No Alterations of Brain Structural Asymmetry in Major Depressive Disorder: An ENIGMA Consortium Analysis
Abstract
Objective:
Asymmetry is a subtle but pervasive aspect of the human brain, and it may be altered in several psychiatric conditions. MRI studies have shown subtle differences of brain anatomy between people with major depressive disorder and healthy control subjects, but few studies have specifically examined brain anatomical asymmetry in relation to this disorder, and results from those studies have remained inconclusive. At the functional level, some electroencephalography studies have indicated left fronto-cortical hypoactivity and right parietal hypoactivity in depressive disorders, so aspects of lateralized anatomy may also be affected. The authors used pooled individual-level data from data sets collected around the world to investigate differences in laterality in measures of cortical thickness, cortical surface area, and subcortical volume between individuals with major depression and healthy control subjects.
Methods:
The authors investigated differences in the laterality of thickness and surface area measures of 34 cerebral cortical regions in 2,256 individuals with major depression and 3,504 control subjects from 31 separate data sets, and they investigated volume asymmetries of eight subcortical structures in 2,540 individuals with major depression and 4,230 control subjects from 32 data sets. T1-weighted MRI data were processed with a single protocol using FreeSurfer and the Desikan-Killiany atlas. The large sample size provided 80% power to detect effects of the order of Cohen’s d=0.1.
Results:
The largest effect size (Cohen’s d) of major depression diagnosis was 0.085 for the thickness asymmetry of the superior temporal cortex, which was not significant after adjustment for multiple testing. Asymmetry measures were not significantly associated with medication use, acute compared with remitted status, first episode compared with recurrent status, or age at onset.
Conclusions:
Altered brain macro-anatomical asymmetry may be of little relevance to major depression etiology in most cases.
Major depressive disorder is a common and debilitating psychiatric disorder characterized by a persistent feeling of sadness or a lack of interest in outside stimuli (DSM-5) (1). The disorder is often characterized by recurrent episodes and can become a chronic condition (2). Worldwide, lifetime prevalence varies considerably. A World Health Organization World Mental Health survey across 18 countries (3) found average lifetime prevalences ranging from 6.6% in Japan to 21.0% in France, with an average lifetime prevalence of 14.6% across high-income countries.
Much of the neurobiology of major depression is unknown, but subtle alterations of brain structure may be involved, and various MRI-based studies have observed regional brain differences between individuals with major depression and healthy control subjects. A recent review of the literature by Zhang et al. (4) described various possible structural alterations in the brains of individuals with major depression, such as case-control differences in the thickness of the medial orbitofrontal cortex and inferior parietal gyrus. However, it was also noted that the results of structural MRI studies in major depression have often been inconsistent (4). This inconsistency is likely due to the use of small study sample sizes in relation to subtle effects, as well as heterogeneity among studies in terms of clinical characteristics and methodology; for example, hardware and software differences between scanners and distinct data processing pipelines can contribute to heterogeneity (5).
In the Enhancing Neuro-Imaging Genetics Through Meta-Analysis (ENIGMA) consortium (http://enigma.ini.usc.edu), researchers from around the world collaborate to analyze many separate data sets jointly and to reduce some of the technical heterogeneity by using harmonized MRI preprocessing protocols. Two recent studies by the ENIGMA consortium’s Major Depressive Disorder Working Group showed differences in cerebral cortical and subcortical brain structures between more than 1,700 individuals with major depression and 7,000 control subjects. Relative to the control group, the major depression group had significantly smaller hippocampal volumes (6). In addition, adults with major depression had thinner cortical gray matter than control subjects in the orbitofrontal cortex, the anterior and posterior cingulate cortex, the insula, and the temporal lobes (7), and adolescents with major depression had a lower total cortical surface area than age-matched control subjects, driven particularly by regional reductions in the medial orbitofrontal cortex and superior frontal gyrus, as well as primary and higher-order visual, somatosensory, and motor surface areas (7).
Left-right asymmetry is an important aspect of human brain organization that may be altered in various psychiatric and neurocognitive conditions, including schizophrenia, autism, and dyslexia (8–10). There are indications that altered brain asymmetry may also play a role in major depression. On a functional level, EEG studies have reported that asymmetry in frontal brain resting activity differs between individuals with major depression and healthy control subjects, although not always in a consistent direction, and is moderated by age and sex (see, e.g., 11–14; reviewed in 15, 16). Such findings have led to the development of stimulation protocols targeting the left dorsolateral prefrontal cortex, which are now used in the clinic for the treatment of major depression (17). Moreover, a recent review of studies based on dichotic listening, visual hemifield analysis, electrophysiology, and neuroimaging concluded that there was evidence for reductions of left frontal and right parietotemporal function in depressive disorders (18). A reduction of left frontal activity is in accordance with approach/withdrawal models of major depression, in which the normal balance of left frontal activity underlying positive reactions to positive stimuli, and right frontal activity underlying negative reactions to negative stimuli, may be disturbed (19, 20).
Some of the average brain anatomical differences between individuals with major depression and control subjects, described in the review by Zhang et al. (4), involved only one of the two hemispheres. Zhang et al. concluded that the right medial orbitofrontal cortex was often found to be thinner, and the volumes of the left middle frontal gyrus and the right thalamus lower, in individuals with major depression than in control subjects (4). The ENIGMA consortium study of the cerebral cortex found that the thickness of the inferior temporal gyrus and caudal anterior cingulate was significantly thinner in adults with major depression only on the right side, but not on the left (7). However, in these analyses it was not tested whether effect sizes of diagnosis were significantly different on the left and right sides, nor was asymmetry quantified as a trait in its own right. Rather, the unilateral patterns were reported on the basis that one hemisphere achieved statistical significance against the null hypothesis of no effect of diagnosis and the other side did not. Such patterns can reflect insufficient statistical power to detect small but uniform bilateral effect sizes and do not necessarily indicate differences in brain laterality per se. Furthermore, to analyze asymmetry alterations in major depression, a post hoc statistical comparison of the left- and right-sided effect sizes reported by the previous studies would not yield the same level of statistical power as can be provided by utilizing the individual-level paired left and right data. Meanwhile, the ENIGMA study of subcortical volumes did not consider left and right hemisphere measures separately, as they were combined for bilateral averages (6).
Brain structural asymmetry in major depression has been investigated only in a small number of individual studies with limited sample sizes. These include a study of gray matter volume of the dorsolateral prefrontal cortex in 39 treatment-naive individuals with major depression, 31 medicated individuals with major depression, and 49 control subjects, in which the treatment-naive individuals had increased rightward asymmetry (i.e., the extent of right > left asymmetry was larger) relative to control subjects (21). Another study reported that the frontal lobe volume was on average less rightward asymmetric in individuals with major depression (N=34) than in control subjects (N=30) (22). No large-scale studies of brain asymmetry in major depression have been performed to date.
To systematically investigate structural asymmetries in the brains of individuals with major depression compared with healthy control subjects, we used data available through the ENIGMA consortium’s Major Depressive Disorder Working Group and targeted brain regional and global hemispheric lateralities as assessed by the asymmetry index (left – right)/(left + right). In healthy populations, some regional brain asymmetries show mean sex differences (23, 24). In addition, major depression is often reported to be more common in women than men; for example, a female-to-male ratio of 1.6:1 was found in a Canadian survey (25). The disorder can also present differently in men and women (25, 26). These observations prompted us to perform secondary analyses separately by sex. Furthermore, as noted above, some structural brain differences between individuals with major depression and control subjects were found to be distinct between adolescent and adult groups (7). Asymmetries of the brain in some subcortical (23) and cortical regions (24) also change with age in healthy populations. We therefore carried out secondary analyses in separate subgroups of individuals with major depression and control subjects under and over age 21 at the time of scanning. Because major depression is a clinically heterogeneous disorder, we also tested whether structural brain asymmetries are different in medicated compared with nonmedicated individuals with major depression, in individuals with acute major depression compared with those in remission, and in individuals with first-episode major depression compared with those with recurrent episodes, as well as whether there are differences by age at onset of the disorder.
Methods
Data Sets
We pooled individual-level data from 32 nonoverlapping data sets collected around the world, of which one data set included only subcortical volumes and all others included both subcortical and cerebral cortical measures. (See Table S1 in the online supplement for the geographic locations and demographic characteristics of the different samples.) All participating sites obtained approval from local institutional review boards and ethics committees, and all study participants provided written informed consent.
In total, the combined data set for cortical measures contained 2,256 individuals with major depression and 3,504 control subjects after local quality control at each center (see below) but before central quality control, which was performed specifically for the present study (further explained below). The combined data set for subcortical measures consisted of 2,540 case subjects and 4,230 control subjects before central quality control. Eleven of the study centers contributing to this analysis were also involved in the previous study of cortical differences between individuals with major depression and control subjects (7), and eight of the study centers contributing to this analysis also contributed to the previous ENIGMA major depression subcortical study (6). The mean age at sampling across data sets was 37.1 years (SD=16.1) for individuals with major depression and 39.0 years (SD=17.3) for control subjects. Among the individuals with major depression, 36% were male, and among the control subjects, 47% were male. Descriptive information, by data set, is presented in Table S1 in the online supplement, and diagnostic instruments are described in Table S2. Data on antidepressant medication use at time of scanning, recurrent episodes, acute or remitted status, and age at onset of major depression are presented, by data set, in Table S3 in the online supplement.
Image Processing
Structural T1-weighted brain MRI scans were acquired at each study site. Images were acquired at different field strengths (1.5-T or 3-T scanners) and with various acquisition parameters, as indicated in Table S4 in the online supplement. All sites then applied harmonized processing and quality control protocols developed or adopted by the ENIGMA consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols). The data used in this study were from the left and right volumes of eight bilaterally paired subcortical structures (strictly, seven subcortical structures plus the lateral ventricles) and thickness and surface area measures for each of 34 bilaterally paired cortical regions, the latter as defined with the Desikan-Killiany atlas (27). In addition, the average cortical thickness and total surface area per entire hemisphere were analyzed. Subcortical segmentation and cortical parcellations were performed with FreeSurfer (version 5.1 or 5.3) (28, 29). Parcellation of cortical gray matter regions were visually inspected and statistically evaluated for outliers following the standardized ENIGMA protocol.
Data Preparation, Visualization, and Statistical Analysis
De-identified data were sent from all data sets to a central analysis team. As a measure of asymmetry for each bilaterally paired measure, we then calculated the asymmetry index (AI) as (L−R)/(L+R), where L and R are the left and right measures, respectively. Thus, positive and negative AI values indicate leftward and rightward asymmetry, respectively. It is important to note that in the definition of the AI, the difference (i.e., L−R) was normalized by use of the bilateral measure as denominator (i.e., L+R), such that the measure does not scale with the overall magnitude of L and R. For this reason, we did not adjust for intracranial volume in our analyses. Furthermore, we were interested in detecting the full extent of any case-control effects on AIs, without removing variance in the AIs that might be correlated with other brain measures potentially affected in major depression.
Quality control at the sites had excluded individual data points. Centrally, individuals with more than four entries missing for the eight subcortical volumes were excluded from the analysis of subcortical regions as having possibly unreliable subcortical data. Similarly, individuals with more than eight missing values out of 34 regional cortical thickness measures were removed altogether from the analysis of cortical thickness, and likewise for surface area measures. Exclusion of subjects by this step varied from 1% of both individuals with major depression and control subjects for the subcortical data to 3% of control subjects for the surface area data. The total remaining numbers were 3,399 control subjects and 2,217 individuals with major depression for the cortical surface areas, 3,427 control subjects and 2,229 individuals with major depression for the cortical thickness values, and 4,185 control subjects and 2,517 individuals with major depression for the subcortical volumes. The numbers of individual missing values then varied by structure from 0.16% missing values for the surface area of the lateral orbitofrontal cortex to 14.3% missing values for the surface area of the entorhinal cortex. Detailed numbers are provided in Table S5 in the online supplement.
To prevent large effects of possible outliers, all AIs were winsorized to 2.2 times the interquartile range, as recommended by Hoaglin and Iglewicz (30). Frequency histograms of each AI are shown in Figure S1 in the online supplement. The per–data set mean values for each AI were computed, and multidimensional scaling plots were created separately for cortical thickness AIs, cortical surface AIs, and subcortical volume AIs, to visualize whether any data sets were obvious outliers in terms of their population-level laterality, as considered over multiple regions.
Using individual-level data from all available data sets, for each structure separately, a linear mixed model was fitted using R, version 3.4.0, with AI as the dependent variable and sex, age, age squared, and diagnosis (major depression or control) as fixed factors, with data set as a random factor (random intercept). Because age and age squared are highly correlated, we made use of the poly() function in R for these two predictors, which created a pair of uncorrelated variables to model age effects (so-called orthogonal polynomials) (31), where one variable was linear and one nonlinear. Model fit was checked visually by inspection of the plots of residuals compared with fitted values, and the QQ plots for the residual values. Cook’s distance plots by data set (R command CookD (lme_model, group=“data set”) were used to visualize whether any of the data sets were obvious outliers at the level of individual structures. To interpret the results of our analysis, we used a false discovery rate of 0.05 within all AIs of a given structural measure, separately within 35 cortical thickness AIs, 35 cortical surface area AIs, and eight subcortical volume AIs. A global false discovery rate assessment was also planned, over all AIs tested in the main analysis of all subjects, but no effects of diagnosis on AIs proved significant within the separate false discovery rate corrections (see the Results section), so a global assessment was not needed. We calculated Cohen’s d for the effect size of diagnosis on each AI as t*sqrt(1/n1+1/n2), where n1 and n2 are the sample sizes of the individuals with major depression and the control subjects, respectively, and t is the t statistic for the diagnosis term in the model for a given AI. Brain anatomical figures were generated using FreeSurfer functions and triangular surface plotting (trisurf) in MATLAB, release R2015b, with the Cohen’s d statistics for cortical regions projected onto the pial surface.
We used the pwr() command in R to calculate a priori the minimal effect size that we had 80% power to detect with the available data. (Because each linear model included multiple predictor variables, including a random effect, the a priori power could not be computed exactly, but this calculation assumed the use of simple t tests to provide a useful indication.) For the cortical measures, we set a significance threshold of 0.001 (roughly 0.05/35 in the context of multiple testing over all 34 regional AIs and one global hemispheric AI). This showed that the indicative minimum effect at 80% power was a Cohen’s d value of 0.112. For the subcortical AIs (corrected at 0.05/8=0.006), the indicative minimum effect at 80% power was a d value of 0.090.
For secondary analysis of the effects of major depression diagnosis on AIs within demographic subsets, we separated the data into females only, males only, individuals ≤21 years of age or >21 years of age at the time of scanning. The same linear mixed model as above was applied to each of these subsets separately, except that the factor “sex” was not included for the male-only or female-only subsets.
Secondary analyses of AIs in relation to clinical variables were carried out within individuals with major depression only (see Table S3 in the online supplement). For binary clinical variables (recurrent versus first episode, medicated versus unmedicated with antidepressants at time of scanning, acute versus remitted), we used the same linear mixed model approach described above, except now replacing the diagnosis status with the binary clinical variable in question. For this purpose we only included data sets with at least 10 individuals with major depression in each subgroup. Age at onset within individuals with major depression was tested as a linear effect on AIs, otherwise using the same linear mixed model as for the main analysis. See Tables S6 and S7 in the online supplement for the sample sizes used for each linear mixed model in these secondary analyses. False-discovery-rate-adjusted p values are presented for the eight (subcortical) or 35 (cortical) AIs within each separate analysis.
Results
Multidimensional scaling plots based on per–data set AI mean values showed that none of the data sets were extreme outliers, viewed across all brain structures (see Figure S2 in the online supplement).
In the main analysis (all individuals with major depression compared with control subjects), no significant effects of diagnosis were found for any of the cortical thickness, cortical surface, or subcortical volume AIs after correction for multiple testing (Tables 1–3; see also Table S5 in the online supplement). The subset analyses by age and sex also showed no significant effects of diagnosis on AIs (see Tables 1–3 and Table S5). A small number of unadjusted p values for effects of diagnosis on AIs were below 0.01, but none survived false discovery rate correction for multiple comparisons. The strongest effect of diagnosis on asymmetry in the main analysis was for superior temporal gyrus thickness asymmetry, with an unstandardized effect of diagnosis on AI (i.e., the mean AI difference between case and control subjects after adjustment for the other model effects) of 0.002, a nominal (unadjusted) p value of 0.003, and a Cohen’s d of 0.085. For this region, the right surface area was larger than the left in control subjects, and also in case subjects but to a lesser extent (see Table S5). Similar effects were found for the caudal anterior cingulate thickness AI (Cohen’s d=0.079; left > right in control subjects and more so in case subjects) and the cuneus surface area asymmetry (Cohen’s d=−0.081; right > left in control subjects and more so in case subjects) (see Table S5). Some of the subset analyses also produced nominally significant effects, such as for hippocampal volume asymmetry in males only (Cohen’s d=−0.112) (see Tables 1–3 and Table S5). However, in the context of multiple testing, these cannot be considered reliable effects. Full model results are included in Table S5.
| All | Males | Females | > Age 21 | ≤ Age 21 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subcortical Region | d | p | d | p | d | p | d | p | d | p |
| Accumbens | 0.0009 | 0.972 | –0.026 | 0.541 | 0.023 | 0.492 | 0.012 | 0.679 | –0.004 | 0.948 |
| Amygdala | 0.0163 | 0.523 | 0.041 | 0.324 | 0.001 | 0.986 | 0.009 | 0.759 | 0.029 | 0.646 |
| Caudate | –0.0159 | 0.534 | –0.018 | 0.654 | –0.011 | 0.737 | –0.008 | 0.771 | –0.072 | 0.258 |
| Hippocampus | –0.0414 | 0.105 | –0.112 | 0.007 | –0.003 | 0.928 | –0.037 | 0.187 | –0.029 | 0.652 |
| Lateral ventricles | 0.0584 | 0.021 | 0.057 | 0.168 | 0.059 | 0.072 | 0.046 | 0.106 | 0.089 | 0.156 |
| Pallidum | –0.0224 | 0.391 | –0.088 | 0.037 | 0.009 | 0.782 | –0.003 | 0.930 | –0.087 | 0.186 |
| Putamen | –0.0278 | 0.286 | –0.099 | 0.018 | 0.012 | 0.723 | –0.025 | 0.382 | –0.026 | 0.691 |
| Thalamus | 0.0010 | 0.969 | 0.019 | 0.640 | –0.016 | 0.631 | 0.002 | 0.934 | –0.029 | 0.647 |
TABLE 1. Effect sizes (Cohen’s d) for the effects of diagnosis on asymmetry indexes of subcortical volumes in individuals with major depression and unaffected control subjectsa
| All | Males | Females | > Age 21 | ≤ Age 21 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortical Region | d | p | d | p | d | p | d | p | d | p |
| Total surface area | 0.017 | 0.543 | –0.035 | 0.475 | 0.052 | 0.188 | 0.017 | 0.580 | 0.069 | 0.295 |
| Banks of superior temporal sulcus | 0.004 | 0.879 | 0.009 | 0.858 | –0.010 | 0.812 | –0.013 | 0.680 | 0.072 | 0.300 |
| Caudal anterior cingulate | –0.022 | 0.434 | –0.079 | 0.109 | –0.012 | 0.763 | –0.030 | 0.336 | 0.015 | 0.817 |
| Caudal middle frontal | 0.058 | 0.036 | –0.024 | 0.634 | 0.104 | 0.009 | 0.044 | 0.152 | 0.139 | 0.035 |
| Cuneus | –0.081 | 0.003 | –0.124 | 0.012 | –0.051 | 0.201 | –0.071 | 0.022 | –0.047 | 0.480 |
| Entorhinal | –0.005 | 0.864 | 0.068 | 0.195 | –0.029 | 0.498 | –0.008 | 0.803 | –0.009 | 0.888 |
| Frontal pole | –0.045 | 0.102 | –0.044 | 0.371 | –0.056 | 0.158 | –0.027 | 0.379 | –0.126 | 0.057 |
| Fusiform | –0.002 | 0.944 | 0.060 | 0.241 | –0.041 | 0.309 | 0.030 | 0.341 | –0.122 | 0.065 |
| Inferior parietal | –0.031 | 0.273 | 0.008 | 0.877 | 0.007 | 0.862 | –0.044 | 0.159 | 0.028 | 0.668 |
| Inferior temporal | 0.037 | 0.185 | 0.057 | 0.246 | 0.035 | 0.379 | 0.029 | 0.343 | 0.085 | 0.197 |
| Insula | 0.046 | 0.098 | 0.072 | 0.141 | 0.013 | 0.744 | 0.065 | 0.035 | 0.008 | 0.899 |
| Isthmus of cingulate | 0.003 | 0.918 | 0.068 | 0.166 | 0.055 | 0.167 | 0.004 | 0.890 | 0.039 | 0.553 |
| Lateral occipital | 0.000 | 0.986 | 0.033 | 0.500 | –0.018 | 0.652 | –0.003 | 0.930 | 0.029 | 0.659 |
| Lateral orbitofrontal | 0.004 | 0.891 | 0.034 | 0.488 | –0.015 | 0.713 | 0.003 | 0.923 | 0.001 | 0.982 |
| Lingual | 0.010 | 0.730 | –0.022 | 0.649 | 0.069 | 0.080 | –0.003 | 0.913 | 0.069 | 0.297 |
| Medial-orbitofrontal | –0.019 | 0.503 | 0.025 | 0.612 | –0.036 | 0.369 | –0.045 | 0.152 | 0.082 | 0.212 |
| Middle temporal | –0.042 | 0.140 | 0.008 | 0.868 | –0.099 | 0.016 | –0.047 | 0.139 | –0.038 | 0.578 |
| Paracentral | 0.031 | 0.261 | 0.020 | 0.689 | 0.030 | 0.463 | 0.041 | 0.190 | 0.027 | 0.686 |
| Parahippocampal | –0.003 | 0.925 | 0.051 | 0.303 | 0.005 | 0.901 | –0.028 | 0.372 | 0.078 | 0.239 |
| Pars opercularis | 0.023 | 0.409 | 0.013 | 0.794 | 0.024 | 0.541 | 0.009 | 0.757 | 0.051 | 0.440 |
| Pars orbitalis | 0.022 | 0.420 | –0.014 | 0.773 | 0.022 | 0.571 | 0.019 | 0.526 | 0.023 | 0.727 |
| Pars triangularis | 0.006 | 0.841 | –0.021 | 0.671 | –0.015 | 0.701 | –0.001 | 0.978 | –0.001 | 0.990 |
| Pericalcarine | –0.031 | 0.265 | 0.018 | 0.718 | –0.036 | 0.372 | –0.025 | 0.424 | –0.015 | 0.826 |
| Postcentral | –0.021 | 0.447 | 0.029 | 0.554 | –0.013 | 0.744 | –0.020 | 0.518 | –0.001 | 0.985 |
| Posterior cingulate | –0.044 | 0.110 | –0.060 | 0.216 | –0.046 | 0.243 | –0.046 | 0.136 | –0.004 | 0.947 |
| Precentral | 0.038 | 0.172 | 0.063 | 0.206 | 0.022 | 0.578 | 0.045 | 0.144 | 0.059 | 0.370 |
| Precuneus | 0.005 | 0.851 | –0.047 | 0.341 | 0.071 | 0.072 | –0.021 | 0.498 | 0.084 | 0.202 |
| Rostral anterior cingulate | 0.014 | 0.626 | –0.064 | 0.198 | 0.045 | 0.266 | 0.018 | 0.566 | –0.005 | 0.941 |
| Rostral middle frontal | –0.062 | 0.026 | –0.094 | 0.055 | –0.026 | 0.506 | –0.073 | 0.017 | –0.005 | 0.944 |
| Superior frontal | 0.064 | 0.021 | 0.107 | 0.032 | 0.047 | 0.242 | 0.061 | 0.050 | 0.061 | 0.358 |
| Superior parietal | –0.020 | 0.463 | –0.121 | 0.014 | 0.040 | 0.316 | –0.015 | 0.620 | –0.021 | 0.748 |
| Superior temporal | 0.017 | 0.565 | –0.052 | 0.309 | 0.052 | 0.206 | 0.009 | 0.789 | 0.029 | 0.676 |
| Supramarginal | 0.038 | 0.177 | 0.030 | 0.558 | 0.031 | 0.441 | 0.064 | 0.043 | –0.093 | 0.166 |
| Temporal pole | –0.003 | 0.908 | 0.079 | 0.112 | 0.003 | 0.950 | –0.016 | 0.609 | 0.041 | 0.530 |
| Transverse temporal | 0.008 | 0.770 | –0.076 | 0.117 | –0.018 | 0.651 | 0.017 | 0.569 | –0.046 | 0.487 |
TABLE 2. Effect sizes (Cohen’s d) for the effects of diagnosis on asymmetry indexes of cortical surface areas in individuals with major depression and unaffected control subjectsa
| All | Males | Females | > Age 21 | ≤ Age 21 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortical Region | d | p | d | p | d | p | d | p | d | p |
| Average thickness | 0.028 | 0.307 | 0.070 | 0.111 | –0.005 | 0.893 | 0.024 | 0.431 | 0.055 | 0.400 |
| Banks of superior temporal sulcus | 0.016 | 0.582 | 0.011 | 0.810 | 0.014 | 0.711 | 0.049 | 0.122 | –0.103 | 0.134 |
| Caudal anterior cingulate | 0.079 | 0.004 | 0.083 | 0.059 | 0.077 | 0.030 | 0.070 | 0.021 | 0.097 | 0.142 |
| Caudal middle frontal | 0.017 | 0.537 | –0.008 | 0.859 | 0.036 | 0.314 | 0.009 | 0.771 | 0.056 | 0.392 |
| Cuneus | –0.027 | 0.325 | –0.020 | 0.655 | –0.025 | 0.477 | –0.019 | 0.529 | –0.029 | 0.660 |
| Entorhinal | 0.010 | 0.733 | 0.018 | 0.691 | 0.006 | 0.868 | 0.004 | 0.894 | 0.046 | 0.492 |
| Frontal pole | 0.016 | 0.563 | 0.054 | 0.215 | –0.018 | 0.609 | 0.020 | 0.513 | 0.051 | 0.442 |
| Fusiform | 0.002 | 0.954 | 0.022 | 0.614 | –0.009 | 0.802 | 0.004 | 0.905 | –0.008 | 0.909 |
| Inferior parietal | –0.052 | 0.057 | –0.048 | 0.273 | –0.045 | 0.209 | –0.050 | 0.104 | –0.070 | 0.290 |
| Inferior temporal | 0.048 | 0.080 | 0.071 | 0.109 | 0.028 | 0.430 | 0.045 | 0.140 | 0.037 | 0.579 |
| Insula | 0.005 | 0.850 | 0.039 | 0.378 | –0.018 | 0.614 | –0.003 | 0.924 | 0.039 | 0.560 |
| Isthmus of cingulate | –0.017 | 0.527 | –0.009 | 0.831 | –0.033 | 0.348 | –0.018 | 0.561 | –0.064 | 0.328 |
| Lateral occipital | –0.013 | 0.645 | –0.087 | 0.048 | 0.045 | 0.202 | –0.027 | 0.370 | 0.022 | 0.738 |
| Lateral orbitofrontal | 0.044 | 0.108 | 0.068 | 0.119 | 0.033 | 0.350 | 0.058 | 0.056 | 0.049 | 0.456 |
| Lingual | 0.019 | 0.482 | –0.027 | 0.544 | 0.051 | 0.152 | 0.035 | 0.246 | –0.030 | 0.648 |
| Medial-orbitofrontal | –0.053 | 0.054 | –0.091 | 0.040 | –0.021 | 0.562 | –0.032 | 0.289 | –0.076 | 0.249 |
| Middle temporal | –0.008 | 0.766 | –0.019 | 0.676 | –0.006 | 0.865 | –0.003 | 0.926 | –0.067 | 0.321 |
| Paracentral | 0.001 | 0.983 | 0.062 | 0.160 | –0.037 | 0.299 | 0.001 | 0.966 | 0.034 | 0.602 |
| Parahippocampal | 0.032 | 0.245 | 0.096 | 0.029 | –0.002 | 0.946 | 0.038 | 0.215 | 0.064 | 0.336 |
| Pars opercularis | –0.041 | 0.135 | –0.091 | 0.038 | –0.024 | 0.494 | –0.065 | 0.032 | 0.096 | 0.145 |
| Pars orbitalis | –0.010 | 0.714 | –0.013 | 0.770 | –0.011 | 0.765 | –0.020 | 0.511 | 0.069 | 0.300 |
| Pars triangularis | –0.004 | 0.891 | 0.017 | 0.693 | –0.024 | 0.501 | –0.006 | 0.832 | 0.053 | 0.423 |
| Pericalcarine | 0.008 | 0.768 | –0.013 | 0.772 | 0.025 | 0.484 | 0.012 | 0.693 | –0.030 | 0.648 |
| Postcentral | 0.047 | 0.091 | 0.063 | 0.151 | 0.026 | 0.461 | 0.044 | 0.149 | 0.064 | 0.330 |
| Posterior cingulate | 0.038 | 0.165 | 0.055 | 0.211 | 0.027 | 0.444 | 0.041 | 0.176 | 0.029 | 0.660 |
| Precentral | 0.026 | 0.336 | 0.011 | 0.800 | 0.025 | 0.475 | 0.016 | 0.592 | 0.044 | 0.509 |
| Precuneus | –0.051 | 0.063 | –0.007 | 0.881 | –0.076 | 0.033 | –0.055 | 0.070 | –0.019 | 0.777 |
| Rostral anterior cingulate | –0.007 | 0.808 | 0.031 | 0.483 | –0.027 | 0.451 | –0.016 | 0.600 | 0.063 | 0.341 |
| Rostral middle frontal | –0.030 | 0.268 | –0.039 | 0.378 | –0.036 | 0.309 | –0.021 | 0.493 | –0.045 | 0.492 |
| Superior frontal | –0.011 | 0.691 | 0.063 | 0.153 | –0.059 | 0.098 | –0.008 | 0.781 | 0.028 | 0.674 |
| Superior parietal | 0.004 | 0.872 | 0.081 | 0.066 | –0.047 | 0.188 | 0.011 | 0.711 | –0.032 | 0.629 |
| Superior temporal | 0.085 | 0.003 | 0.090 | 0.049 | 0.071 | 0.056 | 0.068 | 0.033 | 0.133 | 0.051 |
| Supramarginal | 0.021 | 0.445 | 0.077 | 0.084 | –0.016 | 0.651 | 0.011 | 0.712 | 0.029 | 0.667 |
| Temporal pole | –0.010 | 0.730 | –0.024 | 0.581 | 0.009 | 0.804 | –0.003 | 0.924 | –0.033 | 0.618 |
| Transverse temporal | 0.037 | 0.174 | 0.078 | 0.078 | 0.008 | 0.825 | 0.019 | 0.537 | 0.111 | 0.092 |
TABLE 3. Effect sizes (Cohen’s d) for the effects of diagnosis on asymmetry indexes of cortical thicknesses in individuals with major depression and unaffected control subjectsa
We visualized the Cohen’s d values from the main analyses (all subjects combined, i.e., the leftmost columns of Tables 2 and 3) against a cortical brain image to help assess whether any multiregion patterns were discernible that might have spanned neighboring regions or corresponded with the frontal-occipital or dorso-ventral axes (Figure 1). No clear patterns were visible.
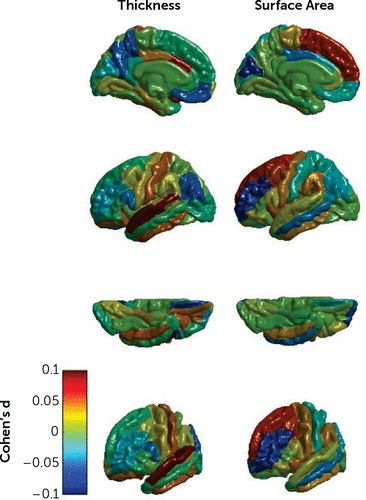
FIGURE 1. Effect sizes for regional asymmetry differences in cortical thickness and surface area between individuals with major depression and unaffected control subjectsa
a A positive effect means that individuals with major depression were more leftward/less rightward asymmetrical than control subjects. From top to bottom: lateral, medial, inferior, fronto-lateral views. Note that all Cohen’s d values ranged from −0.081 to 0.085 and that none of the differences between individuals with major depression and control subjects were significant after adjustment for multiple testing across regions.
The analysis of clinical variable effects on AIs within individuals with major depression (see Tables S6 and S7 in the online supplement) showed only one p value <0.05 after adjustment for multiple testing: the cortical thickness of the fusiform gyrus was more rightward asymmetric in persons using antidepressants at time of scanning (adjusted p value, 0.046). However, this p value was only adjusted within this particular analysis (i.e., 35 cortical thickness AIs tested for effects of medication use) and should be interpreted with care, given the degree of study-wide testing involved. Full results from these analyses can be found in Tables S6 and S7 in the online supplement.
Discussion
In this study, no significant differences of brain structural asymmetry were found between individuals with major depression and unaffected control subjects, for any cerebral cortical or subcortical asymmetry measure, in an unprecedented sample size of over 5,000 subjects. Power analysis indicated that we had 80% power a priori to detect a case-control Cohen’s d of roughly 0.1 for a given AI in the main analysis. However, the strongest effect of diagnosis involved a Cohen’s d value of 0.085 for the superior temporal gyrus thickness AI, which was too subtle to be statistically significant when considering multiple testing, even with this large sample size (adjusted p value, 0.104). There were similarly small and nonsignificant changes of caudal anterior cingulate thickness asymmetry (Cohen’s d=0.079) and cuneus surface area asymmetry (Cohen’s d=−0.081). We are not aware of previous findings in the literature that are concordant with these effects. If differences in the asymmetry of brain structures between individuals with major depression and unaffected control subjects do exist, they were too small to be detected reliably in this analysis. Our study illustrates the importance of taking large-scale and systematic approaches to the study of associations between the brain and disorders.
We found no support for alterations of asymmetry that are consistent with those reported in two previous small studies of the dorsolateral prefrontal cortex (21) or frontal lobe (22). In our data, subregions that are part of the dorsolateral prefrontal cortex showed merely tentative case-control differences for cortical surface area, in opposite directions across subregions (Figure 1). It may therefore be that the earlier studies reported false positive findings in the context of small data sets, although the cortical atlas that we used did not have a perfect equivalent for the measures defined in those studies, and we did not consider gray matter volumes as such. Rather, we studied regional cortical thicknesses and surface areas as distinct measures, which together drive gray matter volumetric measures but have been shown to vary relatively independently (32), such that separate analyses are well motivated.
The possibility remains that altered brain functional or structural asymmetry may be related, as cause, correlate, or effect, to major depression in some etiological subgroups of individuals. The previous ENIGMA consortium analyses of brain structural changes in major depression (in which asymmetry was not investigated) found case-control differences particularly in the context of multiple episodes of depression and/or in relation to age at onset of depression (6, 7). One possibility is therefore that brain changes in major depression may be driven by long-term stress associated with the disorder. After our main analysis, we subdivided the data by sex and age groups, and we also analyzed various clinical variables within individuals with major depression (recurrent versus first episode, on antidepressant medication versus antidepressant-free at time of scanning, acute versus remitted, age at onset) but found no convincing evidence for effects within these subgroups. Sample sizes for these secondary analyses were reduced relative to the main analysis, because of either subsetting or limited availability of clinical variables (see Tables S6 and S7 in the online supplement), and multiple testing for these secondary analyses was substantial. We found one tentative effect involving thickness asymmetry of the fusiform gyrus with respect to medication status of individuals with major depression (false-discovery-rate-adjusted p=0.046). In a previous study, medication-naive persons with major depression (N=37) showed a greater thickness of the left fusiform gyrus than healthy control subjects (N=41) (33), whereas in our analysis, individuals with major depression using antidepressant medication had a rightward change of thickness asymmetry of the fusiform cortex compared with individuals with major depression who were not using antidepressants at the time of scanning. Given the degree of multiple testing in our secondary analyses, and given that this finding has no previous support in the literature, we regard it as tentative. Furthermore, we had no systematic information on past use of medication or other treatments, nor on antidepressant medication dosages at the time of scanning, both of which may be related to disorder duration and severity, so this finding must be interpreted with caution. We did not have information on other diagnostic subtypes, such as melancholia or atypical depression, which may be important with respect to the biological heterogeneity of major depression and will need further research.
While we did not find case-control differences of brain structural asymmetry in this study, functional asymmetries may still play an important role in major depression. Relations between structural and functional variability of the brain are subtle and complex (34–37). As mentioned in the introduction, various studies of depression have reported case-control differences in the asymmetry of frontal electrophysiological patterns (11, 14). The number of pyramidal cells, the number of synapses per cell, and their firing patterns are thought to influence cortical EEG recordings (38). A difference in the number of pyramidal cells may also affect cortical thickness (39). In fact, an inverse relation between cortical thickness and EEG alpha power has been reported for some cortical regions (40). However, a recent meta-analysis of frontal alpha asymmetry as a diagnostic marker in depression (16 studies; major depression group, N=1,883; control group, N=2,161) found no significant difference between individuals with major depression and control subjects (16). Other reviews also point to inconsistencies or problems in studies of frontal alpha asymmetries in depression (15, 41), although most have been studies of the resting state, and there is evidence that EEG differences are stronger during cognitive or emotional processing tasks (42, 43). A recent study that made use of resting-state fMRI reported that certain bilateral changes, which were found by a comparison between 709 individuals with major depression and 725 control subjects, would require a minimum of 400 individuals per group to be detectable, and also that relationships between the brain and clinical variables exhibited poor cross-center reproducibility (44). Clearly, large-scale studies are necessary for brain imaging research on associations with disorders to reach reliable conclusions.
As for asymmetry specifically, it is unclear how altered functional laterality might relate to major depression in terms of cause, effect, or correlation, because of shared underlying factors. The average form of human brain laterality is probably established in the embryo, as indicated by in utero behavioral data (45, 46) as well as neuroanatomical studies of fetuses (47, 48) and gene expression analysis in which left- and right-sided samples from the embryonic central nervous system are contrasted (49–51). The typical form of human brain asymmetry is characterized by left-hemisphere language dominance (in more than 85% of people) (52), right-handedness (also roughly 85% of people) (53), and a particular anatomical pattern involving both subcortical and cerebral cortical features (23, 24). However, brain laterality is also highly variable between individuals. Factors that cause variation around the average form are largely unknown, and heritability estimates are generally low to modest for both functional and structural aspects, while age and sex have significant but subtle effects (10, 23, 24).
We did not consider handedness as a factor in our models, as handedness did not show an effect on brain anatomical laterality in an analysis of over 17,000 subjects from healthy control and population data sets, also performed by the ENIGMA consortium (24). Data on handedness were limited for many of the data sets in the present study.
In a multicenter study such as ours, the between-center variability may result in reduced statistical power relative to an equally sized single-center study, but no single center has been able to collect such large samples alone. In addition, multicenter studies can be representative of real-world heterogeneity, with potentially more generalizable findings than single-center studies (54).
Conclusions
Although this study had a large sample size, with 80% power a priori to detect case-control differences on the order of a Cohen’s d value of 0.1, we found no significant differences between individuals with major depression and control subjects in asymmetries of cerebral cortical thickness and surface area measures, nor for subcortical volume asymmetries. Our study illustrates how high-powered and systematic studies can yield clearer findings in human clinical neuroscience, where previous studies had provided a mixed picture.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed, DSM-5. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: full recovery is the exception rather than the rule. BMC Med 2017; 15:215Crossref, Medline, Google Scholar
3 : The epidemiology of depression across cultures. Annu Rev Public Health 2013; 34:119–138Crossref, Medline, Google Scholar
4 : Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther 2018; 24:994–1003Crossref, Medline, Google Scholar
5 : Intra- and interscanner variability of magnetic resonance imaging based volumetry in multiple sclerosis. Neuroimage 2016; 142:188–197Crossref, Medline, Google Scholar
6 : Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2016; 21:806–812Crossref, Medline, Google Scholar
7 : Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; 22:900–909Crossref, Medline, Google Scholar
8 : Decreased hemispheric connectivity and decreased intra- and inter- hemisphere asymmetry of resting state functional network connectivity in schizophrenia. Brain Imaging Behav 2018; 12:615–630Crossref, Medline, Google Scholar
9 : Asymmetry of fusiform structure in autism spectrum disorder: trajectory and association with symptom severity. Mol Autism 2016; 7:28Crossref, Medline, Google Scholar
10 : Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet 2012; 15:401–413Crossref, Medline, Google Scholar
11 : α Power, α asymmetry, and anterior cingulate cortex activity in depressed males and females. J Psychiatr Res 2012; 46:1483–1491Crossref, Medline, Google Scholar
12 : Electroencephalographic frontal asymmetry and depressive symptoms in the elderly. Biol Psychol 2008; 79:317–322Crossref, Medline, Google Scholar
13 : Long-term stability of frontal electroencephalographic asymmetry in adults with a history of depression and controls. Int J Psychophysiol 2006; 59:107–115Crossref, Medline, Google Scholar
14 : Resting frontal EEG asymmetry patterns in adolescents with and without major depression. Biol Psychol 2018; 132:212–216Crossref, Medline, Google Scholar
15 : Frontal alpha asymmetry as a pathway to behavioural withdrawal in depression: research findings and issues. Behav Brain Res 2015; 292:56–67Crossref, Medline, Google Scholar
16 : Frontal alpha asymmetry as a diagnostic marker in depression: fact or fiction? A meta-analysis. Neuroimage Clin 2017; 16:79–87Crossref, Medline, Google Scholar
17 : Stimulated left DLPFC–nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry 2018; 7:3Crossref, Medline, Google Scholar
18 : Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological, and neuroimaging findings. Neurosci Biobehav Rev 2017; 78:178–191Crossref, Medline, Google Scholar
19 : Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology 2003; 40:106–114Crossref, Medline, Google Scholar
20 : Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology 1998; 35:607–614Crossref, Medline, Google Scholar
21 : Structural asymmetry of dorsolateral prefrontal cortex correlates with depressive symptoms: evidence from healthy individuals and patients with major depressive disorder. Neurosci Bull 2016; 32:217–226Crossref, Medline, Google Scholar
22 : Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res 2000; 100:41–47Crossref, Medline, Google Scholar
23 : Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav 2017; 11:1497–1514Crossref, Medline, Google Scholar
24 : Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA consortium. Proc Natl Acad Sci USA 2018; 115:E5154–E5163Crossref, Medline, Google Scholar
25 : Gender differences in the symptoms of major depressive disorder. J Nerv Ment Dis 2007; 195:905–911Crossref, Medline, Google Scholar
26 : Why is depression more prevalent in women? J Psychiatry Neurosci 2015; 40:219–221Crossref, Medline, Google Scholar
27 : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980Crossref, Medline, Google Scholar
28 : Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage 1999; 9:179–194Crossref, Medline, Google Scholar
29 : FreeSurfer. Neuroimage 2012; 62:774–781Crossref, Medline, Google Scholar
30 : Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc 1987; 82:1147–1149Crossref, Google Scholar
31 : Statistical Models in S. Pacific Grove, Calif, Wadsworth & Brooks/Cole, 1992Google Scholar
32 : Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 2009; 19:2728–2735Crossref, Medline, Google Scholar
33 : Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine 2017; 21:228–235Crossref, Medline, Google Scholar
34 : Brain asymmetry in cortical thickness is correlated with cognitive function. Front Hum Neurosci 2014; 8:877Crossref, Medline, Google Scholar
35 : Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. J Neurosci 2014; 34:6294–6302Crossref, Medline, Google Scholar
36 : Is the planum temporale surface area a marker of hemispheric or regional language lateralization? Brain Struct Funct 2018; 223:1217–1228Medline, Google Scholar
37 : What we know about the brain structure-function relationship. Behav Sci (Basel) 2018; 8:
38 Kenemans L: A Primer on EEG and Related Measures of Brain Activity. Utrecht, the Netherlands, Utrecht University, Department of Experimental Psychology and Psychopharmacology, August 2013Google Scholar
39 : Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci 2004; 24:2247–2258Crossref, Medline, Google Scholar
40 : Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum Brain Mapp 2012; 33:1325–1333Crossref, Medline, Google Scholar
41 : Current source density analysis of resting state EEG in depression: a review. J Neural Transm (Vienna) 2017; 124:109–118Crossref, Medline, Google Scholar
42 : A capability model of individual differences in frontal EEG asymmetry. Biol Psychol 2006; 72:198–207Crossref, Medline, Google Scholar
43 : EEG hemispheric asymmetries during cognitive tasks in depressed patients with high versus low trait anxiety. Clin EEG Neurosci 2010; 41:196–202Crossref, Medline, Google Scholar
44 : Reproducibility of functional brain alterations in major depressive disorder: evidence from a multisite resting-state functional MRI study with 1,434 individuals. Neuroimage 2019; 189:700–714Crossref, Medline, Google Scholar
45 : The developmental origins of laterality: fetal handedness. Dev Psychobiol 2013; 55:588–595Crossref, Medline, Google Scholar
46 : The origin of human handedness and its role in pre-birth motor control. Sci Rep 2017; 7:16804Crossref, Medline, Google Scholar
47 : Reference values for the right and left fetal choroid plexus at 11 to 13 weeks: an early sign of “developmental” laterality? J Ultrasound Med 2013; 32:1623–1629Crossref, Medline, Google Scholar
48 : The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex 2011; 21:1076–1083Crossref, Medline, Google Scholar
49 : Left-right asymmetry of maturation rates in human embryonic neural development. Biol Psychiatry 2017; 82:204–212Crossref, Medline, Google Scholar
50 : Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. eLife 2017; 6:
51 : Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science 2005; 308:1794–1798Crossref, Medline, Google Scholar
52 : Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLoS One 2014; 9:
53 : Hand preference and age in the United States. Neuropsychologia 1992; 30:601–608Crossref, Medline, Google Scholar
54 : Pooling fMRI data: meta-analysis, mega-analysis, and multi-center studies. Front Neuroinform 2009; 3:33Crossref, Medline, Google Scholar



