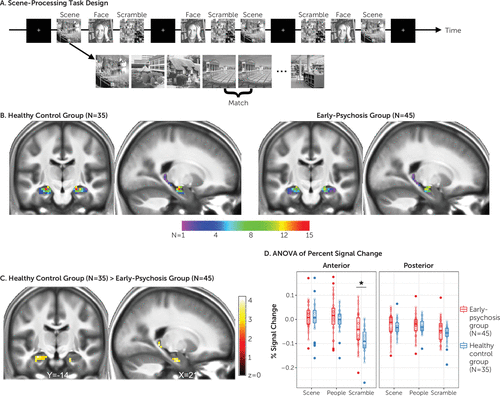
FIGURE 2. Scene-processing task design and hippocampal activation in healthy control subjects and early-psychosis patientsa
a Panel A illustrates the scene-processing task design and an example block. Individuals make a response via button press when an image repeats (“Match”). In panel B, activation of the anterior hippocampus during scene processing is detectable at the individual subject level. The color bar represents the frequency of individuals (N) in each group showing activation in response to scenes. Both images show activation based on the first-level analyses from individual subjects thresholded at z=2.3 and masked by a sample-specific hippocampus region of interest. In panel C, a between-group region-of-interest analysis demonstrates that early-psychosis patients exhibit reduced activation of the hippocampus during scene processing. The p values for these analyses were corrected for multiple comparisons using a semiparametric bootstrap joint testing procedure with a cluster-forming threshold of 0.005 and 2,000 bootstrap samples for a cluster-wise error rate of p<0.05. In panel D, a group-by-hippocampal region-by-condition analysis of variance (ANOVA) of percent signal change data confirms impaired anterior hippocampal activation during scene processing due to elevated baseline function. The asterisk denotes a significant post hoc comparison at p=0.003, corrected for multiple comparisons.
Cerebral Blood Volume Imaging
Gadolinium-enhanced CBV data were acquired on a different day than the fMRI task (average interscan interval: early-psychosis group, 4.96 weeks, SD=10.02; healthy control group, 7.52 weeks, SD=19.36; Wilcoxon W=386, p=0.14). A 3-D T1 fast field echo sequence was used to acquire T1-weighted pre- and postcontrast images. Images were acquired perpendicular to the long axis of the hippocampus. We compared the fractional increase in tissue signal after the contrast agent had thoroughly perfused the microvasculature and equilibrated in the blood.
Statistical Analysis
We conducted region-of-interest analyses to examine the response to scenes within sample-specific left and right hippocampal masks using the PBJ package in R (32) (details concerning mask creation are presented in the online supplement). We first confirmed hippocampal activation in response to scenes within each group by entering the first-level contrast images for scenes into one-sample t tests. To demonstrate scene activation of the anterior hippocampus at the individual level, we constructed overlap maps from the first-level analysis results of each participant (details on overlap map construction are presented in the online supplement). To test the hypothesis of abnormal anterior hippocampal response in early psychosis, we conducted a two-sample t test of the average response to scenes between healthy control subjects and early-psychosis patients. The p values for these analyses were corrected for multiple comparisons using a semiparametric bootstrap joint testing procedure implemented in the PBJ package, with a cluster-forming threshold of 0.005 and 2,000 bootstrap samples for a cluster-wise error rate of p<0.05. This method has been shown to maintain the nominal family-wise error rate more consistently than Gaussian random field theory methods for control of family-wise error, particularly at cluster-forming thresholds greater than 0.001 (32). Percent signal change for each condition was extracted from sample-specific anterior and posterior hippocampus regions of interest using MarsBaR (http://marsbar.sourceforge.net). To confirm the specificity of our findings to the anterior hippocampus, we conducted a group-by-region-by-condition analysis of variance (ANOVA) on percent signal change data in R. Follow-up tests, corrected for multiple comparisons by the Holm method, were conducted to examine significant interactions.
Based on our previous work finding increased anterior hippocampal CBV in schizophrenia (15), we analyzed CBV data (units: mL/mL) averaged across hemispheres to investigate changes specifically in the anterior and posterior slices close to the transitional area. Given our strong a priori hypothesis of anterior hippocampal hyperactivity in psychosis, we performed slice-wise one-tailed Wilcoxon rank sum tests corrected for multiple comparisons using the Holm method. This approach allowed us to more precisely localize the areas along the hippocampal long axis showing hyperactivity in patients.
To test the hypothesis that decreased anterior hippocampal activation in patients during scene processing was associated with increased baseline hippocampal activity, we first computed separate Spearman correlations between anterior hippocampal CBV and percent signal change from the anterior hippocampus during scenes within each group. We then tested whether the correlations differed between groups using a z test of the Fisher-transformed correlations (33). Mean CBV across all anterior slices and percent signal change, averaged across the whole anterior hippocampus region of interest for each subject, were entered into the analysis.
Results
Reduced Anterior Hippocampal Response to Scenes in Early-Psychosis Patients
We found robust anterior hippocampal activation in response to scenes. Both groups showed bilateral activation of the anterior hippocampus in response to scenes, even at a whole brain family-wise error rate of <0.05 (see Figure S1 in the online supplement). Importantly, anterior hippocampal activation was observable at the individual subject level in healthy control subjects and early-psychosis patients (Figure 2B; see also Figure S2 in the online supplement). When we investigated group differences in hippocampal response during scene processing, we found evidence for reduced activation of the hippocampus in early-psychosis patients (Figure 2C). We found similar evidence for reductions in the hippocampus bilaterally when we examined activation in response to scenes alone compared with scrambled images (excluding the face condition; see Figure S3 in the online supplement). To determine whether our findings were specific to the anterior hippocampus, we conducted a group-by-hippocampal region-by-condition ANOVA of percent signal change data. The results of this analysis confirmed that patients in the early stages of psychosis had abnormal activation of the anterior hippocampus (group-by-region-by-condition interaction: F=3.68, p=0.03). Follow-up tests revealed that this effect was driven by elevated baseline activation during the scrambled image condition in the anterior hippocampus (t=−3.42, p=0.003) (Figure 2D). The anterior hippocampal response to scenes was not associated with antipsychotic dosage (in chlorpromazine equivalents) in the patient group (r=−0.02, p=0.92) and did not differ between medicated (N=37, mean=0.06, SD=0.06) and unmedicated (N=8, mean=0.06, SD=0.06) patients (t=−0.34, p=0.74). Anterior hippocampal activation was greater in patients who smoked than in those who were not current smokers (smokers: mean=0.10, SD=0.05; nonsmokers: mean=0.04, SD=0.05; t=3.38, p=0.003).
In concert with the hippocampus, a network of brain regions, including the parahippocampal, medial prefrontal, posterior cingulate, inferior parietal, lateral occipital, and opercular cortices, was activated in response to scenes in both groups (at whole brain family-wise error-corrected p<0.05; see Figure S1 in the online supplement). In contrast to the hippocampus, activation in these brain regions did not differ between the two groups.
Hippocampal Hyperactivity Limited to the Anterior Hippocampus in Early-Psychosis Patients
In the 51 individuals who underwent CBV imaging, we tested the hypothesis that CBV is increased in the anterior hippocampus in early psychosis. We found strong evidence for increased hippocampal CBV in anterior slices 2 (Wilcoxon W=468, p=0.006, corrected) and 3 (W=468, p=0.006, corrected) and moderate evidence in all other slices at uncorrected thresholds (slice 1: W=399, p=0.04; slice 4: W=419, p=0.02; slice 5: W=412, p=0.02; slice 6: W=411, p=0.03) (Figure 3). Anterior hippocampal CBV in the patient group was not associated with antipsychotic dosage (in chlorpromazine equivalents) (r=0.19, p=0.43) and did not differ between medicated and unmedicated patients (t=−0.95, p=0.38). Additionally, anterior hippocampal CBV was similar in current smokers and nonsmokers (t=−0.46, p=0.66).
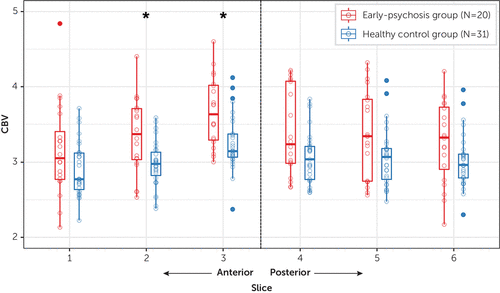
FIGURE 3. Increased anterior hippocampal cerebral blood volume (CBV) in early-psychosis patients relative to healthy control subjectsa
a Asterisks indicate significant Wilcoxon rank sum tests at p=0.006, after Holm correction for multiple comparisons.
Association of Anterior Hippocampal Hyperactivity With Task-Related Responsivity
We then investigated whether the reduced activation of the anterior hippocampus in response to scenes that we found in the early-psychosis patients was associated with increased hippocampal baseline activity. We were able to test this hypothesis in the 51 participants with both fMRI and CBV data. Consistent with our hypothesis, we observed strong evidence of a negative correlation between task activation and CBV in patients (r=−0.50, p=0.01), but not control subjects (r=0.16, p=0.81) (Figure 4). That is, patients with the highest baseline activity in the anterior hippocampus had the lowest activation in response to scenes. The relationship between task activation and CBV differed between patients and control subjects (z=2.33, p=0.01). Although anterior hippocampal activation was higher in smokers compared with nonsmokers (smokers: mean=0.10, SD=0.04; nonsmokers: mean=0.04, SD=0.05), the relationship between fMRI activation and CBV was unrelated to smoking status (t=1.15, p=0.27). Finally, anterior hippocampal fMRI activation was comparable in patients with and without CBV data (t=0.64, p=0.53), suggesting that the findings observed in this smaller sample of 20 patients are representative of the larger patient cohort.
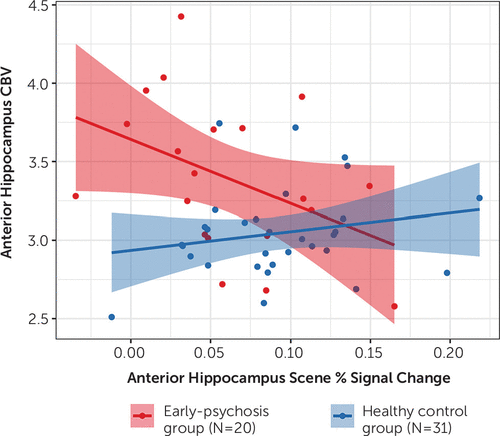
FIGURE 4. Association of increased cerebral blood volume in the anterior hippocampus with decreased activation during scene processing in early psychosisa
a Increased cerebral blood volume in the anterior hippocampus was associated with decreased activation during scene processing in early-psychosis patients (r=−0.50, p=0.01) but not healthy control subjects (r=0.16, p=0.81).
Discussion
Our study provides compelling evidence that anterior hippocampal activation is impaired in early psychosis and that this impairment is related to underlying hyperactivity. These findings form a critical translational bridge between basic research studies of anterior hippocampal activity as a neural substrate for psychosis (1) and functional MRI studies in patients, where it is typically unclear how findings of hyper- versus hypoactivation relate to underlying metabolism and activity.
Preclinical data suggest that the anterior hippocampus, and the subiculum in particular, may be a primary driver in the pathophysiology of psychosis through its modulation of dopaminergic tone (8). Two recent studies using high-resolution fMRI found that the anterior subiculum, rather than the adjacent cornu ammonis or dentate gyrus subfields, is involved in scene perception (28, 34). Consequently, the task used in the present study appears to be uniquely suited to detecting changes in the region hypothesized to induce dopaminergic dysfunction in psychosis. Our findings provide novel evidence that hyperactivity and the associated reduced activation of the anterior hippocampus can be studied with a simple and brief task. However, high-resolution fMRI is needed to confirm anterior subiculum (rather than broad hippocampal) dysfunction during scene processing in psychosis. Notably, scene-processing tasks like the one used in our study can reliably elicit anterior hippocampal activation at the single subject level (27), which suggests the possibility that scene processing could be used as a neuroimaging biomarker for treatment development and early detection of psychosis (9).
Existing preclinical studies support anterior hippocampal hyperactivity both as a therapeutic target and as an early detection marker for psychosis. Prenatal insults to the hippocampus give rise to a psychosis-like phenotype in adolescent and adult rodents (8). Critical work by Grace, Lodge, and colleagues has shown that the hippocampal excitation-inhibition imbalance and hyperdopaminergic state present in this preclinical model can be restored by pharmacological interventions (including benzodiazepines [35], alpha-5 GABA-A [36], and alpha-7 nicotinic receptor modulators [37]), deep brain (38) and vagal nerve stimulation (39), and cell-based therapies (40). In line with this basic research, Tregellas et al. have demonstrated across multiple imaging paradigms that nicotinic agents can reduce hippocampal activation in patients (reviewed in reference 41). Additional work from animal studies has shown that reducing hippocampal hyperactivity in adolescent animals has the potential to forestall illness onset (35) and improve cognitive deficits in adulthood (42). If hippocampal dysfunction in schizophrenia develops in stages (3), then detection of hippocampal hyperactivity before changes in structure occur may offer the best opportunity to intercede and halt illness progression. In order to establish whether the scene-processing task can be used as a biomarker, future work must demonstrate its reliability in patients, sensitivity to illness staging and progression, and response to both existing and novel therapies.
To our knowledge, no studies have yet tested explicitly whether there is impaired anterior hippocampal activation during scene processing in psychosis. Several task-based fMRI studies that have observed altered anterior hippocampus activation in psychosis have used scenes as stimuli (23, 24, 43). Although these studies focused on examining disruption of hippocampus-mediated memory functions, an alternative possibility is that the tasks engaged scene-processing mechanisms within the hippocampus. The present study replicates and extends the recent findings by Francis et al. (24), who reported decreased activation of the anterior hippocampus of early-psychosis patients during a scene-encoding task. Although that study examined scene processing in the context of memory encoding and retrieval, the area of reduced activation they detected in patients is nearly identical to the one reported here. In a separate study, Ragland et al. (23) observed increased activation of the anterior hippocampus during item changes of scenes in patients with schizophrenia. Their task required comparison of fine details in the context of spatial processing, consistent with hypothesized functions of the posterior, rather than anterior, hippocampus (44). Close examination of their data reveals that the increased anterior hippocampal response in patients occurred in the context of no differences in activation for the same contrast in control subjects (see Figure 2 in reference 23). Collectively, this pattern of findings is consistent with underlying hyperactivity in patients manifesting as hypoactivation during tasks that elicit anterior hippocampal activation in healthy control subjects and hyperactivation during tasks that do not activate the anterior hippocampus in healthy control subjects.
Our results provide an important link between findings from persons at high risk for psychosis, where sample sizes are limited by low rates of conversion to clinical psychosis, and studies of patients with chronic psychosis, where long-term medication effects are a significant confounder. We observed increased CBV primarily in the anterior hippocampus of patients in the early stages of psychosis, consistent with previous findings in high-risk individuals (16). Although we observed significant between-group differences in CBV only in the anterior hippocampus, subthreshold differences may be present in the posterior hippocampus. Indeed, two slices in the midbody of the hippocampus (slices 4 and 5; see Figure 4) showed increased CBV that was significant at p<0.05 before correction for multiple comparisons. The majority of patients in the present study were examined during or soon after their first psychotic episode (Table 1). These data provide partial support for the hypothesis that there is a spreading of hippocampal dysfunction during psychosis progression (3, 4). Alternatively, heterogeneity may exist within the patient cohort such that hyperactivity is characteristic of a subset of patients, rather than serving as a global illness biomarker. Longitudinal studies are needed to clarify which of these possibilities is more likely.
The strengths of our study include a large cohort of patients in the early stages of a nonaffective psychotic illness and the use of multimodal neuroimaging of anterior hippocampal function. Our study is limited primarily by the relatively small number of individuals for whom both fMRI and CBV data were available. However, to our knowledge, this sample is the largest CBV imaging data set acquired in patients with a psychotic disorder. Seminal work by Schobel, Small, and colleagues (14, 16) (schizophrenia patients, N=18, and psychotic disorder, N=10, respectively) and previous work by our group (15, 45) (schizophrenia patients, N=15) has examined hippocampal hyperactivity in prodromal or high-risk individuals and in individuals with established schizophrenia, but the present study is the first to demonstrate anterior hippocampal hyperactivity in early psychosis. In comparison to fMRI, CBV imaging presents several challenges that likely preclude its use as a repeatable measure of hippocampal function for longitudinal studies of psychosis, illness staging, and assessment of treatment effects. Many patients decline to participate in a CBV study because of its invasive nature (28% of those eligible for the present study). CBV imaging also has weight and kidney function requirements that can limit the participation of patients treated with antipsychotic medications. An additional limitation is that we observed a relationship between CBV and task-related activation only in the patient group. It is possible that there is not enough CBV variability within the healthy control subjects to detect such an association. However, the absence of a correlation in healthy control subjects is consistent with other hippocampus-related deficits in schizophrenia (46). Finally, we did not examine how the present findings relate to possible alterations in other brain regions, including medial prefrontal cortex function (17). Future studies should examine whether there is an association between GABA concentration in the medial prefrontal cortex and task-related anterior hippocampal dysfunction.
In summary, using a multimodal imaging approach, we found novel evidence for anterior hippocampal dysfunction in early psychosis. Longitudinal studies are needed to examine the relationship between the observed functional differences and subsequent structural changes in the hippocampus and outcomes of patients with psychosis.
1 : Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008; 31:234–242Crossref, Medline, Google Scholar
2 : Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol Psychiatry 2017; 81:874–885Crossref, Medline, Google Scholar
3 : GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res 2015; 167:4–11Crossref, Medline, Google Scholar
4 : Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 2018; 23:1764–1772Crossref, Medline, Google Scholar
5 : Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull 2012; 38:927–935Crossref, Medline, Google Scholar
6 : Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry 1999; 46:589–599Crossref, Medline, Google Scholar
7 : A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 2009; 29:2344–2354Crossref, Medline, Google Scholar
8 : The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr Bull 2019; 45:148–157Crossref, Medline, Google Scholar
9 : Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry 2014; 76:111–119Crossref, Medline, Google Scholar
10 : Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. Neuroimage Clin 2018; 20:1106–1114Crossref, Medline, Google Scholar
11 : Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev 2011; 35:1175–1185Crossref, Medline, Google Scholar
12 : Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br J Psychiatry 2009; 194:18–24Crossref, Medline, Google Scholar
13 : The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity: a combined functional MRI and electrophysiological study. J Neurosci 2009; 29:2428–2439Crossref, Medline, Google Scholar
14 : Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 2009; 66:938–946Crossref, Medline, Google Scholar
15 : Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin 2014; 5:359–364Crossref, Medline, Google Scholar
16 : Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78:81–93Crossref, Medline, Google Scholar
17 : Prefrontal GABA levels, hippocampal resting perfusion, and the risk of psychosis. Neuropsychopharmacology 2018; 43:2652–2659Crossref, Medline, Google Scholar
18 : Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar
19 : Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
20 : A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 2011; 12:585–601Crossref, Medline, Google Scholar
21 : Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull 2013; 39:358–365Crossref, Medline, Google Scholar
22 : Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry 2005; 187:500–509Crossref, Medline, Google Scholar
23 : Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. Neuroimage Clin 2016; 13:82–88Crossref, Medline, Google Scholar
24 : Functional neuroanatomical correlates of episodic memory impairment in early phase psychosis. Brain Imaging Behav 2016; 10:1–11Crossref, Medline, Google Scholar
25 : Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry 2007; 64:999–1014Crossref, Medline, Google Scholar
26 : Anterior hippocampus: the anatomy of perception, imagination, and episodic memory. Nat Rev Neurosci 2016; 17:173–182Crossref, Medline, Google Scholar
27 : Evidencing a place for the hippocampus within the core scene processing network. Hum Brain Mapp 2016; 37:3779–3794Crossref, Medline, Google Scholar
28 : Ultra-high-field fMRI reveals a role for the subiculum in scene perceptual discrimination. J Neurosci 2017; 37:3150–3159Crossref, Medline, Google Scholar
29 : Impaired associative inference in the early stage of psychosis. Schizophr Res 2018; 202:86–90Crossref, Medline, Google Scholar
30 : Diverse definitions of the early course of schizophrenia: a targeted literature review. NPJ Schizophr 2018; 4:21Crossref, Medline, Google Scholar
31 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, New York State Psychiatric Institute, Biometrics Research, 2001Google Scholar
32 : Robust spatial extent inference with a semiparametric bootstrap joint inference procedure. Biometrics (Epub ahead of print, July 8, 2019)Google Scholar
33 : Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed. Mahwah, NJ, Lawrence Erlbaum Associates, 2003Google Scholar
34 : Investigating the functions of subregions within anterior hippocampus. Cortex 2015; 73:240–256Crossref, Medline, Google Scholar
35 : Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci Biobehav Rev 2016; 70:260–270Crossref, Medline, Google Scholar
36 : The role of α5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des 2014; 20:5069–5076Crossref, Medline, Google Scholar
37 : α7 Nicotinic receptor-modulating agents reverse the hyperdopaminergic tone in the MAM model of schizophrenia. Neuropsychopharmacology 2018; 43:1712–1720Crossref, Medline, Google Scholar
38 : Hippocampal deep brain stimulation reverses physiological and behavioural deficits in a rodent model of schizophrenia. Int J Neuropsychopharmacol 2013; 16:1331–1339Crossref, Medline, Google Scholar
39 : Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci 2014; 34:9261–9267Crossref, Medline, Google Scholar
40 : Cell-based therapies for the treatment of schizophrenia. Brain Res 2017; 1655:262–269Crossref, Medline, Google Scholar
41 : Targeting neuronal dysfunction in schizophrenia with nicotine: evidence from neurophysiology to neuroimaging. J Psychopharmacol 2017; 31:801–811Crossref, Medline, Google Scholar
42 : Treatment with levetiracetam improves cognition in a ketamine rat model of schizophrenia. Schizophr Res 2018; 193:119–125Crossref, Medline, Google Scholar
43 : Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr Res 2012; 138:157–163Crossref, Medline, Google Scholar
44 : Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014; 15:655–669Crossref, Medline, Google Scholar
45 : Increased hippocampal blood volume and normal blood flow in schizophrenia. Psychiatry Res 2015; 232:219–225Crossref, Medline, Google Scholar
46 : Verbal learning and hippocampal dysfunction in schizophrenia: a meta-analysis. Neurosci Biobehav Rev 2018; 86:166–175Crossref, Medline, Google Scholar



