Psychedelics and Psychedelic-Assisted Psychotherapy
Abstract
Objective:
The authors provide an evidenced-based summary of the literature on the clinical application of psychedelic drugs in psychiatric disorders.
Methods:
Searches of PubMed and PsycINFO via Ovid were conducted for articles in English, in peer-reviewed journals, reporting on “psilocybin,” “lysergic acid diethylamide,” “LSD,” “ayahuasca,” “3,4-methylenedioxymethamphetamine,” and “MDMA,” in human subjects, published between 2007 and July 1, 2019. A total of 1,603 articles were identified and screened. Articles that did not contain the terms “clinical trial,” “therapy,” or “imaging” in the title or abstract were filtered out. The 161 remaining articles were reviewed by two or more authors. The authors identified 14 articles reporting on well-designed clinical trials investigating the efficacy of lysergic acid diethylamide (LSD), 3,4-methylenedioxymethamphetamine (MDMA), psilocybin, and ayahuasca for the treatment of mood and anxiety disorders, trauma and stress-related disorders, and substance-related and addictive disorders as well as in end-of-life care.
Results:
The most significant database exists for MDMA and psilocybin, which have been designated by the U.S. Food and Drug Administration (FDA) as “breakthrough therapies” for posttraumatic stress disorder (PTSD) and treatment-resistant depression, respectively. The research on LSD and ayahuasca is observational, but available evidence suggests that these agents may have therapeutic effects in specific psychiatric disorders.
Conclusions:
Randomized clinical trials support the efficacy of MDMA in the treatment of PTSD and psilocybin in the treatment of depression and cancer-related anxiety. The research to support the use of LSD and ayahuasca in the treatment of psychiatric disorders is preliminary, although promising. Overall, the database is insufficient for FDA approval of any psychedelic compound for routine clinical use in psychiatric disorders at this time, but continued research on the efficacy of psychedelics for the treatment of psychiatric disorders is warranted.
“Timothy Leary’s dead…”
—The Moody Blues, 1968
Although hallucinogens derived from plants have been used in religious practices for centuries, it was not until 1938 that the Swiss chemist Albert Hofmann synthesized the first synthetic hallucinogen, lysergic acid diethylamide (LSD), while working with the pharmaceutical company Sandoz (1, 2). On April 16, 1943, during a series of experiments, Hofmann serendipitously came into physical contact with LSD, which resulted in “an uninterrupted stream of fantastic pictures, extraordinary shapes with intense, kaleidoscopic play of colors” (1). In 1947, Sandoz began to market LSD under the trade name Delysid as an adjunctive psychotherapy medication and as an agent for experimental study on the nature of psychoses (1).
In 1960, Harvard psychologist Timothy Leary began experiments under the Harvard Psilocybin Project to determine whether psilocybin was an effective adjuvant agent in psychotherapy. Leary also experimented with LSD and eventually became a polarizing figure who was dismissed from Harvard, along with his colleague Richard Alpert, in 1963. The last of the Sandoz patents for the production of LSD expired in 1963, and illicit production of LSD increased as it was being used widely in medically unsupervised settings (1). In 1965, governments in Europe and the United States raised concerns about the general public’s use of LSD and psilocybin. The U.S. Congress passed the Drug Abuse Control Amendments, which made the sale and manufacture of LSD without a license a misdemeanor and forced all researchers who had not been granted Investigational New Drug exemptions by the U.S. Food and Drug Administration (FDA) to relinquish their supplies of LSD (1). Clinical experimentation and research with psychedelics consequently decreased and were ultimately halted by the Controlled Substances Act of the Comprehensive Drug Abuse Prevention and Control Act of 1970.
Although Timothy Leary died in 1996, the lyrics by Ray Thomas of the Moody Blues almost three decades earlier were prescient: psychedelic research was indeed dead after the passage of the Controlled Substances Act. The following year, President Richard Nixon declared the “War on Drugs,” and much of the experimentation in psychedelics moved underground in counterculture movements that spread across the United States and Europe.
Over the course of the past decade, there has been a resurgence of research on the potential therapeutic benefits of psychedelic compounds, with the number of published review articles and clinical trial reports steadily increasing. Research on these compounds has been supported by diverse organizations ranging from the United Kingdom Medical Research Council, a nationally funded health agency, to the Multidisciplinary Association for Psychedelic Studies (MAPS), a nonprofit organization that was founded in 1986 to increase the knowledge base of psychedelic substances. Additional support has come from the Heffter Research Institute, a nonprofit scientific organization founded in 1993 that promotes research with the classic hallucinogens and related compounds, and the Beckley Foundation, a U.K.-based research and nongovernmental organization focused on pioneering psychedelic research and evidence-based drug policy reform. These organizations have helped fund many pivotal trials and often work with regulatory agencies, including the FDA and the European Medicines Agency, to ensure that studies conform to the requisite regulatory guidelines for eventual approval of clinical use. Contemporary psychedelic drug research has been conducted at leading academic research universities around the world, including Johns Hopkins University, New York University, University of California, Los Angeles, Imperial College London, University of Zurich, and University of Basel. Recently, Johns Hopkins University and Imperial College London established centers for psychedelic research, which aim to investigate the effects of psychedelic drugs on the mind, the brain, and psychiatric disorders.
The U.S. Drug Enforcement Administration (DEA) currently classifies LSD, ayahuasca, psilocybin, and 3,4-methylenedioxymethamphetamine (MDMA) as Schedule I substances, reflecting a lack of any accepted medical use or safety data and their potential for abuse. This review is intended to summarize the evidence base, including all of the available research in the scientific literature, for the safety and efficacy of psychedelic compounds in the treatment of psychiatric disorders.
Methods
Searches were conducted of PubMed and PsycINFO via Ovid for English-language articles in peer-reviewed journals reporting on “psilocybin,” “lysergic acid diethylamide,” “LSD,” “ayahuasca,” “3,4-methylenedioxymethamphetamine,” and “MDMA,” in human subjects, for publication dates from January 1, 2007, through July 1, 2019. We chose to focus the review on these four compounds because they have recently received notable media coverage for their therapeutic potential (3–5). A total of 1,603 articles were identified and screened. Articles that did not contain the terms “clinical trial,” “therapy,” or “imaging” in the title or abstract were filtered out, resulting in a total of 161 articles for further review. To achieve a comprehensive summary of relevant clinical findings, our summary was not limited to these randomized clinical trials but also included open-label trials and investigations in healthy volunteers. We identified 14 articles reporting on well-designed clinical trials investigating the efficacy of LSD, MDMA, psilocybin, and ayahuasca for use in the treatment of mood and anxiety disorders, trauma- and stress-related disorders, and substance use disorders as well as for end-of-life care. Methodological strengths and limitations of studies evaluating the use of psychedelics in psychiatric disorders were identified and are summarized below for each drug. The review has been supplemented with information from texts on the history of the use of psychedelics in psychiatry and information on clinical techniques used in studies, such as psychedelic psychotherapy. Information about ongoing or planned clinical trials has been included with ClinicalTrials.gov registration information. The methodology flow chart is presented in the online supplement.
Psychedelic Compounds
The psychedelics can be divided into four classes based on their pharmacological profiles and chemical structures: classic psychedelics (serotonin 2A [5-HT2A] receptor agonists), empathogens or entactogens (mixed serotonin and dopamine reuptake inhibitors and releasers), dissociative anesthetic agents (N-methyl-d-aspartate [NMDA] antagonists), and atypical hallucinogens, which affect multiple neurotransmitter systems (6). In this review we discuss three classic psychedelics (LSD, psilocybin, and ayahuasca) and one entactogen (MDMA) in detail. The dissociative anesthetic ketamine has been the subject of previous publications from the American Psychiatric Association Work Group on Biomarkers and Novel Treatments (7, 8) and will be compared and contrasted with these compounds in the section comparing the psychedelic compounds later in the review.
Psilocybin
Psilocybin is a plant alkaloid derived from tryptamine precursors and found in a variety of mushroom species (9). It has been used by native peoples of Central and South America within a sacramental context for centuries to facilitate spiritual experiences (10). In the 1950s, psychedelic mushrooms were introduced to Western culture when amateur mycologist R. Gordon Wasson and his wife, pediatrician Valentina Wasson, published a story in Life magazine describing their experience with psilocybin during participation in a Mazatecan ceremony in Mexico. The psychoactive compounds psilocybin and psilocin were first isolated from the mushroom species Psilocybe mexicana through collaborative research by mycologist Roger Heim and Albert Hofmann and his colleagues at Sandoz Laboratories (1). After determining the molecular structures of these compounds, Sandoz began the synthetic chemical production of psilocybin, eliminating the previously required cultivation of mushrooms (1).
Psilocybin is actively metabolized to psilocin, a serotonin transporter inhibitor and 5-HT2A receptor partial agonist with <40% activation efficacy; it also binds to the 5-HT2C, 5-HT1A, and 5-HT1B receptors, with binding affinities in descending order (11, 12). When taken at high doses (0.3–0.6 mg/kg), it can cause mild to profound changes in sensory perception, including synesthesia, euphoria, sensory illusions, and auditory and visual hallucinations. These effects are dose dependent and last 3 to 6 hours (13–15). Unpleasant effects can include feelings of a seemingly “unending experience,” as well as nausea, vomiting, and transient headaches (16–18).
Systematic investigation into psilocybin began in 1962, when Walter Pahnke and Timothy Leary conducted the “Marsh Chapel Experiment,” also known as the “Good Friday Experiment” (19, 20). In this randomized controlled trial, Protestant divinity student volunteers (N=20; 10 per group) received psilocybin or a placebo (niacin) to evaluate the potential entheogenic properties of psilocybin. While the active and control drugs had differing physiological properties that likely challenged the blinding of the experiment, measurement of participants’ responses with an eight-category scale for mystical experiences confirmed the hypothesized effect of psilocybin (p<0.05).
Leary and colleagues also conducted the “Concord Prison Experiment” to determine whether psilocybin-assisted group psychotherapy could reduce rates of recidivism after a period of incarceration (21). In this open-label study, prison inmates (N=32) participated in two psilocybin-assisted group psychotherapy sessions, each with a dose of 20–70 mg, followed by a series of psychotherapy sessions. Despite initial reports by Leary that psilocybin significantly reduced rates of recidivism, a later reanalysis by Doblin found that the recidivism rate of the experimental group was not significantly lower than that of the general prison population (20, 22).
Recently, there has been a resurgence in psilocybin research in the United States and Europe in the treatment of refractory mood disorders, refractory obsessive-compulsive disorder, end-of-life anxiety, and tobacco and alcohol use disorders. Carhart-Harris et al. (23) conducted an open-label pilot study evaluating the feasibility and efficacy of psilocybin-assisted psychotherapy for patients (N=12) with moderate to severe depression (defined as a score >17 on the Hamilton Depression Rating Scale [HAM-D]) and treatment-refractory depression (no improvement after trials of two different classes of antidepressant medication lasting at least 6 weeks within the current episode). Participants were given two oral doses of psilocybin in association with psychotherapy sessions, 7 days apart; they received a low dose (10 mg) of psilocybin at the first session and a higher dose (25 mg) at the second session. During the psilocybin sessions, therapists used a nondirective, supportive approach. All assessment measures were performed at baseline and at 1 week and 3 months after the second psilocybin-assisted psychotherapy session. The primary measure for efficacy was the Quick Inventory of Depressive Symptomatology (QIDS). QIDS depression scores were significantly decreased from baseline to 1 week and 3 months after treatment. The mean change in QIDS score was −11.8 (SD=4.9; p=0.002) at 1 week and −9.2 (SD=6.0; p=0.003) at 3 months. Secondary measures included the HAM-D and the Beck Depression Inventory (BDI). At the 1-week follow-up, categorical remission (defined as a score ≤9 on the BDI) was achieved by eight patients (67%). At the 3-month follow-up, categorical response (a 50% reduction in BDI score) was achieved by seven patients (58%), and five patients (42%) remained in complete remission.
In the same sample, functional MRI (fMRI) scans were performed at baseline and again the morning after the high-dose psilocybin-assisted psychotherapy session (24). One day before and 1 day after their psilocybin sessions, patients were shown images of faces with fearful, happy, or neutral expressions selected from the Karolinska Directed Emotional Faces set. Patients who received psilocybin showed increased amygdalar responses to fearful compared with neutral faces 1 day after treatment, and this response predicted positive clinical outcome 1 week later. Heightened amygdalar activity following psilocybin administration was interpreted as evidence of a different antidepressant mechanism of action than that of patients treated with selective serotonin reuptake inhibitors (SSRIs), who have shown diminished amygdalar response to emotional stimuli. Further fMRI research has demonstrated that psilocybin acutely disrupts default mode network connectivity, inducing temporary neuroplastic states that may make an individual more susceptible and receptive to cognitive functions and content accessed with coadministered nondirective supportive psychotherapy (25, 26).
Mood and adjustment disorders comorbid with cancer diagnoses are debilitating and are associated with poor clinical outcomes (27). Grob et al. (28) performed a randomized clinical trial (N=12, 11 of them women) investigating the safety and efficacy of psilocybin for the treatment of anxiety in patients with advanced-stage breast (N=4), colon (N=3), ovarian (N=2), peritoneal (N=1), or salivary gland (N=1) cancers or multiple myeloma (N=1). Each subject acted as his or her own control and had two treatment sessions in random order spaced several weeks apart: one session with a moderate dose of psilocybin (0.2 mg/kg) and the other with active placebo (niacin 250 mg). While there was no significant change in the self-reported State-Trait Anxiety Inventory (STAI) state score, STAI trait scores were significantly decreased at follow-up assessments 1 month (p=0.001) and 3 months (p=0.03) after the second treatment session. BDI scores did not change from baseline (1 day before placebo administration) to the 2-week follow-up assessment, but they dropped significantly by 1 month (p=0.05) and remained significantly different at 6 months (p=0.03).
A similar but larger double-blind randomized crossover study by Griffiths et al. (18) (N=51) investigated the effects of psilocybin, administered in two sessions, on depression and anxiety syndromes in patients with terminal cancer who also had a DSM-IV diagnosis of an anxiety or mood disorder. The primary cancer types were breast (N=13), upper aerodigestive tract (N=7), gastrointestinal (N=4), genitourinary (N=18), hematologic malignancies (N=8), and other (N=1). Participants were excluded if they were taking psychoactive prescription medications (e.g., SSRIs, monoamine oxidase inhibitors, benzodiazepines). During the psilocybin sessions, participants received a high dose (22 mg/70 kg) or a low dose (1 mg or 3 mg/70 kg) of psilocybin, with the low dose serving as an active control. Participants were crossed over to receive the alternative dose in a second session 5 weeks later.
Before the first psilocybin session, participants met with study monitors to discuss “meaningful aspects” of their lives. During dosing sessions, therapists provided a supportive presence and encouraged participants to “trust, let go, and be open” to the experience, but otherwise were nondirective. The data showed that high-dose but not low-dose psilocybin produced large and significant decreases in depression and anxiety symptoms after 5 weeks, and this effect persisted through 6-month follow-up. A clinically significant response was defined as a decrease of ≥50% in score on the GRID-HAM-D-17 or the HAM-A relative to baseline, and scores below threshold level (≤7) defined symptom remission on each measure. The 6-month response rate was 78% for depressive syndromes using the GRID-HAM-D-17 and 83% for anxiety syndromes using the HAM-A; remission scores were achieved by 65% of participants on the GRID-HAM-D-17 and by 57% on the HAM-A.
A double-blind placebo-controlled (using niacin) randomized controlled crossover study by Ross et al. (29) (N=29) evaluated the efficacy of a single high dose of psilocybin (0.3 mg/kg) in conjunction with medication-assisted psychotherapy in patients with cancer-related anxiety and depressive symptoms as measured by the Hospital Anxiety and Depression Scale (HADS), with subscales for anxiety (HADS-A) and depression (HADS-D). Approximately two-thirds of the patients had advanced (stages II–IV) cancer, and the types of cancer included breast or reproductive (59%), gastrointestinal (17%), hematologic (14%), and other (10%). The BDI and the STAI state and trait scales were also administered at baseline and at regular intervals during the study. After 7 weeks, the placebo group was crossed over to psilocybin and the active psilocybin group to placebo. Medication-assisted psychotherapy included preparatory psychotherapy, medication dosing sessions, and postdosing integrative psychotherapy. During medication-assisted psychotherapy sessions, participants were encouraged to lie comfortably on a couch, to wear eye shades, to listen to preselected music, and to direct their thoughts toward their internal experience. Two study therapists, typically one male and one female, were present and available for psychological and medical support throughout the duration of the experimental sessions.
There were significant reductions in all of the primary measures (HADS total, HADS-A, HADS-D, BDI, STAI state, STAI trait) in the psilocybin group compared with the control group immediately after the experimental session, and these reductions were maintained until crossover of the control group at week 7. The psilocybin-first group had significant within-group reductions compared with baseline in anxiety and depression at all six time points, including the final time point at 26 weeks after dosing. Before being crossed over to psilocybin, the placebo-first group had no sustained significant reductions on any of the primary measures. Immediately after receiving psilocybin, the placebo-first group had significant within-group reductions in depression and anxiety symptoms on five of six primary measures. These reductions persisted and were present at all three time points, including the final time point at 26 weeks after dose 2 (approximately 6.5 months). At follow-up, 6.5 months after the active psilocybin intervention, 60%−80% of participants had sustained their responder status on depression and anxiety scales (defined as a reduction ≥50% in score on the measure compared with baseline).
There is preliminary evidence that psilocybin may be efficacious in the treatment of substance use disorders. An open-label study by Johnson et al. (30) enrolled participants who wanted to quit smoking (N=15) in a 15-week course of smoking cessation treatment coupled with psilocybin administration. The first 4 weeks of treatment consisted of cognitive-behavioral therapy, assigning a target quit date, and keeping a smoking diary. Psilocybin was administered at weeks 5 and 7, with an optional third psilocybin session at week 13. Participants were given a moderate dose of psilocybin (20 mg/70 kg) during the first experimental session and received a higher dose of psilocybin (30 mg/70 kg) at their second and third experimental sessions, unless they requested a moderate dose of psilocybin. The target quit date coincided with the first psilocybin session. During the sessions, research staff provided nondirective interpersonal support and did not deliver smoking cessation–specific content. Smoking abstinence was verified at all data collection points using exhaled carbon monoxide (CO level ≤6 ppm) and urinary cotinine measurements (level <200 ng/mL). At the 6-month follow-up, 12 of the 15 participants (80%) were laboratory-verified as abstinent; 10 participants (67%) remained abstinent at 12 months, and nine (75%) at 2.5 years. The pilot study has been extended to include 95 participants and should be completed by 2021 (ClinicalTrials.gov identifier 01943994).
Bogenschutz et al. (31) evaluated open-label psilocybin for the treatment of individuals who met DSM-IV criteria for alcohol dependence and had at least two heavy drinking days in the previous 30 days (N=10). Participants also received psychotherapy, which included 14 sessions: seven sessions of motivational enhancement therapy, three preparation sessions, two psilocybin-assisted psychotherapy sessions, and two debriefing sessions. Participants received their first dose of psilocybin (0.3 mg/kg) after their first four psychotherapy sessions and their second dose (0.4 mg/kg) after their next four sessions, which was followed by four more psychotherapy sessions.
The primary outcome measures were the Stages of Change Readiness and Treatment Eagerness Scale, the Alcohol Abstinence Self-Efficacy Scale, the Penn Alcohol Craving Scale, and the Profile of Mood States. Two therapists were present throughout the psilocybin sessions, and their interactions with the participants were supportive and nondirective. Abstinence was not biologically verified and was based on self-report. The study found that abstinence significantly increased after the first psilocybin session at 4 weeks and was largely sustained through 36 weeks. Bogenschutz et al. are currently conducting a randomized clinical trial investigating the efficacy of psilocybin for treating alcohol dependence. The study is projected to enroll 180 participants and is expected to be completed in 2020 (ClinicalTrials.gov identifier 02061293).
Central to psilocybin-assisted therapy is the notion that participant response correlates with a psilocybin-induced “mystical” or “spiritual” experience. In the studies described above, the investigators noted correlations between symptom reduction and the participants’ appraisals of their psilocybin experiences as personally meaningful, as reflected by their scores on the 30-item Mystical Experience Questionnaire (MEQ-30) (18, 30, 31). The MEQ-30 is a validated measure of mystical experience (32) that assesses seven domains of mystical experiences: internal unity, external unity, noetic quality (feeling of perception or revelation during the experience), sacredness, positive mood, transcendence of time/space, and ineffability (difficulty of communicating or describing the experience to others) (33). Confirmatory factor analyses have demonstrated the reliability and validity of the instrument, and external and convergent validity have been demonstrated by latent variable scores positively predicting psilocybin-related changes in attitudes, behavior, and well-being (32).
Mystical experiences have many names—religious experiences, transcendental experiences, transforming moments, epiphanies—but are all characterized by personal transformations that lead to dramatic or “quantum” changes in a person’s sense of self and behavior (34). In a prospective study, Griffiths et al. (34) examined the long-term effects of a psilocybin-related mystical experience in individuals with no prior use of psilocybin when combined with meditation or spiritual practices. The total scores on the MEQ-30 and the Spiritual Experiences Scale both indicated healthy psychological functioning at 6-month follow-up, with the intensity of the psilocybin-induced mystical experience making the most significant contribution to the effect.
Although practitioners recognize that the acute presentation of a psilocybin-intoxicated individual closely resembles psychosis, hallucinogens such as psilocybin are not thought to precipitate a new psychotic illness but rather may unmask a psychotic disorder in those who are susceptible (35, 36). In an analysis of 110 healthy study volunteers from 227 psilocybin administrations, researchers found no evidence of hallucinogen persisting perception disorder, prolonged psychosis, or other long-term impairment of functioning in any subjects (37). Much of the research on the sequelae from psilocybin and other classic psychedelic use is from studies that screen participants for a history of psychiatric problems, regulate the dosage of the drug, and administer the drug in a controlled setting. These safeguards are intended to minimize the potential for adverse events.
Contrast this with the potential effects of psilocybin in an uncontrolled community setting. In an online survey (38) of almost 2,000 people who answered positively to the question of whether, after taking psilocybin mushrooms, they “ever had a psychologically difficult or challenging experience (i.e., a bad trip)—that is, have you experienced significant fear, anxiety, or distress or anything else that you found psychologically difficult,” 39% of respondents reported that the experience was one of the most challenging experiences of their lifetime. Twenty-four percent of participants reported psychological symptoms lasting 1 week or longer (i.e., fear, anxiety, depression, or paranoia), 10% reported persistent symptoms for more than 1 year, and 7.6% sought professional help for psychological symptoms. Although this online survey is not rigorous enough to serve as a guide for clinical practice, it nevertheless points out potential concerns with the use of psychedelics in uncontrolled settings (6).
In 2018, the FDA designated psilocybin a “breakthrough therapy” for treatment-resistant depression, giving it priority consideration in the regulatory process (39). At this time, Compass Pathways, a London-based life sciences company, is starting phase 2B clinical trials in Europe and North America in 216 patients across 12–15 research sites for treatment-resistant depression, with additional phase 3 studies (40–42). The Usona Institute, a U.S. nonprofit medical research organization, is also planning phase 2 and 3 FDA-registration multisite trials to investigate psilocybin as a treatment for depression, anxiety, and mood disorders associated with end of life (43). Two ongoing phase 2 randomized clinical trials are investigating psilocybin’s effects in patients with a diagnosis of obsessive-compulsive disorder to replicate and extend the initial findings of a study by Moreno et al. (44) (published in 2006, outside the search date criteria for this review) (ClinicalTrials.gov identifiers 03300947 and 03356483). Additional studies are investigating psilocybin for the treatment of cocaine use disorder (ClinicalTrials.gov identifier 04052568), opioid use disorder (ClinicalTrials.gov identifier 04161066), anorexia nervosa (ClinicalTrials.gov identifier 04052568), and depression in early Alzheimer’s disease (ClinicalTrials.gov identifier 04123314).
Lysergic Acid Diethylamide (LSD)
LSD is an ergot derivative best known for its ability to induce powerful psychedelic, spiritual, and mystical experiences (1, 45, 46). LSD has been described as a psychoadjuvant or “nonspecific amplifier of the unconscious,” with effects that include weakening ego identification, accelerating and broadening thought processes and content, promoting novel thought associations, and modifying one’s interpretations and understanding of relationships and objects (47–49). It can induce feelings of closeness to others, enhance emotional empathy, enhance sociality, and acutely impair fear recognition (50). At moderate to high doses, LSD enhances sensory perception, which can lead to illusions, dreamlike waking imagery, synesthesia, alterations in sound perception, and mystical experience (48, 51–53).
The hallucinogenic effects of LSD are thought to be mediated by several mechanisms: partial agonism at the 5-HT2A receptor, binding to the 5-HT1A, 5-HT2C, and 5-HT2B receptors (with affinity in descending order), and binding at dopamine D2 receptors. It also causes glutamate release in the frontal cortex and increased functional connectivity and excitability in thalamic and cortical structures (11, 54–58). LSD does not interact with monoamine transporters and is more potently bound than all other tryptamines to the 5-HT2A and 5-HT1A receptors (11). Other pharmacodynamic and pharmacokinetic mechanisms of LSD have been extensively explored (59) but are outside the scope of this review.
Starting in the 1940s and continuing through the 1960s, there was a rise in the number of studies on potential uses of LSD in healthy volunteers as well as in treating psychiatric disorders (16, 60). Observed psychological outcomes were initially thought to mimic schizophrenia, suggesting LSD as a potential model for psychosis (1, 47, 61). Recent studies have shown that psychotic symptoms associated with LSD ingestion are more likely in healthy volunteers with premorbid schizoid and paranoid traits and persons with a family history of schizophrenia (62). A large epidemiologic study of 130,000 adults in the United States did not find a link between psychedelic use (including LSD and psilocybin) and mental health problems or suicidal behavior (63).
Studies have noted the experiential effects of LSD-induced behavioral changes in individuals with substance use disorders, and LSD has been recognized as a potential treatment for alcohol use disorder (64). Several research groups have described LSD’s potential for symptom alleviation in individuals with mood disorders and in pain syndromes associated with end-of-life care (16, 45, 65). Although preliminary LSD trials produced generally positive outcomes, clinical research on the therapeutic use of LSD was cut short in 1968, when the Drug Abuse Control Amendments were modified to make possession of LSD a misdemeanor and the sale of LSD a felony. LSD is currently classified as a Schedule I drug under the Controlled Substances Act (66, 67).
Recently there have been a few small open-label studies outside the United States investigating LSD for the treatment of mood disorders, anxiety in the terminally ill, and migraine headaches (16, 68). A group of Swiss and German researchers, Gasser et al. (48), conducted a randomized controlled trial to examine the safety and efficacy of LSD-assisted psychedelic psychotherapy in patients with anxiety associated with medical disease (N=12), including malignancy, Parkinson’s disease, celiac disease, and ankylosing spondylitis. The primary outcome measure was the STAI trait and state forms completed at baseline, at 1 week, and at 2-month and 12-month follow-ups. At baseline, all participants scored >40 on the STAI state and trait, and half met DSM-IV criteria for generalized anxiety disorder. Participants were tapered off of antidepressant and antianxiety medications and received psychotherapy supplemented by two LSD-assisted psychedelic psychotherapy sessions spaced 2 to 3 weeks apart. Eight participants received a moderate dose of LSD (200 μg), and four participants received a low dose (20 μg), which was intended to act as an active placebo.
At the 2-month follow-up, mean trait anxiety did not significantly change in the high-dose LSD group compared with the placebo group, but mean state anxiety was significantly decreased in the high-dose LSD group compared with the low-dose (placebo) group. Comparing trait and state anxiety scores at baseline with those at the 2-month follow-up yielded effect sizes of 1.1 and 1.2, respectively. All four participants in the low-dose (placebo) group experienced increases in trait anxiety over time, and two of them also had increases in state anxiety (69).
Swiss researchers Schmid and Liechti et al. (69, 70) reported on short-term and long-term follow-ups after healthy volunteers (N=16) were given a single moderate dose of LSD (200 μg) as part of a randomized double-blind placebo-controlled crossover study with two experimental sessions. During the experimental sessions, participants rested in hospital beds and had the option of listening to music on headphones (no alternative entertainment was offered, and no specific guidance or therapy was provided). Participants were asked to complete the Persisting Effects Questionnaire (71), the Mysticism Scale, lifetime version, the Death Transcendence Scale, and the NEO Five-Factor Inventory at study screening and again 1 month and 12 months after their LSD session.
One and 12 months after LSD administration, the Persisting Effects Questionnaire showed significant increases in positive attitudes about life or self, positive mood changes, altruistic/positive social effects, positive behavioral changes, and well-being/life satisfaction that participants attributed to their LSD experience. The Mysticism Scale total score was increased, with significant increases in introvertive and extrovertive factor scores. The Death Transcendence Scale total score and mysticism subscale scores were also significantly increased at 1 and 12 months, and the NEO Five-Factor Inventory ratings of conscientiousness were significantly higher at 12 months. After 12 months, 10 of 14 participants (71%) rated their LSD experience “among the 10 most meaningful experiences” in their lives, and five participants rated it “among the five most spiritually meaningful experiences” in their lives. This study suggested positive effects of LSD on attitudes, mood, and behavior, which may have implications for the treatment of psychiatric disorders (70).
Neuroimaging researchers Mueller et al. (72) conducted a double-blind placebo-controlled randomized crossover study investigating the effects of LSD (100 μg) on amygdalar activity during processing of fearful stimuli in healthy subjects (N=20). At the point of anticipated peak effect, 2.5 hours after LSD ingestion, participants underwent fMRI scans while viewing images of faces depicting various degrees of fear, anger, happiness, or neutral expressions taken from the Ekman and Friesen series of Pictures of Facial Affect. All participants were crossed over to the other condition and scanned with the same protocol. Compared with placebo, LSD produced a significant decrease in left amygdalar reactivity to fearful stimuli and impaired recognition of fearful faces, but it did not affect recognition of neutral, happy, or angry faces. It was also noted that LSD administration was associated with decreased activity in the right medial prefrontal cortex compared with placebo. The investigators interpreted the results as indicating that LSD may modify the processing of biases toward negative stimuli, which play a role in depression and anxiety disorders. They also suggested that LSD might be useful for reducing perceptions of negative emotions, ameliorating social cognitive deficits, and facilitating therapeutic alliance.
Recently, there has been emerging interest in microdosing LSD, the practice of taking doses below the perceptual threshold at 3- to 5-day intervals in an effort to trigger a cellular response. Mainstream media publications and subjective reports have suggested that microdosing LSD at 10–20 μg might induce positive effects, such as promoting creativity and enhancing mood, without the full experience of psychedelic effects (73, 74). Currently, there is no available scientific evidence to support the practice of microdosing. In fact, LSD doses of 13 and 26 μg (N=20) have been shown to produce measurable subjective and physiological effects with minimal effects on cognition and creativity (75). It is worth highlighting that low-dose LSD (20 μg) received by the active placebo group in the Gasser et al. study mentioned (48) above was associated with worsening anxiety in people with comorbid medical illness. While this finding may be attributable to resampling over time or placebo nonexpectancy, it may also be ascribed to microdosing. The Beckley Foundation intends to study the neurobiological and clinical effects of LSD microdosing as a strategy for cognitive enhancement in an upcoming investigation, but specific details were unavailable at the time of writing.
While the current LSD clinical research is limited, there are several new clinical investigations on the horizon in Switzerland. These studies will examine LSD as a treatment for patients suffering from anxiety with or without a life-threatening disease (ClinicalTrials.gov identifier 03153579), LSD-assisted psychotherapy for patients with illness-related anxiety (ClinicalTrials.gov identifier 00920387), and LSD-induced altered states of consciousness (ClinicalTrials.gov identifier 03321136).
Ayahuasca
Ayahuasca is a decoction prepared through the combination of Banisteriopsis caapi and Psychotria viridis, two plants native to the Amazon basin (76–79). Ingested orally, the mixture is known to induce effects by actions of β-carboline alkaloids (namely, harmine derivatives) found in Banisteriopsis caapi and N,N-dimethyltryptamine (DMT) in Psychotria viridis (76, 78). The preparation works synergistically, in that β-carboline alkaloids inhibit monoamine oxidase A (MAO-A) (80), preventing peripheral degradation of DMT, a serotonin transporter and norepinephrine transporter inhibitor as well as releaser of 5-HT and agonist at 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT2B receptors (with affinity in descending order) (11, 80, 81). The environment in which the substance is ingested, the user’s expectations, and pharmacodynamic interactions of the decoction’s components are all thought to influence outcomes associated with ayahuasca ingestion (77).
Ayahuasca is associated with a wide range of subjective effects, including auditory and visual hallucinations, altered sensorium, altered spatial perceptions, and euphoria (77, 82), as well as mystical and noetic experiences (77). Psychotic episodes have been documented in association with ayahuasca intoxication, usually in persons with a personal or family history of mood disorders, psychotic disorders, or substance use disorders (36, 60, 83). These ayahuasca-induced psychoses are not generally prolonged. It has been documented that psychoses can be mitigated by screening individuals for preexisting psychiatric disorders, but conclusions regarding the relationship between ayahuasca and prolonged psychotic episodes are drawn from small sample sizes, therefore limiting generalizability (60, 84).
Ayahuasca consumption has been associated with traditional practices among indigenous groups of the northwestern Amazon region, but the past several decades have seen a growing international interest in its possible therapeutic effects (77, 85). The U.S. Supreme Court has sanctioned the use of ayahuasca for religious and spiritual practices (86) by groups such as União do Vegetal and Santo Daime, but clinical trials in the United States remain nonexistent because DMT, a component of ayahuasca, is a Schedule I controlled substance.
Clinical investigations with ayahuasca outside the United States have begun in the past several years. Brazilian researchers Osório et al. (87) conducted a small (N=6) open-label clinical trial investigating the efficacy of ayahuasca in patients with depression who had not responded to at least one trial of an antidepressant medication. All patients met criteria for major depressive disorder based on the Structured Clinical Interview for DSM-IV and were admitted to a psychiatric unit for 2 weeks for drug washout prior to ayahuasca administration. The HAM-D and Montgomery-Åsberg Rating Scale (MADRS) were administered 10 minutes before ayahuasca administration and again 40, 80, 140, and 180 minutes afterward, with follow-up assessments 1, 7, 14, and 21 days later. Participants drank a standard dose (2.2 mL/kg) of ayahuasca (containing 0.8 mg/mL DMT, 0.21 mg/mL harmine, and no harmaline as measured by gas chromatography/mass spectrometry) prepared by the Santo Daime community. All participants were discharged from the psychiatric unit 24 hours after ayahuasca administration. Mean HAM-D score was reduced by 62% 1 day after drug administration (p=0.01), with an even more pronounced reduction of 72% (p=0.01) 7 days after drug administration. The mean MADRS score was reduced by 82% at 7 days (p=0.009), with a sustained effect at 21 days. Investigators noted that the most significant antidepressant effects were observed for expressed sadness, pessimistic thinking, suicidal ideation, and difficulty concentrating.
Given the positive therapeutic signal of their pilot study, the same research team conducted a replication study with a larger sample (N=17) (88). The mean baseline HAM-D score for this group was 19.4, and the mean baseline MADRS score was 25.6. Symptoms, as measured by both scales, significantly decreased acutely, starting 80 minutes after drug administration. At 21-day follow-up, the mean HAM-D score was 7.56, representing a highly statistically significant mean change of −11.4 points (p<0.0005). Positive findings in the earlier study were replicated, but because neither study was randomized, double-blinded, or placebo-controlled, the results must be viewed as preliminary. Although vomiting occurred in about half the participants, participants generally described the ayahuasca session as a pleasant experience, and no serious adverse events were observed in either study.
Currently, the data are insufficient to support the use of ayahuasca in the clinical setting. The clinical research involving ayahuasca, which includes promising preliminary results for the treatment of depression, is limited by several factors, including lack of chemical analyses to confirm the exact ingredients in the ayahuasca drink used in the studies. A multitude of additional compounds have been described across indigenous preparations, including, among others, caffeine, nicotine, cocaine, and scopolamine (78). In assessing the aforementioned studies, one must be cognizant of the fact that ayahuasca was administered as a nonstandardized concoction. Randomized clinical trials using pharmacologically pure compounds are necessary to advance our knowledge about the therapeutic potential of ayahuasca.
3,4-Methylenedioxymethamphetamine (MDMA)
MDMA is a ring-substituted phenethylamine with structural similarities to amphetamine and mescaline. MDMA was synthesized by Merck & Co. in 1912 as a potential therapeutic agent to decrease clotting time and to prevent hemorrhaging (89). The compound did not prove efficacious for use as a hemostatic drug, but its psychotropic properties were recognized. Chemist Alexander Shulgin resynthesized MDMA in 1976, and the first published report characterizing the psychoactive effects of MDMA appeared in 1978 (90).
Despite the lack of systematic research into its efficacy and safety, some psychotherapists began using MDMA to improve the outcome of psychotherapy sessions with the goal of enhancing their patients’ insights and understanding of their psychological problems. MDMA was associated with feelings of emotional well-being and was described as “penicillin for the soul” (90).
These psychoactive properties encouraged MDMA’s use as a recreational drug. In the early to mid-1980s, MDMA was illicitly synthesized and distributed under the street name “Ecstasy” and became popular for facilitating an altered emotional state at dance parties called “raves.” Because of concerns about abuse liability and neurotoxicity, the DEA emergently classified MDMA as a temporary Schedule I substance in 1985, and then permanently classified it as such in 1988.
MDMA and other 3,4-methylenedioxy- substituted phenethylamines have been postulated to represent a new class of pharmacological agents, termed entactogens, with effects only partially overlapping those of psychostimulants and serotonergic hallucinogens (91–93). The effects of MDMA are believed to be mediated by a number of mechanisms, including monoamine release, serotonin and norepinephrine transporter reuptake inhibition, monoamine oxidase inhibition, partial agonism of serotonin receptors (5-HT2A, 5-HT1A, and 5-HT2C receptors), and increase in blood concentrations of oxytocin (94–98). To date, studies with healthy volunteers have confirmed that MDMA produces an easily controlled and reversible state of altered consciousness characterized by euphoria, empathy, well-being, insightfulness, extraversion, positive mood, gregariousness, feelings of authenticity, increased access to emotionally intense material, increased interpersonal trust, and compassion for oneself and others (96, 99–103). In the clinical population, anxiety has been reported in a majority of study participants, and painful emotions such as grief, fear, and rage are not uncommon in participants with a diagnosis of PTSD (104–106).
The first double-blind placebo-controlled MDMA study in the United States was conducted in 1994 (107) and was followed up by two additional phase 1 trials (91, 108). A single dose of MDMA causes transient but tolerable increases in heart rate, blood pressure, and body temperature in healthy subjects (109). Subsequent placebo-controlled studies in Europe confirmed these general safety and tolerability findings and demonstrated that the processing of contextual information is left intact after MDMA ingestion (110, 111).
A double-blind fMRI randomized clinical trial in healthy volunteers (N=9) (112) showed that during peak drug effect, MDMA decreased amygdalar reactivity in response to angry faces but not fearful faces and enhanced ventral striatum activity in response to happy faces from the Ekman and Friesen series of Pictures of Facial Affect. Volunteers receiving MDMA were also better able to verify positive facial expressions and found it more difficult to identify negative ones, compared with volunteers who received placebo. These findings of reduced response to threat and enhanced responses to reward provided important insights into MDMA’s effects on emotional information processing (112, 113).
In 2010, Mithoefer et al. (106) completed the first phase 2 randomized controlled trial investigating the efficacy of MDMA in treating chronic PTSD (N=23). The study enrolled adults with a DSM-IV-TR diagnosis of chronic PTSD. Inclusion criteria also included treatment-resistant symptoms (defined as a score ≥50 on the Clinician-Administered PTSD Scale [CAPS]) and previous failure of at least 3 months of an SSRI or selective serotonin-norepinephrine reuptake inhibitor in addition to 6 months of psychotherapy (the specific type of psychotherapy was not specified). Study participants received two experimental sessions of either manualized MDMA-assisted psychotherapy with active drug (125 mg orally with an optional supplemental dose of 62.5 mg) (N=12) or placebo (N=8). The manualized therapy was developed for the study based on principles of Holotropic Breathwork (114) and LSD psychotherapy (115), and it emphasized a nondirective supportive approach (104, 105).
The primary outcome measure was mean change in CAPS total scores measured at baseline, 4 days after each experimental session, and 2 months after the second experimental session. Baseline mean CAPS scores were 79.6 (SD=8.1) for the placebo group and 79.2 (SD=6.6) for the MDMA group (p=0.966). Three to 5 days after the first experimental session, the participants’ CAPS scores were 74.1 (SD=10.3) for the placebo group and 37.8 (SD=8.4) for the MDMA group (p=0.013). Three to 5 days after the second experimental session, CAPS scores were 66.8 (SD=8.0) for the placebo group and 29.3 (SD=6.5) for the MDMA group (p=0.002). Two months after the second experimental session, CAPS scores were 59.1 (SD=9.4) for the placebo group and 25.5 (SD=7.7) for the MDMA group (p=0.013). A significantly greater proportion of the MDMA group (10 of 12, 83.3%) than the placebo group (2 of 8, 25%) met criteria for categorical response (reduction ≥30% from baseline in CAPS score). All placebo-treated participants were offered the option of subsequent open-label crossover. Seven of eight chose to cross over, and all seven had a clinical response 4–6 weeks after two MDMA sessions. The mean change in CAPS score in this group (N=7) was −31.7 (SD=15) (p<0.05).
CAPS scores obtained 17–74 months after the two MDMA-assisted psychotherapy sessions were examined in a prospective long-term follow-up study (116). Sixteen participants completed all measures over 3.5 years (duration of follow-up: mean=45.4 months, SD=17.3). Among completers, no significant change was observed in mean CAPS scores from the point of exit from the trial (mean=24.6, SD=18.6) to the final follow-up assessment (mean=23.7, SD=22.8). On average, the group maintained statistically and clinically significant PTSD symptom relief, suggesting a potential for durable therapeutic effect from MDMA-assisted psychotherapy.
Most recently, Mithoefer et al. (105) completed a three-dose phase 2 double-blind randomized controlled trial investigating the efficacy and dose-response relationship of MDMA-assisted psychotherapy for the treatment of chronic PTSD in service personnel, firefighters, police officers, and veterans (N=26). All participants had a diagnosis of PTSD for at least 6 months, had a baseline CAPS total score ≥50, and had failed to respond to, or tolerate, previous pharmacotherapy or psychotherapy trials. Participants were required to taper and remain off of psychotropic medications during study participation. Participants were randomly assigned to receive MDMA at a low dose (30 mg; N=7), a moderate dose (75 mg; N=7), or a high dose (125 mg; N=12) in two blinded psychotherapy sessions spaced 1 month apart. In all of the MDMA sessions, participants had the option of receiving a supplemental dose of half of the initial dose 1.5–2 hours after the initial dose. During the MDMA sessions, two therapists, a male and female co-therapy team, performed manualized MDMA psychotherapy (the same nondirective supportive therapy approach used in the pilot study described above). The primary outcome measure was the mean change in CAPS score from baseline to 1 month after the second experimental MDMA session. The moderate- and high-dose groups had significantly greater reductions in PTSD symptom severity from baseline than the low-dose group (low-dose group: −11.4, SD=12.7; moderate-dose group: −58.3, SD=9.8; p=0.0005; high-dose group: −44.3, SD=28.7; p=0.004). No significant differences were found between the moderate- and high-dose groups (p=0.185). Remission was achieved in six of the seven participants (86%) in the moderate-dose group and seven of the 12 participants (58%) in the high-dose group, compared with two of the seven participants (29%) in the low-dose group. Additionally, compared with the low-dose group, more participants in the moderate- and high-dose groups met criteria for clinical response (defined as a reduction >30% from baseline in CAPS score): 29% in the low-dose group, 100% in the moderate-dose group, and 67% in the high-dose group.
In 2016, the FDA approved the MAPS investigators’ design for two phase 3 clinical trials investigating MDMA for the treatment of PTSD (117). In 2017, the FDA designated MDMA as a “breakthrough therapy” based on its use in assisting psychotherapy for the treatment of PTSD, giving it priority consideration in the regulatory process (118).
Additional trials investigating the efficacy of MDMA for social anxiety disorder in adults with autism spectrum disorder (ClinicalTrials.gov identifier 02008396) and for anxiety associated with a life-threatening illness (ClinicalTrials.gov identifier 02427568) have been completed but are outside the scope of this review.
Comparison of the Psychological Effects and Neurobiology of the Psychedelic Compounds
The classic psychedelics are subdivided into phenethylamines and tryptamines. The tryptamines include the synthetic ergoline LSD as well as the plant-derived indoleamines psilocybin and DMT. The phenethylamines include MDMA and mescaline. The tryptamines share their core structure with the neurotransmitter serotonin (5-HT) and modulate multiple targets, including 5-HT receptors, monoamine transporters, and trace-amine-associated receptors (11). The entactogen MDMA (a phenethylamine) is pharmacologically related to mescaline, amphetamine, and methamphetamine and acts as a serotonin agonist and releases both dopamine and norepinephrine (119). The dissociative anesthetic ketamine, which has psychedelic properties, is an NMDA receptor antagonist that has shown antidepressant efficacy across multiple clinical trials and efficacy in decreasing suicidal ideation (7, 8, 120). While not a classic psychedelic, ketamine can cause dose-dependent dissociation, alterations in the perception of sight and sound, derealization, “mystical-type” effects, paranoia, and transient confusion (121–124).
The molecular structures of MDMA, psilocybin, LSD, ayahuasca, and ketamine are depicted in Figure 1.
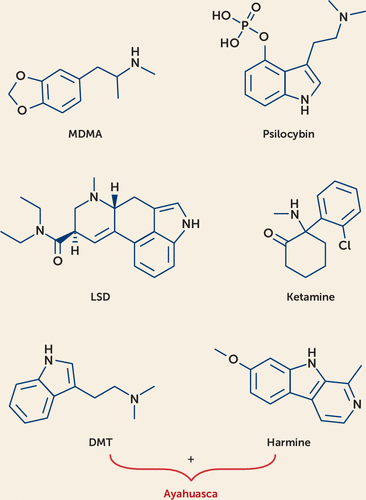
FIGURE 1. Molecular structure of psychedelic compoundsa
a MDMA is a phenethylamine, psilocybin and DMT are indoleamines, LSD is an ergoline, and ketamine is a cyclohexanone. Molecular structures are from PubChem (National Center for Biotechnology Information, U.S. National Library of Medicine) and rendered in the ChemDoodle software program.
While the structures and pharmacological profiles of these compounds are distinct, the psychological effects overlap. Examples of the cognitive, perceptual, emotional, and social relatedness effects of the psychedelics, as well as their primary pharmacological mechanisms of action, are provided in Table 1, organized by compound as classified by Garcia-Romeu et al (6).
| Effects | |||||||
|---|---|---|---|---|---|---|---|
| Class and Compound | Primary Mechanism of Action | Cognition | Perception | Negative Emotions | Positive Emotions | Social Relatedness | Other Compounds |
| Classic psychedelics | |||||||
| LSD, psilocybin, and ayahuasca (DMT) | Serotonin 5-HT2A and 5-HT2C receptor agonist | Increased cognitive flexibility (53), creative thinking (51), and insightfulness (52); distractibility and disorganized behavior (49, 51, 53, 62) | Changes in visual perception (51, 53); mystical experiences (6, 12, 34, 52); paranoia (53); hallucinations, depersonalization, derealization (51, 62, 69) | Anxiety (29, 51, 69); labile mood with anxiety (34) | Increase in well-being and life satisfaction (70); positive mood (60, 71) or blissful state (52, 53, 69) | Enhanced empathy (50); prosocial attitudes and behaviors (34); openness and trust (69) | Mescaline |
| Entactogens | |||||||
| MDMA | Serotonin 5-HT2A agonist; mixed serotonin, norepinephrine, and dopamine reuptake inhibition and release | Deficits in spatial memory (111); mild impairment on psychomotor tasks (92) | Changes in body perception, slight visual and auditory alterations, no hallucinations (92) | Distrust and hostility (103); anxiety (93, 101, 103, 105) | Increased trust and sense of a greater meaning in life (100); euphoria (92, 103) and well-being (92) | Increased connectedness toward others (91, 99, 102); increased empathy (96, 100, 103) | MDA, MDEA |
| Dissociative anesthetics | |||||||
| Ketamine | NMDA antagonist | Deficits in vigilance, verbal fluency, delayed recall, and tests of frontal lobe function (121) | Derealization, depersonalization (8, 120, 121, 124); illusions in all sensory domains and perceptual alterations (121) | Amotivation, emotional dulling, hostility (121); anxiety (121, 123) | Improved mood (7, 8, 120, 123) | Emotional withdrawal (121) | Dextromethorphan, phencyclidine (PCP), and nitrous oxide |
TABLE 1. Primary pharmacological mechanisms of action of the psychedelic compounds and their cognitive, perceptual, emotional, and social relatedness effectsa
As shown in the table, some of the psychological effects of the classic psychedelic compounds, MDMA, and ketamine are similar, whereas the primary underlying neurobiological processes are distinct. These divergent pharmacological profiles provide an opportunity to understand the neurobiology of the different psychological effects and the potential to use these different psychological effects in the treatment of psychiatric disorders.
Among the classic psychedelics, LSD has the greatest affinity for the 5-HT2A receptor (which is associated with psychoactive effects of the classic psychedelics), and only LSD binds with submicromolar affinity to the α1 adrenergic and has affinity for the D1–3 dopaminergic receptors (11). Visual perceptual changes in study subjects who have ingested LSD are associated with increased functional connectivity in the visual cortex, and the effects on consciousness (i.e., sense of self) are correlated with decreased connectivity between the parahippocampus and retrosplenial cortex within the default mode network (125). Comparing this profile to the simple tryptamine psilocybin, LSD is 10 to 100 times more potent than psilocybin at the 5-HT1A and 5-HT2 receptors and is more potent at α adrenergic and dopaminergic receptors, whereas psilocybin is a more potent inhibitor of the serotonin transporter (11).
The entactogen MDMA overlaps in chemical structure with methamphetamine and mescaline and has the biological effects of epinephrine, dopamine, and serotonin (126). Derealization may occur in individuals using MDMA, but unlike the classic psychedelic compounds, hallucinations are rare (119). This pharmacological profile leads to psychological effects that overlap with those that occur with the serotonergic hallucinogens, including positive emotions and euphoria. MDMA shares the autonomic and cardiovascular effects of a methamphetamine, such as increased energy, tachycardia, increased systolic and diastolic blood pressure, and tachypnea. While MDMA has been singled out as an entactogen for its ability to create a feeling of closeness or connection with others and increasing emotional empathy (127), classic psychedelics also have the ability to increase feelings of openness and trust (128).
The dissociative anesthetics (ketamine, phencyclidine, and nitrous oxide) also have psychological properties in common with the classic psychedelics (see Table 1). In the majority of recent depression studies, ketamine has been administered by intravenous infusion at a rate of 0.5 mg/kg over 40 minutes without adjunctive psychotherapy (7). Recently, a subgroup of clinicians have been administering ketamine via sublingual or intramuscular routes, at relatively higher doses than previously reported in the literature, to treat a wide array of psychiatric illnesses, including depression, anxiety, PTSD, and existential issues. This technique has been termed ketamine-assisted psychotherapy. Ketamine-assisted psychotherapy is not currently well defined, and there is limited objective evidence to support its use at this time (129).
Ketamine is an NMDA antagonist that causes an increased activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors and indirectly enhances dopaminergic (D2) and serotonergic (5-HT2) activity (130). Ketamine reduces the contribution of NMDA receptors to afferent information from internal and external sensory inputs and causes glutamatergic overactivity, and limbic cortical disinhibition indirectly enhances dopaminergic and serotonergic activity (130). While there has been debate on whether ketamine’s acute antidepressant effect requires normal function of the endogenous opioid system (131) or opioid system activation, through direct and/or indirect action at the mu-opioid receptors (132), ketamine’s dissociative effects are primarily attributed to its NMDA receptor antagonism (132).
Like ketamine, the classic psychedelics are also potent modulators of glutaminergic activity in prefrontal circuits (133). Vollenweider and Kometer (133) compared the classic psychedelic psilocybin with ketamine and showed that the drugs produced an overlapping set of psychological effects on the five-dimension Altered States of Consciousness Scale. Psilocybin showed dose-dependent (0.15–0.27 mg/kg by mouth) increases in the dimensions of visionary restructuralization (i.e., visual illusions and hallucinations) and oceanic boundlessness (described as a blissful state and experience of unity), whereas ketamine, in a dose-dependent manner (6–12 μg/kg per minute intravenously) influenced dimensions of anxious ego-disintegration (described as a sense of disembodiment and impaired self-control) as well as vivid imagery and changing meaning of percepts (i.e., visual restructuralization) and experience of unity (e.g., “oceanic boundlessness”). These researchers assert that there is a common mechanism of action that modulates glutaminergic transmission in the prefrontal-limbic circuit that leads to neuroplastic adaptations via the AMPA receptor, which are the basis for the antidepressant efficacy of both psilocybin and ketamine (133).
Despite knowledge about pharmacodynamic profiles of the psychedelics, there remains debate about how they alter consciousness and mood (11). Vollenweider suggests that psilocybin induces metabolic changes, including hyperfrontality (i.e., increased cerebral blood flow to the prefrontal cortex), and alters thalamocortical synaptic transmission through activation of 5-HT2A receptors in the cortico-striato-thalamo-cortical loop (133–135). Vollenweider and his colleagues propose that the disruption of thalamic gating disables the filtering of sensory and cognitive information, which leads to perceptual alterations during the psychedelic experience (35, 49, 134). Carhart-Harris and his colleagues suggest that psilocybin and other classic psychedelics are associated with hypofrontality (decreased blood flow to the prefrontal cortex) and decreased connectivity and neural activity in key regions of the default mode network immediately after drug administration (26). He proposes that these physiological alterations drive the mind toward a more primitive state of entropy or disorder that is suppressed during normal waking consciousness and allows for the disruption of stereotyped patterns of thought and behavior. As the mind becomes more flexible, the individual may challenge automatic thoughts and develop new perspectives (26).
The research-informed theories of Vollenweider and Carhart-Harris are not exclusive and raise new questions about the role of cerebral perfusion, thalamic gating, connectivity, and serotonin in psychiatric disorders. Furthermore, they demonstrate how the psychedelics’ unique and diverse pharmacological profiles, which only partially overlap, may be utilized to better inform our understanding of neuroscience.
Psychedelic-Assisted Psychotherapy
The number of studies using psychedelic-assisted psychotherapy has increased, leading to variable methodologies across studies. The two most widely utilized psychotherapy paradigms are psycholytic therapy and psychedelic therapy (16, 115). Psycholytic therapy, which evolved in Europe from the 1950s to the 1970s, took the form of psychoanalytically informed talk therapy with low to moderate doses of LSD (30–200 μg), which were administered over several sessions. The sessions were believed to offer greater access to the unconscious with the goal of facilitating a discharge of emotionally charged psychic tension (136). Psychedelic therapy, which developed simultaneously in the United States with the existential and humanistic schools of psychology, used preparatory therapy followed by one or several high doses of a psychedelic (>250 μg LSD) to create an “overwhelming and transcendent experience,” which was then processed in integrative therapy after the drug-facilitated session (136). The goal was to gain novel insights into the patient’s condition (136). The recent MDMA studies have used a hybrid of psycholytic therapy and psychedelic therapy, and the majority of recent psilocybin studies have implemented versions of psychedelic therapy, which has recently been closely aligned with transpersonal psychology (18, 23, 29, 104).
Psychedelic-assisted psychotherapy, which includes the spectrum of psycholytic and psychedelic therapy, typically employs three types of sessions: preparatory, medication (one to three sessions with moderate to high doses of a psychedelic), and integration sessions (137). During the preparatory sessions, the therapist or co-therapist team engages the patient to explore his or her life history and to help the patient understand his or her symptoms and intentions, with an emphasis on the potential for emotional and psychological growth. They also educate the patient about what to expect during the psychedelic session, and they work to develop a sufficient therapeutic alliance (3, 115). During the medication session, the patient is ideally accompanied by a male-female co-therapy team, which has been widely adopted in MDMA studies (104). The male-female co-therapist dyad maintains integrity and safety for the therapeutic relationship, which should not be underappreciated given the history of sexual abuse that occurred during psychotherapy with MDMA in the 1980s (138).
The psychedelic drug is administered in a comfortable room with a reclining chair or bed in an environment that is decorated and appointed so that it will feel familiar and not intimidating in the way a medical office or institutional laboratory might. After drug ingestion, the patient is encouraged to focus his or her attention inward and is offered the option of listening to music and wearing eye shades (3, 29, 104, 115). For the next 6–8 hours, the therapists listen empathically to the patient and maintain a nonthreatening, neutral therapeutic stance. The drug effects and the patient’s thought content drive the experience. The therapists’ goal is to facilitate a sense of safety, trust, and openness (3, 104). After the medication session, during the integration sessions, the therapists work with the patient to interpret the content of the psychedelic experience into meaningful long-term change through identifying insights or interpreting thoughts or ideas that arose during the psychedelic session (3, 115, 137).
Little is known about the intrapsychic processes and mechanisms by which psychedelic drugs are presumed to work in facilitating psychotherapy or general mental health. It is believed that the therapeutic effect is a result of the interaction between the drug and the mindset of the patient (together often referred to as “set”), the external conditions (often referred to as “setting”), and the therapist(s) (1, 104, 136). It is believed that a therapeutic set and setting make adverse outcomes less likely even when challenging and painful experiences arise. Furthermore, working through a painful experience is an important part of the therapeutic process, just as “peak mystical experience” can be, and should not be considered an adverse event.
Currently, it is unclear whether one psychotherapy approach is better than another. Psychedelics might be used to catalyze or augment widely accepted structured therapies, such as prolonged exposure therapy, cognitive processing therapy, and acceptance and commitment therapy, or less structured treatments, such as dynamic therapy and psychoanalysis. Furthermore, it is unclear whether it is the psychedelic drug itself, the psychedelic-assisted psychotherapy experience, or drug-facilitated enhancements in the therapeutic alliance that promote change (136). While a statistical association between mystical experiences and resolution of symptoms has been reported, the lack of qualitative analysis of various elements of individual psychotherapy sessions used in combination with psychedelic drug sessions limits external validity and, in turn, our understanding of the cognitive or emotional processes that lead to favorable outcomes.
The Potential for Abuse
All the drugs reviewed here, except ketamine, are currently classified by the DEA as Schedule I controlled substances under the Controlled Substances Act. As noted earlier, this classification was created by the U.S. Congress in 1970 to diminish the availability of drugs of abuse: “Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse” (139). Other drugs under this classification include heroin, marijuana, methaqualone, and mescaline. Psychedelic drugs have remained Schedule I drugs for almost 50 years. Ketamine is classified as a Schedule III substance, which is for drugs with an accepted medical use (e.g., anesthesia) and a potential for abuse.
In 2010, the United Kingdom’s Independent Scientific Committee on Drugs published a study that directly addressed the prevalence and severity of adverse effects of potential drugs of abuse on a nine-category matrix of harm (140). They derived scores estimating the magnitude of overall harm to users (and to others) for each drug and substance of abuse. At the top of the list was alcohol, with a harm score of 72, followed by heroin, with a score of 55, then crack cocaine, with a score of 54. Benzodiazepines and ketamine both had a harm score of 15, and methadone’s score was 13. Ecstasy, LSD, and psilocybin were at the bottom of the list with harm scores of 9, 7, and 5, respectively. This publication was extremely controversial, although not without support, and eventually led to the dismissal of the lead author, David Nutt, from Britain’s Advisory Council on the Misuse of Drugs. In response to this criticism, Nutt and his colleagues refined their methodology and used a multicriteria decision analysis to again evaluate the harmfulness of drugs, both to the individual and to society (140). The results were similar, with alcohol, heroin, and crack cocaine having the highest overall harm scores and Ecstasy, LSD, and psilocybin ranking at the bottom of the list. Given that the societal harm scores were influenced by data from economic costs, health records, police records, and an expert group approach, their generalizability is limited by availability of the analyzed substances in specific countries.
A National Institute on Drug Abuse (NIDA) “DrugFacts” brochure states that certain hallucinogens (e.g., PCP) are potentially addictive and can produce drug cravings and tolerance over time (141). However, hallucinogens are not associated with uncontrollable drug-seeking behavior (141) and animals cannot be trained to self-administer hallucinogens (142). Other hallucinogens (e.g., DMT in the form of ayahuasca tea) do not lead to addiction or tolerance (141). Medical administration of hallucinogens should include careful consideration of the appropriate dosage, patient screening, and appropriate preparation of the patient, including preparation and follow-up of psychedelic-assisted psychotherapy sessions in accordance with an approved procedure based on research evidence (143).
Another NIDA DrugFacts brochure acknowledges research evidence of the abuse potential of MDMA in animals, albeit to a lesser degree than cocaine (144). While MDMA self-administration models in animals suggest patterns of episodic use at irregular intervals, the observed potential for abuse seems to be less than that for amphetamine and methamphetamine (145). The prospective long-term follow-up study of individuals with PTSD who received MDMA (N=19; described above [116]) reported that no study participants developed a substance abuse problem (with any illicit drug) during the follow-up period of 7–17 months, suggesting that, at least in research settings, MDMA can be administered with minimal risk that patients will subsequently seek out and self-administer “street Ecstasy.” However, further evaluation of MDMA’s long-term risks is needed (116).
Researchers at Johns Hopkins University recently evaluated the abuse potential of medically administered psilocybin (143) and determined that, if approved as a medication, psilocybin would be appropriate for Schedule IV classification. Other substances currently classified as Schedule IV include benzodiazepines and hypnotics with a relatively low potential for abuse and dependence.
The available evidence supports a plan for further research into the abuse potential of psychedelic compounds, with consideration of both their therapeutic potential and their risk of abuse or misuse. Future research on psychedelic compounds should include measures of drug-seeking behavior over time, urine drug screens to monitor illicit drug use, and efforts to determine which patient populations may be vulnerable to developing new (or to experiencing relapse of preexisting) substance use disorders.
Recommendations for Future Research
With the increased interest in psychedelic research and the FDA’s fast-tracking of psychedelic compounds, this would be an appropriate time for the National Institutes of Health, in conjunction with the FDA and other funding agencies, such as MAPS, the Usona Institute, and the Heffter Research Institute, to conduct a series of international symposia on clinical trial methodology in psychedelic drug research. Sellers et al. (119) reviewed the challenges inherent in conducting psychedelic research, and their analysis could serve as a road map for these meetings. They describe multiple confounders and biases in psychedelic trials. They highlight the difficulty in blinding; the lack of data on the acute and chronic dose response (as the drugs can have very different psychological effects at different doses); patient biases and expectancy (including in studies that include patients with prior hallucinogenic use and do not account for that in the analyses); highly selected patient populations, which limits generalizability; and the exclusion of patients with known risk factors (e.g., personal or family history of psychosis), which limits the understanding of the true risks of the drugs in the routine clinical care of a psychiatric patient population.
Sellers et al. also express their concern that many of the studies’ dependent variables, such as the Hallucinogen Rating Scale and Altered States of Consciousness Scale, are incompletely characterized and do not have established predictive validity or utility. They assert that many of the commonly used scales in these studies are not validated patient report outcome measures and have not been shown to be surrogate markers of any therapeutic outcome measure. This is a fair criticism of many of the scales. Some scales, however, such as the MEQ-30, have been validated in experimental studies with controlled doses of psilocybin (32), although even the MEQ-30 was validated using a narrow range of drug doses and was restricted to one hallucinogenic compound. More rigorous analysis of the treatment assessment scales is needed in order to qualify them as patient report outcome measures in clinical trials.
Research will also be limited by the fact that there is not currently a rigorous definition of some of the clinical techniques used in these trials (e.g., psychedelic-assisted psychotherapy) and that expectations and the participants’ prior drug experiences are important variables in the response to psychedelic-assisted therapy (6). Future research should also focus on the pharmacodynamic and pharmacokinetic profiles of these agents, with close attention paid to the dose-response relationship and side effects.
Finally, more studies focusing on abuse potential are needed, particularly as the potential for abuse relates to more vulnerable populations. Such studies will be important in assessing the risk these drugs may pose in routine clinical use and could be instrumental in meeting FDA requirements for changing the classification of psychedelics (119).
Conclusions
The published scientific evidence, although somewhat limited (Table 2), supports continued investigation of psychedelic compounds for treating psychiatric disorders, but it does not yet support the use of any of these drugs for patient care by clinical practitioners outside the research setting.
| Compound and Study | Design | Diagnosis | N | Dose | Placebo or Control | Psychedelic Sessions | Primary Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| MDMA | ||||||||
| Mithoefer et al. (106) | Randomized double-blind crossover | PTSD | 23 | 125 mg, plus optional 62.5 mg | Lactose | 2 | CAPS | Significant reduction in PTSD symptom severity. The mean change in CAPS scores 2 months after the second experimental session was −53.7 for the MDMA group and −20.5 for the placebo group. |
| Mithoefer et al. (116) | Follow-up | PTSD | 19 | N/A | N/A | N/A | CAPS | Significant and sustained reduction in PTSD symptom severity at 74 months. |
| Mithoefer et al. (105) | Randomized double-blind dose-response crossover | PTSD | 26 | 30 mg, 75 mg, or 125 mg, plus optional 1/2 initial dose | 30 mg MDMA active control | 2 | CAPS | Significant reduction in PTSD symptom severity. The mean change in CAPS score 1 month after the second experimental session was −58.3 for the 75 mg group, −44.3 for the 125 mg group, and −11.4 for the 30 mg group. |
| Psilocybin | ||||||||
| Carhart-Harris et al. (23) | Open-label | Treatment-resistant depression | 12 | 10 mg, and 25 mg 2 weeks later | None | 2 | QIDS | Significant reduction in depressive symptoms. The mean change in QIDS score was −11.8 at 1 week and −9.2 at 3 months after the experimental session. |
| Grob et al. (28) | Randomized double-blind placebo | Cancer-related anxiety and depression | 12 | 0.2 mg/kg | Niacin | 1 | STAI, BDI | Sustained decrease in STAI scores for the entire 6-month follow-up, which reached significance at 1 and 3 months after treatment. The mean BDI score dropped by almost 30% after 1 month and reached significance at 6-month follow-up. |
| Griffiths et al. (18) | Randomized double-blind crossover | Cancer-related depression and anxiety | 51 | 22 or 30 mg/70 kg | Psilocybin, 1 or 3 mg/70 kg | 1 | HAM-A, HAM-D | At 6-month follow-up, the overall rate of clinical response was 78% on the HAM-D and 83% on the HAM-A; the overall rate of symptom remission was 65% on the HAM-D and 57% on the HAM-A. |
| Ross et al. (29) | Randomized double-blind crossover | Cancer-related anxiety and depression | 29 | 0.3 mg/kg | Niacin | 1 | HADS, STAI, BDI | At 6.5-month follow-up, after all participants had received psilocybin, 60%–80% of participants had clinically significant sustained reductions in depression or anxiety, sustained benefits in existential distress and quality of life, and improved attitudes toward death. |
| Johnson et al. (30) | Open-label | Tobacco use disorder | 15 | 20 mg/70 kg or 30 mg/70 kg | None | 2–3 | Laboratory-verified abstinence | At 6-month follow-up, 80% of participants were laboratory-verified as abstinent. |
| Johnson et al. (148) | Follow-up | Tobacco use disorder | 15, 12 | N/A | N/A | 0 | Laboratory-verified abstinence | At 1-year follow-up, 10/15 (67%) participants were laboratory-verified as abstinent, and at 2.5-year follow-up, 9/12 (75%) participants were laboratory-verified as abstinent. |
| Bogenschutz et al. (31) | Open-label | Alcohol use disorder | 10 | 0.3 mg/kg, and 0.4 mg/kg 4 weeks later | None | 2 | AASE | Abstinence measured using the AASE increased significantly after psilocybin administration. Gains were largely maintained at 36-week follow-up, and the intensity of the first psilocybin session predicted changes in drinking in weeks 5–8. |
| LSD | ||||||||
| Gasser et al. (48) | Randomized double-blind crossover | Anxiety associated with life-threatening disease | 12 | 200 μg | LSD 20 μg | 2 | STAI | Significant reduction in STAI state score at 2-month follow-up. The mean change in STAI state score was −11.6, and this reduction in state anxiety was sustained at 12-month follow-up. |
| Schmid et al. (70) | Randomized double-blind crossover | Healthy subjects | 16 | 200 μg | Not specified | 1 | PEQ, MS | A moderate dose of LSD induced a subjectively meaningful experience with lasting positive effects: positive attitudes about life and/or self, positive mood changes, altruistic/positive social effects, and positive changes in well-being/life satisfaction. |
| Ayahuasca | ||||||||
| Osório et al. (87) | Open-label | Major depression with failure of one antidepressant | 6 | 2.2 mL/kg (0.8 mg/mL DMT, 0.21 mg/mL harmine) | None | 1 | HAM-D, MADRS | HAM-D scores were reduced by 62% 1 day after drug administration and by 72% at 7 days. MADRS scores were reduced by 82% at 7 days, with sustained effects at 21 days. |
| Sanches et al. (88) | Open-label | Major depression with failure of one antidepressant | 17 | 2.2 mL/kg (0.8 mg/mL DMT, 0.21 mg/mL harmine) | None | 1 | HAM-D, MADRS | Significant reductions in HAM-D and MADRS scores 1, 7, 14, and 21 days after drug administration. The mean change in HAM-D score 21 days after drug administration was −11.4. |
TABLE 2. Recent psychedelic clinical trialsa
There is currently a paucity of novel pharmacological mechanisms in the treatment of many psychiatric disorders, and some commentators have called for a “disruptive pharmacology” to investigate new treatments with novel mechanisms using drugs that have previously been restricted by the FDA, including psychedelic agents (146). While we support research on the medical applications of these compounds, we are realistic about the need for more clinical trials using rigorous and validated methodology in controlled settings to address concerns about the potential for substance abuse and significant medical and psychiatric sequelae in vulnerable populations. Research has been hampered by the fact that there is not a rigorous definition of psychedelic-assisted psychotherapy and the fact that the expectations and personal experiences of the study subjects are important variables in the response to psychedelic-assisted therapy (6). These variables can be difficult to account for in a clinical trial, but they should be a part of the future research agenda.
The FDA’s breakthrough designation of MDMA for the treatment of PTSD and psilocybin for the treatment of depression reflects the drugs’ potential to treat resistant psychiatric disorders. Recent trials have also shown that psilocybin may be effective for treating anxiety disorders, substance use disorders, and emotional suffering associated with facing the end of one’s life. Clinical research data with psilocybin is particularly interesting, as it shows that several sessions of psilocybin-assisted psychotherapy can lead to antidepressant effects that persist for weeks to months. This modality of treatment might provide a therapeutic advantage over current standards of care, such as transcranial magnetic stimulation, electroconvulsive therapy, or ketamine infusion therapy, each of which requires multiple visits per week to achieve antidepressant effect and often requires multiple visits per month to sustain remission (8). While LSD and ayahuasca currently have less scientific evidence to support their use in the clinical setting, the data available at the time of this review clearly support future controlled trials to evaluate their efficacy and safety.
Of some concern is that the use of these compounds appears to be outpacing evidence-based research. The practice of microdosing LSD or psilocybin—taking low doses of psychedelics below the perceptual threshold at regular intervals (approximately once every 3–5 days) to enhance creativity, productivity, mood, or the therapeutic alliance—has become increasingly popular in recent years (4, 74, 147). The growing popularity of microdosing in the general (non–psychiatrically ill) population raises additional questions about psychedelics that might be encountered in clinical practice.
In his 1979 autobiography entitled LSD: My Problem Child (1), Albert Hofmann described his concerns about the potential overenthusiasm for LSD among the public: “This joy at having fathered LSD was tarnished after more than ten years of uninterrupted scientific research and medicinal use when LSD was swept up in the huge wave of an inebriant mania that began to spread over the Western world, above all the United States, at the end of the 1950s.” At the time, the recreational use of LSD was increasing and had societal consequences that led to the restriction of these potentially promising psychedelic compounds from further research as treatments for psychiatric disorders. Psychedelic drugs acquired a negative reputation when they were available to the public through underground channels, without medical indication or regulation. The nascent body of data reviewed here should be leveraged to inform next-step research that asks meaningful questions about the therapeutic potential and the abuse potential of psychedelic-assisted psychotherapy in standardized clinical trials, as well as about the potential therapeutic and adverse effects of psychedelic drugs used as monotherapy.
This area of research, involving drugs with pharmacological actions different from those associated with current antidepressant medications, has the potential to advance our understanding of the neurobiological processes and therapeutic outcomes achieved by patients with a variety of mood and anxiety spectrum disorders. As we have pointed out, there are significant limitations in the study methodologies, and the available evidence base includes the use of nonrepresentative samples (relative to the general population) through self-selection of individuals into clinical trials who may be biased toward expecting beneficial effects, including mystical experience related to ingestion of psychedelics; crossover study designs rather than parallel-group designs, precluding between-group comparisons for long-term follow-up outcomes with participants who received placebo; inconsistencies in medication dosing between studies; and blinding methods compromised by the pronounced effects of the psychedelic interventions. These limitations notwithstanding, the preliminary data on the therapeutic potential of psychedelic drugs support further research.
1 : LSD, My Problem Child: Reflections on Sacred Drugs, Mysticism, and Science. Santa Cruz, Calif, Multidisciplinary Association for Psychedelic Studies (MAPS), 2009Google Scholar
2 : Acid Dreams: The Complete History of LSD: The CIA, The Sixties, and Beyond. New York, Grove Press, 1992Google Scholar
3 Pollan M: My adventure with trip doctors. New York Times Magazine, May 15, 2018Google Scholar
4 Williams J: A strait-laced writer explores psychedelics, and leaves the door of perception ajar. New York Times, May 14, 2018Google Scholar
5 McClelland M: The psychedelic miracle: how some doctors are risking everything to unleash the healing power of MDMA, ayahuasca, and other hallucinogens. Rolling Stone, March 9, 2017Google Scholar
6 : Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol 2016; 24:229–268Crossref, Medline, Google Scholar
7 : A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 2017; 74:399–405Crossref, Medline, Google Scholar
8 : Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172:950–966Link, Google Scholar
9 : Plants of The Gods: Their Sacred, Healing, and Hallucinogenic Powers. Rochester, Vt, Healing Arts Press, 1992Google Scholar
10 : The Wondrous Mushroom: Mycolatry in Meso-America. New York, McGraw-Hill, 1980Google Scholar
11 : Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 2016; 26:1327–1337Crossref, Medline, Google Scholar
12 : Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther 2019; 197:83–102Crossref, Medline, Google Scholar
13 : Metabolism of psilocybin and psilocin: clinical and forensic toxicological relevance. Drug Metab Rev 2017; 49:84–91Crossref, Medline, Google Scholar
14 : The pharmacology of psilocybin. Addict Biol 2002; 7:357–364Crossref, Medline, Google Scholar
15 : High dose psilocybin is associated with positive subjective effects in healthy volunteers. J Psychopharmacol 2018; 32:770–778Crossref, Medline, Google Scholar
16 : Therapeutic Applications of Classic Hallucinogens, in Behavioral Neurobiology of Psychedelic Drugs Current Topics in Behavioral Neurosciences. Edited by Halberstadt AL, Vollenweider FX, Nichols DE. Berlin, Heidelberg, Springer, 2016Crossref, Google Scholar
17 : Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend 2012; 123:132–140Crossref, Medline, Google Scholar
18 : Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 2016; 30:1181–1197Crossref, Medline, Google Scholar
19 : Pahnke’s “Good Friday Experiment”: a long-term follow-up and methodological critique. Journal of Transpersonal Psychology 1991; 23:1–28Google Scholar
20 : Prisoner behavior change and experimental mysticism: two classic studies from the Harvard Psilocybin Project, in Sacred Mushroom of Visions: Teonanácatl: A Sourcebook on the Psilocybin Mushroom. Edited by Metzner R, Darling DC. Rochester, Vt, Park Street Press, 2006Google Scholar
21 : Timothy Leary: A Biography. New York, Harcourt, 2006Google Scholar
22 : Dr. Leary’s Concord Prison Experiment: a 34-year follow-up study. J Psychoactive Drugs 1998; 30:419–426Crossref, Medline, Google Scholar
23 : Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 2016; 3:619–627Crossref, Medline, Google Scholar
24 : Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry 2015; 20:311–319Crossref, Medline, Google Scholar
25 : Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA 2012; 109:2138–2143Crossref, Medline, Google Scholar
26 : The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 2014; 8:20Crossref, Medline, Google Scholar
27 : Evidence-based treatment of depression in patients with cancer. J Clin Oncol 2012; 30:1187–1196Crossref, Medline, Google Scholar
28 : Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 2011; 68:71–78Crossref, Medline, Google Scholar
29 : Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016; 30:1165–1180Crossref, Medline, Google Scholar
30 : Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 2014; 28:983–992Crossref, Medline, Google Scholar
31 : Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015; 29:289–299Crossref, Medline, Google Scholar
32 : Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol 2015; 29:1182–1190Crossref, Medline, Google Scholar
33 : Factor analysis of the Mystical Experience Questionnaire: a study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig 2012; 51:721–737Crossref, Medline, Google Scholar
34 : Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol 2018; 32:49–69Crossref, Medline, Google Scholar
35 : Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci 2008; 29:445–453Crossref, Medline, Google Scholar
36 : Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clin Neuropharmacol 2012; 35:235–243Crossref, Medline, Google Scholar
37 : Acute, subacute, and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol 2011; 25:1434–1452Crossref, Medline, Google Scholar
38 : Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol 2016; 30:1268–1278Crossref, Medline, Google Scholar
39 Hartman S: Psilocybin could be legal for therapy by 2021. Rolling Stone, November 18, 2018. https://www.rollingstone.com/culture/culture-news/psilocybin-legal-therapy-mdma-753946/Google Scholar
40 COMPASS: Our clinical trials: treatment-resistant depression study. August 18, 2018. https://compasspathways.com/research-clinical-trials/Google Scholar
41 Brodwin E: A startup backed by Peter Thiel has churned out 20,000 doses of magic mushrooms, and is making more. Business Insider, July 23, 2018Google Scholar
42 Kunzman K: FDA approves landmark psilocybin trial for treatment-resistant depression. MD Magazine (mdmag.com), August 24, 2018. https://www.mdmag.com/medical-news/fda-approves-landmark-psilocybin-trial-for-treatmentresistant-depressionGoogle Scholar
43 Usona Institute: Usona Research. 2018 http://www.usonainstitute.org/research/Google Scholar
44 : Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 2006; 67:1735–1740Crossref, Medline, Google Scholar
45 : Psychedelic drugs and mystical experience. Int Psychiatry Clin 1969; 5:149–162Medline, Google Scholar
46 : Psychedelics. Pharmacol Rev 2016; 68:264–355Crossref, Medline, Google Scholar
47 Abramson HA: The Use of LSD in Psychotherapy, in Transactions of a Conference on d-Lysergic Acid Diethylamine (LSD-25). New York: Josiah Macy Jr. Foundation Publications, 1959Google Scholar
48 : Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 2014; 202:513–520Crossref, Medline, Google Scholar
49 : LSD increases primary process thinking via serotonin 2A receptor activation. Front Pharmacol 2017; 8:814Crossref, Medline, Google Scholar
50 : LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology 2016; 41:2638–2646Crossref, Medline, Google Scholar
51 : Dreams and psychedelics: neurophenomenological comparison and therapeutic implications. Curr Neuropharmacol 2017; 15:1032–1042Crossref, Medline, Google Scholar
52 : Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology (Berl) 2017; 234:1499–1510Crossref, Medline, Google Scholar
53 : The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 2016; 46:1379–1390Crossref, Medline, Google Scholar
54 : The role of 5-HT2A, 5-HT 2C, and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 2015; 232:275–284Crossref, Medline, Google Scholar
55 : Tolerance to LSD and DOB induced shaking behaviour: differential adaptations of frontocortical 5-HT(2A) and glutamate receptor binding sites. Behav Brain Res 2015; 281:62–68Crossref, Medline, Google Scholar
56 : Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 2015; 277:99–120Crossref, Medline, Google Scholar
57 : The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol 2017; 27:451–457Crossref, Medline, Google Scholar
58 : Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand 2017; 136:648–657Crossref, Medline, Google Scholar
59 : The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther 2008; 14:295–314Crossref, Medline, Google Scholar
60 : The current state of research on ayahuasca: a systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J Psychopharmacol 2016; 30:1230–1247Crossref, Medline, Google Scholar
61 : Experimental schizophrenia-like symptoms. Am J Psychiatry 1952; 108:572–578Link, Google Scholar
62 : d-Lysergic acid diethylamide (LSD) as a model of psychosis: mechanism of action and pharmacology. Int J Mol Sci 2016; 17:E1953Crossref, Medline, Google Scholar
63 : Psychedelics not linked to mental health problems or suicidal behavior: a population study. J Psychopharmacol 2015; 29:270–279Crossref, Medline, Google Scholar
64 : A new adjunct to the treatment of alcoholism: the hallucinogenic drugs. Q J Stud Alcohol 1958; 19:406–417Crossref, Medline, Google Scholar
65 : The Human Encounter With Death. New York, EP Dutton, 1977Google Scholar
66 : A review of lysergic acid diethylamide (LSD) in the treatment of addictions: historical perspectives and future prospects. Curr Drug Abuse Rev 2014; 7:146–156Crossref, Medline, Google Scholar
67 : Efficacy and enlightenment: LSD psychotherapy and the Drug Amendments of 1962. J Hist Med Allied Sci 2014; 69:221–250Crossref, Medline, Google Scholar
68 : Dark classics in chemical neuroscience: lysergic acid diethylamide (LSD). ACS Chem Neurosci 2018; 9:2331–2343Crossref, Medline, Google Scholar
69 : Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 2015; 78:544–553Crossref, Medline, Google Scholar
70 : Long-lasting subjective effects of LSD in normal subjects. Psychopharmacology (Berl) 2018; 235:535–545Crossref, Medline, Google Scholar
71 : Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006; 187:268–283Crossref, Medline, Google Scholar
72 : Acute effects of LSD on amygdala activity during processing of fearful stimuli in healthy subjects. Transl Psychiatry 2017; 7:e1084Crossref, Medline, Google Scholar
73 Kuchler H: How Silicon Valley rediscovered LSD. Financial Times, August 10, 2017Google Scholar
74 : The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys. Rochester, Vermont, Park Street Press, 2011Google Scholar
75 : Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biol Psychiatry 2019; 86:792–800Crossref, Medline, Google Scholar
76 : Subjective effects and tolerability of the South American psychoactive beverage ayahuasca in healthy volunteers. Psychopharmacology (Berl) 2001; 154:85–95Crossref, Medline, Google Scholar
77 : The Antipodes of the Mind: Charting the Phenomenology of the Ayahuasca Experience. New York, Oxford University Press, 2010Google Scholar
78 : New World tryptamine hallucinogens and the neuroscience of ayahuasca, in Behavioral Neurobiology of Psychedelic Drugs. Edited by Halberstadt AL, Vollenweider FX, Nichols DE. Berlin, Heidelberg, Springer, 2018, pp 283–311Google Scholar
79 : The healing vine: ayahuascaas medicine in the 21st century, in Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments, vol 1. Edited by Winkelman MJ, Roberts TB. Westport, Conn, Praeger/Greenwood, 2007Google Scholar
80 : Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs 1999; 31:171–177Crossref, Medline, Google Scholar
81 : Psychedelics and the human receptorome. PLoS One 2010; 5:e9019Crossref, Medline, Google Scholar
82 : Serotonergic hallucinogen-induced visual perceptual alterations. Curr Top Behav Neurosci 2018; 36:257–282Crossref, Medline, Google Scholar
83 : Ayahuasca, dimethyltryptamine, and psychosis: a systematic review of human studies. Ther Adv Psychopharmacol 2017; 7:141–157Crossref, Medline, Google Scholar
84 : Hallucinogen persisting perception disorder and risk of suicide. J Pharm Pract 2016; 29:431–434Crossref, Medline, Google Scholar
85 : Ayahuasca Religions: A Comprehensive Bibliography and Critical Essays. Santa Cruz, Calif, Multidisciplinary Association for Psychedelic Studies (MAPS), 2009Google Scholar
86 : Report on psychoactive drug use among adolescents using ayahuasca within a religious context. J Psychoactive Drugs 2005; 37:141–144Crossref, Medline, Google Scholar
87 : Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Br J Psychiatry 2015; 37:13–20Crossref, Google Scholar
88 : Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol 2016; 36:77–81Crossref, Medline, Google Scholar
89 : MDMA-assisted therapy: a new treatment model for social anxiety in autistic adults. Prog Neuropsychopharmacol Biol Psychiatry 2016; 64:237–249Crossref, Medline, Google Scholar
90 : Ecstasy: The Complete Guide. Edited by Holland J. Rochester, Vermont, Park Street Press, 2001Google Scholar
91 : Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002; 162:396–405Crossref, Medline, Google Scholar
92 : Human pharmacology of 3,4-methylenedioxymethamphetamine (“Ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol 2000; 20:455–466Crossref, Medline, Google Scholar
93 : MDMA effects consistent across laboratories. Psychopharmacology (Berl) 2014; 231:3899–3905Crossref, Medline, Google Scholar
94 : Increased oxytocin concentrations and prosocial feelings in humans after Ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci 2009; 4:359–366Crossref, Medline, Google Scholar
95 : Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA). Psychopharmacology (Berl) 2017; 234:2883–2895Crossref, Medline, Google Scholar
96 : Multifaceted empathy of healthy volunteers after single doses of MDMA: a pooled sample of placebo-controlled studies. J Psychopharmacol 2017; 31:589–598Crossref, Medline, Google Scholar
97 . Pharmacology of MDMA- and Amphetamine-Like New Psychoactive Substances. Berlin, Heidelberg, Springer, pp 1-22Google Scholar
98 : 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 2003; 63:1223–1229Crossref, Medline, Google Scholar
99 : The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): controlled studies in humans and laboratory animals. Neurosci Biobehav Rev 2015; 57:433–446Crossref, Medline, Google Scholar
100 : Positive psychology in the investigation of psychedelics and entactogens: a critical review. Neuropharmacology 2018; 142:179–199Crossref, Medline, Google Scholar
101 : The potential dangers of using MDMA for psychotherapy. J Psychoactive Drugs 2014; 46:37–43Crossref, Medline, Google Scholar
102 : Effects of 3,4-methylenedioxymethamphetamine on socioemotional feelings, authenticity, and autobiographical disclosure in healthy volunteers in a controlled setting. J Psychopharmacol 2016; 30:378–387Crossref, Medline, Google Scholar
103 : Effects of Ecstasy on cooperative behaviour and perception of trustworthiness: a naturalistic study. J Psychopharmacol 2014; 28:1001–1008Crossref, Medline, Google Scholar
104 : A Manual for MDMA-Assisted Psychotherapy in the Treatment of Posttraumatic Stress Disorder. Santa Cruz, Calif, Multidisciplinary Association for Psychedelic Studies (MAPS), 2013Google Scholar
105 : 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018; 5:486–497Crossref, Medline, Google Scholar
106 : The safety and efficacy of +/−3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 2011; 25:439–452Crossref, Medline, Google Scholar
107 : Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res 1996; 73:103–107Crossref, Medline, Google Scholar
108 : The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend 2001; 65:97–101Crossref, Medline, Google Scholar
109 : Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 2012; 224:363–376Crossref, Medline, Google Scholar
110 : Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int J Neuropsychopharmacol 2014; 17:371–381Crossref, Medline, Google Scholar
111 : Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 2007; 189:557–563Crossref, Medline, Google Scholar
112 : Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009; 207:73–83Crossref, Medline, Google Scholar
113 : MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology (Berl) 2014; 231:4219–4229Crossref, Medline, Google Scholar
114 : Measure of significance of holotropic breathwork in the development of self-awareness. J Altern Complement Med 2015; 21:796–803Crossref, Medline, Google Scholar
115 : LSD Psychotherapy, 4th ed. Ben Lomond, Calif, Multidisciplinary Association for Psychedelic Studies (MAPS), 2008Google Scholar
116 : Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol 2013; 27:28–39Crossref, Medline, Google Scholar
117 Multidisciplinary Association for Psychedelic Studies (MAPS): FDA agrees to phase 3 trials of MDMA-assisted psychotherapy for PTSD. November 29, 2016. https://maps.org/research/mdma/ptsd/6480-fda-agrees-to-phase-3-trials-of-mdma-assisted-psychotherapy-for-ptsdGoogle Scholar
118 Multidisciplinary Association for Psychedelic Studies (MAPS): A phase 3 program of MDMA-assisted psychotherapy for the treatment of severe posttraumatic stress disorder. 2017. https://maps.org/research/mdma/ptsd/phase3Google Scholar
119 : Studies with psychedelic drugs in human volunteers. Neuropharmacology 2018; 142:116–134Crossref, Medline, Google Scholar
120 : Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 2018; 175:327–335Link, Google Scholar
121 : Subanesthetic effects of the noncompetitive NMDA antagonist ketamine in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
122 : Therapeutic infusions of ketamine: do the psychoactive effects matter? Drug Alcohol Depend 2014; 136:153–157Crossref, Medline, Google Scholar
123 : The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 2014; 12:444–461Crossref, Medline, Google Scholar
124 : Novel psychotherapeutics: a cautiously optimistic focus on hallucinogens. Expert Rev Clin Pharmacol 2018; 11:1–3Crossref, Medline, Google Scholar
125 : Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA 2016; 113:4853–4858Crossref, Medline, Google Scholar
126 : The pharmacology and toxicology of “Ecstasy” (MDMA) and related drugs. CMAJ 2001; 165:917–928Medline, Google Scholar
127 : Progress and promise for the MDMA drug development program. Psychopharmacology (Berl) 2018; 235:561–571Crossref, Medline, Google Scholar
128 : Modern clinical research on LSD. Neuropsychopharmacology 2017; 42:2114–2127Crossref, Medline, Google Scholar
129 : Ketamine assisted psychotherapy (KAP): patient demographics, clinical data, and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs 2019; 51:189–198Crossref, Medline, Google Scholar
130 : Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology 2018; 142:30–40Crossref, Medline, Google Scholar
131 : Caution against overinterpreting opiate receptor stimulation as mediating antidepressant effects of ketamine (letter). Am J Psychiatry 2019; 176:249Link, Google Scholar
132 : Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 2018; 175:1205–1215Link, Google Scholar
133 : The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 2010; 11:642–651Crossref, Medline, Google Scholar
134 : Phenomenology, structure, and dynamic of psychedelic states, in Behavioral Neurobiology of Psychedelic Drugs. Edited by Halberstadt AL, Vollenweider FX, Nichols DE. Berlin, Heidelberg, Springer Berlin Heidelberg, 2018, pp 221–256Google Scholar
135 : The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 2007; 32:1876–1887Crossref, Medline, Google Scholar
136 : Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions. Int Rev Psychiatry 2018; 30:291–316Crossref, Medline, Google Scholar
137 . The influence of therapists’ first-hand experience with psychedelics on psychedelic-assisted psychotherapy research and therapist training. Journal of Psychedelic Studies. 2018.Crossref, Google Scholar
138 : The early use of MDMA (“Ecstasy”) in psychotherapy (1977–1985). Drug Sci Policy Law 2018; 4 (https://doi.org/10.1177/2050324518767442)Crossref, Google Scholar
139 US Department of Justice, Drug Enforcement Administration, Diversion Control Division: Controlled Substance Schedules, List of Controlled Substances. 2018. http://www.deadiversion.usdoj.gov/schedules/Google Scholar
140 : Drug harms in the UK: a multicriteria decision analysis. Lancet 2010; 376:1558–1565Crossref, Medline, Google Scholar
141 : Hallucinogens. April 22, 2019. https://www.drugabuse.gov/publications/drugfacts/hallucinogensGoogle Scholar
142 : Hallucinogens. Pharmacol Ther 2004; 101:131–181Crossref, Medline, Google Scholar
143 : The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 2018; 142:143–166Crossref, Medline, Google Scholar
144 National Institute on Drug Abuse: MDMA (Esctasy/Molly). June 6, 2018. https://www.drugabuse.gov/publications/drugfacts/mdma-ecstasymollyGoogle Scholar
145 : Predicting the abuse liability of entactogen-class, new and emerging psychoactive substances via preclinical models of drug self-administration, in Neuropharmacology of New Psychoactive Substances (NPS): The Science Behind the Headlines. Edited by Baumann MH, Glennon RA, Wiley JL. Cham, Switzerland, Springer International Publishing, 2017, pp 145–164Google Scholar
146 : Disruptive psychopharmacology. JAMA Psychiatry (Epub ahead of print, July 36, 2019)Google Scholar
147 Gregoire C: Everything you wanted to know about microdosing (but were afraid to ask): a leading psychedelic researcher explains what’s really behind the trend. Huffington Post, January 13, 2016. https://www.huffpost.com/entry/psychedelic-microdosing-research_n_569525afe4b09dbb4bac9db8Google Scholar
148 : Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 2017; 43:55–60Crossref, Medline, Google Scholar



