Mitigation of Olanzapine-Induced Weight Gain With Samidorphan, an Opioid Antagonist: A Randomized Double-Blind Phase 2 Study in Patients With Schizophrenia
Abstract
Objective:
Preclinical evidence and data from a proof-of-concept study in healthy volunteers suggest that samidorphan, an opioid antagonist, mitigates weight gain associated with olanzapine. This study prospectively compared combination therapy of olanzapine plus either samidorphan or placebo for the treatment of schizophrenia.
Methods:
This was an international, multicenter, randomized phase 2 study of olanzapine plus samidorphan in patients with schizophrenia. The study had a 1-week open-label olanzapine lead-in period followed by a 12-week double-blind treatment phase in which patients were randomly assigned in a 1:1:1:1 ratio to receive olanzapine plus placebo (N=75) or olanzapine plus 5 mg (N=80), 10 mg (N=86), or 20 mg (N=68) of samidorphan. The primary aims were to confirm that the antipsychotic efficacy of olanzapine plus samidorphan was comparable to olanzapine plus placebo, to assess the effect of combining olanzapine with samidorphan on olanzapine-induced weight gain, and to assess the overall safety and tolerability of olanzapine plus samidorphan.
Results:
Antipsychotic efficacy, as assessed by total score on the Positive and Negative Syndrome Scale (PANSS), was equivalent across all treatment groups. Treatment with olanzapine plus samidorphan resulted in a statistically significant lower weight gain (37% lower weight gain compared with olanzapine plus placebo). The least square mean percent change from baseline in body weight was 4.1% (2.9 kg) for the olanzapine plus placebo group and 2.6% (1.9 kg) for the olanzapine plus samidorphan group (2.8% [2.1 kg] for the 5 mg group, 2.1% [1.5 kg] for the 10 mg group, and 2.9% [2.2 kg] for the 20 mg group). Adverse events reported at a frequency ≥5% in any of the olanzapine plus samidorphan groups and occurring at a rate ≥2 times greater than in the olanzapine plus placebo group were somnolence, sedation, dizziness, and constipation. Other safety measures were comparable between the olanzapine plus samidorphan groups and the olanzapine plus placebo group.
Conclusions:
The antipsychotic efficacy of olanzapine plus samidorphan was equivalent to that of olanzapine plus placebo, and olanzapine plus samidorphan was associated with clinically meaningful and statistically significant mitigation of weight gain compared with olanzapine plus placebo. Olanzapine plus samidorphan was generally well tolerated, with a safety profile similar to olanzapine plus placebo.
In comparative effectiveness trials of schizophrenia, olanzapine is one of the most efficacious antipsychotics, and in long-term studies it has a lower rate of discontinuation due to lack of efficacy compared with other first-line antipsychotics (1–3). In the landmark Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, only 15% of patients treated with olanzapine discontinued treatment because of lack of efficacy, compared with 24%−28% of patients treated with other antipsychotics (2). In addition, short-term trials of olanzapine have reported significant symptom improvement in patients with schizophrenia (4, 5) as well as significantly sustained response in relapse prevention (6). However, the clinical utility of olanzapine has been limited by an association with comparatively greater weight gain and metabolic dysregulation (7, 8). Overall, body weight gain of ≥7% has been observed in 22%−86% of olanzapine-treated patients, depending on prior antipsychotic exposure and duration of treatment (2, 3, 9).
Preclinical studies have provided evidence for a critical role of the opioid system in mediating food reward, feeding behavior, and metabolism. For example, decrease in weight gain has been reported in μ-, κ-, and δ-opioid receptor knockout mice, despite no differences in caloric intake in μ- and κ-opioid receptor knockouts (10–12). Thus, adding an opioid antagonist to CNS-active drugs may mitigate metabolic dysregulation. Samidorphan is a new compound that has been demonstrated in vivo to function as a µ-opioid antagonist (13). In vitro, samidorphan binds with high affinity to human μ-, κ-, and δ-opioid receptors and acts as an antagonist at μ-opioid receptors and a partial agonist at κ- and δ-opioid receptors (14, 15). Samidorphan was found in both preclinical and phase 1 clinical studies to mitigate weight gain associated with olanzapine (16, 17). In a recent proof-of-concept study in healthy volunteers, mean weight gain over the course of 3 weeks was significantly less in patients treated with olanzapine plus samidorphan compared with those treated with olanzapine alone (2.2 kg [SD=1.4] compared with 3.1 kg [SD=1.9]) (17). The combination drug formulation of olanzapine plus samidorphan is intended to provide the antipsychotic efficacy of olanzapine while mitigating the weight gain and concomitant metabolic abnormalities commonly associated with olanzapine alone.
In this proof-of-concept, placebo-controlled phase 2 study, we evaluated the efficacy, safety, and tolerability of the combination of olanzapine and samidorphan in patients with schizophrenia. The study compared the antipsychotic efficacy and safety of olanzapine plus fixed doses of samidorphan relative to olanzapine plus placebo, with a specific safety focus on the evaluation of the hypothesized weight gain mitigation effect of olanzapine plus samidorphan relative to olanzapine plus placebo.
Methods
This was an international multicenter safety, tolerability, and dose-ranging phase 2 study conducted from June 2013 to March 2015. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines agreed on by the International Conference on Harmonization, 1997. The study protocol, amendments, and informed consent forms were approved by independent ethics committees or institutional review boards for all sites.
Study Design
The primary aims of the study were to assess the antipsychotic efficacy and safety of olanzapine in combination with samidorphan compared with olanzapine plus placebo for the treatment of schizophrenia, including adverse events and weight gain. Secondary goals were 1) to explore the relationship between weight mitigation effects of the olanzapine plus samidorphan combination and the amount of weight gained over the course of 1-week exposure to open-label olanzapine prior to randomization, and 2) to assess whether samidorphan exhibited a dose effect when combined with olanzapine.
The study consisted of a 1-month screening phase, after which patients were switched from their current treatment to receive 1 week of open-label olanzapine. After the olanzapine lead-in phase, patients entered a 12-week double-blind treatment phase (hereafter referred to as the treatment phase) consisting of open-label olanzapine (5–20 mg/day) coadministered with a blinded dose of study medication (samidorphan at 5 mg/day, 10 mg/day, or 20 mg/day, or placebo), followed by a 12-week extension phase as well as a 4-week safety follow-up. The 1-week open-label olanzapine lead-in was utilized to detect early weight gain for subsequent stratification in randomization, to identify the early-weight-gain population, and to test patient toleration of olanzapine. The olanzapine dosage was selected and titrated by study investigators on the basis of patient needs throughout the study and in line with current clinical practice. Patients who completed the olanzapine lead-in were enrolled in the treatment phase and were randomly assigned in a 1:1:1:1 ratio to one of four treatment groups: olanzapine plus samidorphan at 5 mg/day, 10 mg/day, or 20 mg/day or olanzapine plus matched placebo (see Figure S1 in the online supplement). Randomization was stratified by weight change during the 1-week olanzapine lead-in period into three strata: no weight gain, weight gain <1 kg, and weight gain ≥1 kg.
After completing the 12-week double-blind treatment phase, patients transitioned to the extension phase, where they received 12 weeks of active treatment with olanzapine plus samidorphan. Patients who had been assigned to one of the active samidorphan arms continued on the same dosage of samidorphan, and those who had been on placebo started 20 mg/day of samidorphan in addition to olanzapine (see Figure S1). In the extension phase, patients and study personnel were aware that all patients would receive olanzapine plus samidorphan, but they were blind to the dosage that patients received. The transition of patients from placebo to olanzapine plus samidorphan in the extension phase was done in a blinded manner via an interactive web response system.
Patients who exhibited early weight gain were identified at the end of the 1-week olanzapine lead-in period, as it is known that patients who exhibit early weight gain with olanzapine treatment are likely to gain weight overall (18).
Patients
To be included in the study, patients had to be 18–50 years of age; have a diagnosis of schizophrenia (based on DSM-IV-TR criteria); be clinically stable (a score ≤80 on the Positive and Negative Syndrome Scale [PANSS] and a score ≤3 on the Clinical Global Impressions [CGI] severity scale); have maintained a stable body weight for ≥3 months prior to screening (≤5% change by history); and have a body mass index in the range of 17–30 at screening. Patients had not been exposed to olanzapine, clozapine, mesoridazine, chlorpromazine, or thioridazine for more than 1 week within 1 year prior to screening, or at any time in the 3 months before screening. The use or anticipated use of over-the-counter drugs for weight reduction, systemic steroids, or antipsychotic medications, among others, within 60 days before screening was prohibited. Patients were excluded if they were started on their first antipsychotic treatment within the past 12 months or had symptoms lasting <2 years.
Patients who were taking antipsychotic medication at the time of screening were tapered off of that medication within 2 weeks after initiation of the treatment phase. All patients provided written informed consent before entering the study.
Study Assessments
The primary efficacy endpoint was absolute change in PANSS total score from baseline (randomization) to the end of the treatment phase (week 12) for all of the olanzapine plus samidorphan groups compared with the olanzapine plus placebo group. Secondary endpoints included percent change in body weight and the proportion of patients who exhibited significant weight gain (≥7% and ≥10%) from baseline to the end of the treatment phase. Efficacy, safety, and change in body weight were also assessed throughout the 12-week extension phase.
Safety evaluations were carried out over the full study period and included adverse events, clinical laboratory tests (chemistry, hematology, and urinalysis), ECG, vital signs, and physical examination. Blood samples were taken at screening, during the olanzapine lead-in phase, and throughout the active study period for assessment of lipid and glycemic measures.
Statistical Analysis
The efficacy population included all patients who underwent randomized treatment assignment and received at least one dose of study drug and had at least one postbaseline PANSS assessment. Absolute change from baseline to week 12 in PANSS score was analyzed using a two-sided mixed model for repeated measures (MMRM). For the primary analysis, all patients in the olanzapine plus samidorphan treatment group were pooled for comparison with those in the olanzapine plus placebo group, with an equivalence margin of 10 points. Analyses of individual dosage levels were to be performed only if equivalence was demonstrated. Percent change in body weight from baseline to week 12 for the olanzapine plus samidorphan treatment group and the individual olanzapine plus samidorphan treatment groups was also analyzed using an MMRM model. The proportion of patients with significant weight gain (≥7% and ≥10% change from baseline) was analyzed using the Cochran-Mantel-Haenszel method to adjust for the olanzapine lead-in period weight change strata. Descriptive statistics were generated for the change from baseline in PANSS score and weight.
Percent change in body weight and proportion of patients with significant weight gain were evaluated in all patients as well as a subset of patients exhibiting early weight gain (>0 kg). The early-weight-gain analysis population included all patients who gained weight during the 1-week open-label olanzapine lead-in period and had at least one postbaseline weight assessment. Safety was assessed in all patients who underwent randomized treatment assignment and received at least one dose of study drug.
A sample size of 280 patients (70 per treatment group for olanzapine plus placebo and olanzapine plus samidorphan at 5 mg/day, 10 mg/day, and 20 mg/day) was determined to provide 95% power to demonstrate equivalence in change in PANSS score from randomization to week 12 of all patients receiving olanzapine plus samidorphan compared with those receiving olanzapine plus placebo, with an equivalence margin of 10 points.
Results
Baseline Characteristics and Disposition
Overall, 347 patients were enrolled and received at least one dose of olanzapine. Of these, 89% (N=309) completed the 1-week olanzapine lead-in period; the main reasons for discontinuation during the lead-in period were patient decision (N=11; 3.2%) and adverse events (N=3; 0.9%) (Figure 1). After completion of the olanzapine lead-in period, patients were randomly assigned in a 1:1:1:1 ratio to receive olanzapine plus placebo (N=75), olanzapine plus samidorphan at 5 mg/day (N=80), at 10 mg/day (N=86), or at 20 mg/day (N=68). All 309 patients received at least one dose of study drug and were included in the safety analysis. Of these, 300 patients had at least one postbaseline PANSS assessment and were included in the analysis. In total, 195 (65.0%) of the 300 patients experienced early weight gain during the olanzapine lead-in phase and were included in the early-weight-gain analysis. Altogether, 221 patients completed the treatment phase: 165 (70.5%) patients in the olanzapine plus samidorphan group and 56 (74.7%) patients in the olanzapine plus placebo group. The proportion of patients who discontinued early was similar between the olanzapine plus samidorphan and olanzapine plus placebo groups; 29.5% (N=69) and 25.3% (N=19), respectively. The most common reasons for discontinuation were patient decision (olanzapine plus samidorphan, 9.4%; olanzapine plus placebo, 5.3%), adverse events (olanzapine plus samidorphan, 9.0%; olanzapine plus placebo, 4.0%), and lost to follow-up (olanzapine plus samidorphan, 6.4%; olanzapine plus placebo, 12.0%) (Figure 1).
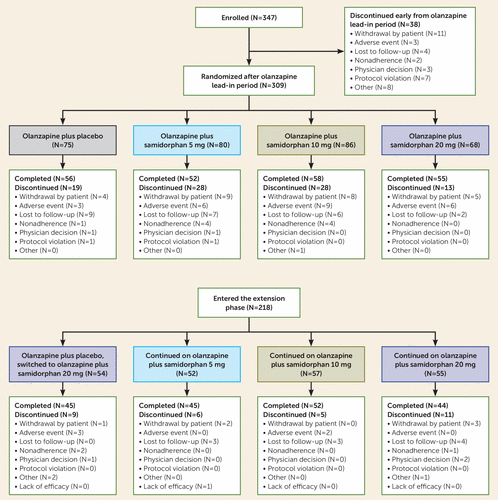
FIGURE 1. Patient disposition in the 12-week treatment and extension phases in a study of olanzapine plus samidorphan compared with olanzapine plus placeboa
a Olanzapine dosages were determined by the investigators and ranged from 5 to 20 mg/day.
Of the patients who completed the treatment phase, 218 (98.6%) enrolled in the extension phase; those who had previously received olanzapine plus placebo were switched to olanzapine plus samidorphan at 20 mg/day (N=54), and patients who had received olanzapine plus samidorphan continued on the dosage they received in the treatment phase (N=164) (Figure 1). Altogether, 85.8% (N=187) of patients completed the extension phase and 14.2% (N=31) discontinued, the majority of whom were lost to follow-up (4.6%).
The baseline demographic and clinical characteristics were similar between the olanzapine plus samidorphan and olanzapine plus placebo groups; 74.8% and 70.7%, respectively, were males; the mean age was 38.4 years and 40.3 years, respectively; and the mean body mass index was 25.2 and 25.1, respectively. The mean baseline PANSS score was 62.8 and 62.7 in the olanzapine plus samidorphan and olanzapine plus placebo group, respectively, and the mean CGI severity score was 2.9 in both groups (see Table S1 in the online supplement).
The average olanzapine dosage was 11.5 mg/day during the treatment phase and was similar across the four treatment groups: 11.1 mg/day, 10.9 mg/day, and 12.1 mg/day for the olanzapine plus samidorphan at 5 mg/day, 10 mg/day, and 20 mg/day groups, respectively, and 11.8 mg/day for the olanzapine plus placebo group.
Efficacy
In the treatment phase, PANSS score was maintained from randomization through week 12 (Figure 2, Table 1). There were no differences in PANSS score between the olanzapine plus samidorphan and olanzapine plus placebo groups. At baseline, the PANSS score for the olanzapine plus samidorphan group was 62.0 (SD=9.7), compared with 62.0 (SD=10.4) for the olanzapine plus placebo group. At week 12, the least square mean of the change from baseline in PANSS score was −2.2 (95% CI=−3.2, −1.3) for the olanzapine plus samidorphan group, compared with −2.9 (95% CI=−4.5, −1.3) for the olanzapine plus placebo group. The least square mean difference between the groups was 0.6 points (95% CI=−1.2, 2.5), within a predefined equivalence margin of 10. The mean PANSS score remained stable from week 12 (the extension study baseline) to week 24; the mean change from week 12 to the end of the extension phase for the olanzapine plus samidorphan treatment group was −1.7 (SD=5.9).
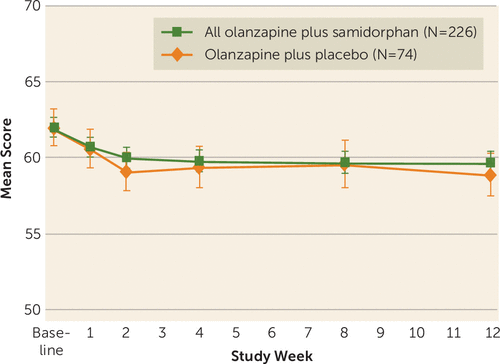
FIGURE 2. Positive and Negative Syndrome Scale scores for patients treated with olanzapine plus samidorphan or with olanzapine plus placebo, by visita
a The graph presents observed data. Error bars indicate standard error. The least square mean difference between groups at week 12 was 0.6 (95% CI=−1.2, 2.5). Table 1 presents mean values and least square mean change and difference relative to olanzapine plus placebo.
| Measure | Olanzapine Plus Placebo (N=74) | Olanzapine Plus Samidorphan 5 mg (N=75) | Olanzapine Plus Samidorphan 10 mg (N=83) | Olanzapine Plus Samidorphan 20 mg (N=68) | All Olanzapine Plus Samidorphan(N=226) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Baseline score | 62.0 | 10.4 | 60.9 | 10.0 | 62.3 | 9.6 | 62.9 | 9.4 | 62.0 | 9.7 |
| LSM | 95% CI | LSM | 95% CI | LSM | 95% CI | LSM | 95% CI | LSM | 95% CI | |
| Change from baseline to week 12 | −2.9 | −4.5, −1.3 | −1.5 | −3.2, 0.1 | −2.7 | −4.2, −1.1 | −2.5 | −4.2, −0.9 | −2.2 | −3.2, −1.3 |
| Difference compared with olanzapine plus placebo | 1.3 | −1.0, 3.6 | 0.2 | −2.0, 2.5 | 0.3 | −2.0, 2.6 | 0.6 | −1.2, 2.5 | ||
TABLE 1. Total score and change from baseline on the Positive and Negative Syndrome Scale for patients treated with olanzapine plus samidorphan or with olanzapine plus placeboa
Weight Changes
During the treatment phase, the mean percent change in body weight at week 12 was 37% lower in the olanzapine plus samidorphan group (least square mean percent change from baseline to week 12, 2.6%; absolute change, 1.9 kg) compared with patients in the olanzapine plus placebo group (4.1% and 2.9 kg). The least square mean difference between groups was −1.5% (95% CI=−2.5, −0.4, p=0.006) and −1.0 kg (95% CI=−1.8, −0.2, p=0.018) (Figure 3, Table 2).
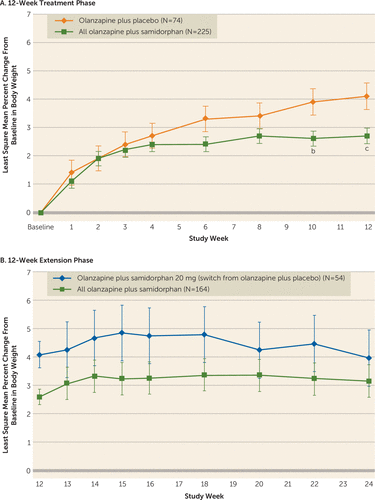
FIGURE 3. Change in body weight from baseline for patients treated with olanzapine plus samidorphan or with olanzapine plus placebo, by visit, during the 12-week treatment and extension phasesa
a Error bars indicate standard error. Table 2 presents mean values and least square mean change and difference relative to olanzapine plus placebo.
b p<0.05 compared with olanzapine plus placebo.
c p<0.01 compared with olanzapine plus placebo.
| Treatment Phaseb | Extension Phasec | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Weight (kg) | Percent Change From Baseline to Week 12 | Difference Versus Olanzapine Plus Placebo | Baseline Weight (kg) | Percent Change From Baseline to Week 25 | |||||||||
| Group | N | Mean | SD | LSM | 95% CI | LSM | 95% CI | p | N | Mean | SD | Mean | SD |
| Olanzapine plus placebo | 74 | 76.0 | 12.4 | 4.1 | 3.2, 5.0 | 54 | 78.8 | 13.3 | 0.1 | 3.6 | |||
| Olanzapine plus samidorphan 5 mg | 75 | 78.3 | 13.9 | 2.8 | 1.8, 3.7 | −1.3 | −2.6, −0.0 | 0.049 | 52 | 79.4 | 14.7 | −0.1 | 3.6 |
| Olanzapine plus samidorphan 10 mg | 83 | 77.4 | 13.6 | 2.1 | 1.3, 3.0 | −1.9 | −3.2, −0.7 | 0.003 | 57 | 78.6 | 14.4 | 1.2 | 4.7 |
| Olanzapine plus samidorphan 20 mg | 67 | 75.8 | 12.7 | 2.9 | 1.9, 3.8 | −1.2 | −2.5, 0.1 | 0.072 | 54 | 77.9 | 13.9 | 0.2 | 3.6 |
| All olanzapine plus samidorphan | 225 | 77.2 | 13.4 | 2.6 | 2.1, 3.1 | −1.5 | −2.5, −0.4 | 0.006 | 163 | 78.6 | 14.3 | 0.5 | 4.1 |
TABLE 2. Change in body weight from baseline for patients treated with olanzapine plus samidorphan or with olanzapine plus placebo during the 12-week treatment and extension phasesa
The risk of patients gaining ≥10% of baseline body weight was 2.7 times greater for the olanzapine plus placebo group compared with the olanzapine plus samidorphan group (odds ratio=2.73, 95% CI=1.11, 6.67, p=0.023). The risk of patients gaining ≥7% of baseline body weight was 1.6 times greater for the olanzapine plus placebo group compared with the olanzapine plus samidorphan group, but the difference was not statistically significant (odds ratio=1.56, 95% CI=0.76, 3.20, p=0.227) (Figure 4).
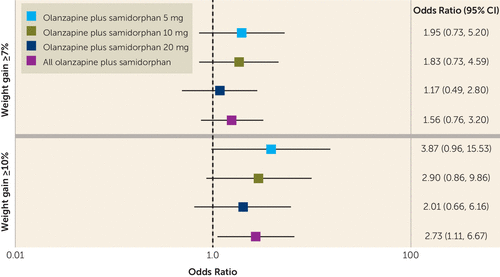
FIGURE 4. Proportion of patients exhibiting significant weight gain by week 12 in a study of olanzapine plus samidorphan compared with olanzapine plus placeboa
a The figure reports odds ratios for the olanzapine plus placebo group compared with the olanzapine plus samidorphan groups.
During the extension phase, the mean percent change in body weight remained stable and was similar across all olanzapine plus samidorphan dosages. There was a 0.5% (SD=4.1) increase for patients who received continuous olanzapine plus samidorphan treatment, compared with a 0.1% (SD=3.6) increase for those who were switched from olanzapine plus placebo to olanzapine plus samidorphan at 20 mg/day (Figure 3).
Analysis of individual dosage groups.
Patients who were treated with olanzapine plus samidorphan at 5 mg/day (least square mean: 2.8%; least square mean difference compared with olanzapine plus placebo: −1.3, 95% CI=−2.6, −0.0, p=0.049) and at 10 mg/day (least square mean: 2.1%; least square mean difference compared with olanzapine plus placebo: −1.9, 95% CI=−3.2, −0.7, p=0.003) had a significantly lower percent change in weight from randomization to week 12 compared with the olanzapine plus placebo group (least square mean: 4.1%) (Figure 3). Although the change in weight for patients in the group receiving olanzapine plus samidorphan at 20 mg/day was lower, the difference was not statistically significant compared with the olanzapine plus placebo group (least square mean: 2.9%; least square mean difference: −1.2%, 95% CI=−2.5, 0.1, p=0.072).
Analysis of olanzapine dosage and body weight.
A nonsignificant dose-dependent increase in weight was observed in olanzapine-treated patients, with a mean percent change in body weight at week 12 of 3.3%, 4.1%, and 4.8% for those with mean daily olanzapine doses <10 mg, ≥10–<15 mg, and ≥15 mg, respectively. There was not a dose-dependent effect of olanzapine when given with samidorphan, with changes in body weight at week 12 of 2.6%, 2.6%, and 0.4% for those with mean daily olanzapine doses <10 mg, ≥10–<15 mg, and ≥15 mg, respectively.
Patients exhibiting early weight gain.
Altogether, 195 of 300 (65.0%) patients had early weight gain during the olanzapine lead-in phase and were included in the early-weight-gain analysis.
During the treatment phase, the mean percent change in body weight at week 12 was 51% lower in the olanzapine plus samidorphan group (least square mean percent and absolute change from baseline to week 12, 2.6% and 1.9 kg, respectively) compared with patients in the olanzapine plus placebo group (least square mean change from baseline to week 12, 5.3% and 3.8 kg). The least square mean difference between the olanzapine plus samidorphan and olanzapine plus placebo groups was −2.7% (95% CI=−4.0, −1.4, p<0.001) and −1.9 kg (95% CI=−2.9, −0.9, p<0.001), respectively.
The risk of patients gaining ≥10% of baseline body weight was 4.1 times greater (odds ratio=4.10, 95% CI=1.4, 12.3, p=0.008) for the olanzapine plus placebo group compared with the olanzapine plus samidorphan group. The risk of patients gaining ≥7% of baseline body weight was 2.2 times greater for the olanzapine plus placebo group compared with the olanzapine plus samidorphan group, but the difference was not statistically significant (odds ratio=2.21, 95% CI=0.9–5.3, p=0.07).
During the extension phase, in the early-weight-gain population there was a slight mean percent increase in body weight (0.2%) in both the olanzapine plus samidorphan continuous treatment group and the group switched from olanzapine plus placebo to olanzapine plus samidorphan at 20 mg/day.
Safety
Adverse events.
No deaths, suicides, or suicide attempts were reported during the conduct of the trial.
Overall, during the olanzapine lead-in phase, 57 patients (16.4%) experienced an adverse event. One serious adverse event of worsening of schizophrenia symptoms was reported, which led to study drug discontinuation.
During the treatment phase, 54.3% (N=127) and 54.7% (N=41) of patients in the olanzapine plus samidorphan and olanzapine plus placebo groups, respectively, reported adverse events; most were mild to moderate in severity (Table 3). Common adverse events that were reported at a rate ≥5% in any of the olanzapine plus samidorphan groups and that occurred at a rate of at least twofold greater than in the olanzapine plus placebo group were somnolence, sedation, dizziness, and constipation (Table 3). Serious adverse events were reported in 13 patients: two patients (2.7%) in the olanzapine plus placebo group and 11 (4.7%) in the olanzapine plus samidorphan groups. A total of 24 patients discontinued the study because of adverse events: three patients (4.0%) in the olanzapine plus placebo group and 21 (9.0%) in the olanzapine plus samidorphan group.
| Olanzapine Plus Placebo (N=75) | Olanzapine Plus Samidorphan 5 mg (N=80) | Olanzapine Plus Samidorphan 10 mg (N=86) | Olanzapine Plus Samidorphan 20 mg (N=68) | All Olanzapine Plus Samidorphan (N=234) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | N | % | N | % | N | % | N | % | N | % |
| Any adverse event | 41 | 54.7 | 38 | 47.5 | 46 | 53.5 | 43 | 63.2 | 127 | 54.3 |
| Adverse event by highest severity | ||||||||||
| Mild | 22 | 29.3 | 23 | 28.8 | 31 | 36.0 | 28 | 41.2 | 82 | 35.0 |
| Moderate | 17 | 22.7 | 13 | 16.3 | 13 | 15.1 | 13 | 19.1 | 39 | 16.7 |
| Severe | 2 | 2.7 | 2 | 2.5 | 2 | 2.3 | 2 | 2.9 | 6 | 2.6 |
| Drug-related adverse events | ||||||||||
| Related to samidorphan/placebo or olanzapine | 30 | 40.0 | 28 | 35.0 | 36 | 41.9 | 35 | 51.5 | 99 | 42.3 |
| Related to samidorphan/placebo | 20 | 26.7 | 20 | 25.0 | 27 | 31.4 | 26 | 38.2 | 73 | 31.2 |
| Related to olanzapine | 26 | 34.7 | 25 | 31.3 | 27 | 31.4 | 30 | 44.1 | 82 | 35.0 |
| Deaths | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Any serious adverse event | 2 | 2.7 | 3 | 3.8 | 4 | 4.7 | 4 | 5.9 | 11 | 4.7 |
| Adverse events leading to study discontinuation | 3 | 4.0 | 6 | 7.5 | 9 | 10.5 | 6 | 8.8 | 21 | 9.0 |
| Nervous system disorders | ||||||||||
| Somnolence | 3 | 4.0 | 10 | 12.5 | 11 | 12.8 | 8 | 11.8 | 29 | 12.4 |
| Sedation | 3 | 4.0 | 0 | 0.0 | 4 | 4.7 | 8 | 11.8 | 12 | 5.1 |
| Dizziness | 1 | 1.3 | 0 | 0.0 | 3 | 3.5 | 6 | 8.8 | 9 | 3.8 |
| Headache | 4 | 5.3 | 3 | 3.8 | 1 | 1.2 | 1 | 1.5 | 5 | 2.1 |
| Investigations | ||||||||||
| Increased weight | 9 | 12.0 | 8 | 10.0 | 7 | 8.1 | 6 | 8.8 | 21 | 9.0 |
| Metabolism and nutrition disorders | ||||||||||
| Increased appetite | 6 | 8.0 | 5 | 6.3 | 5 | 5.8 | 6 | 8.8 | 16 | 6.8 |
| Gastrointestinal disorders | ||||||||||
| Nausea | 4 | 5.3 | 5 | 6.3 | 4 | 4.7 | 5 | 7.4 | 14 | 6.0 |
| Dry mouth | 4 | 5.3 | 2 | 2.5 | 5 | 5.8 | 6 | 8.8 | 13 | 5.6 |
| Constipation | 1 | 1.3 | 0 | 0.0 | 5 | 5.8 | 2 | 2.9 | 7 | 3.0 |
| Psychiatric disorders | ||||||||||
| Insomnia | 4 | 5.3 | 2 | 2.5 | 2 | 2.3 | 1 | 1.5 | 5 | 2.1 |
TABLE 3. Patients with common adverse events during the 12-week treatment phase in a study of olanzapine plus samidorphan compared with olanzapine plus placebo (safety population)a
In the extension phase, 40.9% (N=67) of patients had adverse events, and serious adverse events were reported in three patients (none were considered to be related to study drug). Five patients had adverse events that led to study discontinuation: two in the olanzapine plus samidorphan group and three in the olanzapine plus placebo group who switched to olanzapine plus samidorphan at 20 mg/day in the extension phase.
Somnolence, sedation, dizziness, and constipation occurred more frequently in patients receiving samidorphan, and no clinically significant trends were observed for other adverse events, laboratory values, vital signs, or ECG results in any of the treatment arms.
Analysis of individual dosage groups.
During the treatment phase, patients who received olanzapine plus samidorphan at 5 mg/day (N=38; 47.5%) experienced fewer adverse events, those who received olanzapine plus samidorphan at 10 mg/day (N=46; 53.5%) experienced a similar number of adverse events, and those who received olanzapine plus samidorphan at 20 mg/day (N=43; 63.2%) experienced a greater number of adverse events compared with patients who received olanzapine plus placebo (N=41; 54.7%). Table 3 provides a breakdown of adverse events by samidorphan dosage.
Metabolic parameters.
Mean changes in lipid and glycemic parameters from baseline to the last postbaseline assessment were highly variable across all treatment groups (Table 4). It should be noted that fasting status was not confirmed before sample collection.
| Olanzapine Plus Placebo (N=75) | Olanzapine Plus Samidorphan 5 mg (N=80) | Olanzapine Plus Samidorphan 10 mg (N=86) | Olanzapine Plus Samidorphan 20 mg (N=68) | All Olanzapine Plus Samidorphan (N=234) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Cholesterol (mg/dL) | ||||||||||
| Baseline | 174.6 | 30.5 | 177.3 | 34.1 | 175.3 | 29.3 | 170.5 | 31.1 | 174.6 | 31.5 |
| Change from baseline | 8.9 | 26.8 | 3.3 | 29.2 | 3.1 | 29.3 | 5.0 | 24.6 | 3.8 | 27.9 |
| Total cholesterol, fasting (mg/dL) | ||||||||||
| Baseline | 174.6 | 30.1 | 176.4 | 34.7 | 175.1 | 29.5 | 170.0 | 31.7 | 174.1 | 32.0 |
| Change from baseline | 8.5 | 27.4 | 5.1 | 27.2 | 3.5 | 29.5 | 4.6 | 24.8 | 4.4 | 27.3 |
| LDL cholesterol, fasting (mg/dL) | ||||||||||
| Baseline | 103.1 | 24.7 | 106.5 | 33.0 | 105.3 | 31.1 | 99.0 | 26.2 | 103.9 | 30.5 |
| Change from baseline | 11.5 | 21.9 | 5.8 | 23.1 | 6.5 | 20.0 | 10.3 | 20.9 | 7.4 | 21.3 |
| HDL cholesterol (mg/dL) | ||||||||||
| Baseline | 53.5 | 14.7 | 52.2 | 16.2 | 52.6 | 17.7 | 50.1 | 14.9 | 51.7 | 16.4 |
| Change from baseline | –3.2 | 9.4 | –2.0 | 9.1 | –1.6 | 12.9 | –2.9 | 7.3 | –2.1 | 10.3 |
| Triglycerides, fasting (mg/dL) | ||||||||||
| Baseline | 119.4 | 72.7 | 114.9 | 68.8 | 120.3 | 90.3 | 123.4 | 90.4 | 119.4 | 83.3 |
| Change from baseline | 6.5 | 75.6 | 20.4 | 60.5 | –6.2 | 95.5 | –0.7 | 78.3 | 4.0 | 80.9 |
| Glucose (mg/dL) | ||||||||||
| Baseline | 92.6 | 13.3 | 89.9 | 12.5 | 92.2 | 16.0 | 90.9 | 10.7 | 91.0 | 13.4 |
| Change from baseline | 4.1 | 20.2 | 3.8 | 21.0 | 7.0 | 34.1 | 5.2 | 17.4 | 5.4 | 25.8 |
| Insulin (µIU/mL) | ||||||||||
| Baseline | 17.1 | 24.2 | 16.6 | 27.7 | 16.8 | 18.4 | 16.4 | 41.8 | 16.6 | 29.8 |
| Change from baseline | 10.8 | 52.1 | 10.6 | 48.8 | 0.08 | 23.9 | 4.7 | 47.1 | 5.0 | 40.8 |
TABLE 4. Change from baseline in metabolic parameters in the 12-week treatment phase in a study of olanzapine plus samidorphan compared with olanzapine plus placebo (safety population)a
Discussion
Treatment with olanzapine plus samidorphan resulted in antipsychotic efficacy equivalent to olanzapine plus placebo, as assessed by PANSS total score from baseline to week 12 within a predefined equivalence margin of 10. The effect of olanzapine plus samidorphan in mitigating olanzapine-induced weight gain was observed through multiple endpoints, including percent change in body weight from baseline and the proportion of patients who gained ≥10% of baseline body weight (representative of a clinically meaningful weight gain). Additionally, there was an early and sustained stabilizing effect of olanzapine plus samidorphan on body weight. Overall, there was less weight gain with olanzapine plus samidorphan treatment than with olanzapine plus placebo, and the differences were statistically significant. A larger treatment effect was seen in patients who experienced early weight gain. After 12 weeks of treatment with olanzapine plus samidorphan, in the full study population there was 37% less weight gain from baseline compared with the olanzapine plus placebo group, and patients who gained weight during the olanzapine lead-in phase (early weight gain) had 51% less weight gain from baseline compared with the olanzapine plus placebo group. Consistent with these observations, patients in the olanzapine plus placebo group were 2.7-fold more likely to gain ≥10% of baseline weight than those in the olanzapine plus samidorphan group, and patients in the olanzapine plus placebo group who experienced early weight gain were 4.1-fold more likely to gain ≥10% of baseline weight than those in the olanzapine plus samidorphan group.
Antipsychotic-induced weight gain generally has a rapid onset and can occur in the first few weeks of treatment (19, 20)—an effect that was seen in all treatment groups during the first 2 weeks of this study. Thus, the addition of samidorphan mitigates olanzapine-associated weight gain but does not completely prevent it. Rather, treatment with olanzapine plus samidorphan changed the trajectory of weight gain over the remainder of the study period: patients in the olanzapine plus placebo group continued to gain weight throughout the treatment phase, whereas treatment with olanzapine plus samidorphan mitigated further weight gain. In addition, during the extension phase, treatment with olanzapine plus samidorphan was not associated with continued weight gain in patients who were switched from olanzapine plus placebo to olanzapine plus samidorphan at 20 mg/day, and the trend of weight gain from the first 12-week treatment phase when receiving olanzapine plus placebo was reversed in these patients. Of note, when examining the effects of potential weight-mitigating pharmacotherapies, efficacy has been more pronounced when testing begins before the onset of weight gain (21, 22). Therefore, we focused on testing the prevention of weight gain and have designed our ongoing clinical studies to evaluate this approach, as opposed to treatment of established weight gain or obesity associated with antipsychotics. The mitigation of weight gain by samidorphan appears to be specific to weight gain associated with use of olanzapine, as samidorphan was not found to be associated with significant weight changes in monotherapy in patients with a binge-eating disorder (23), in patients with alcohol dependence (24), or in healthy volunteers (17). Somnolence, sedation, dizziness, and constipation occurred more frequently in patients receiving samidorphan. Other safety measures for olanzapine plus samidorphan were comparable to olanzapine plus placebo.
This study has informed dosage selection for further study. Olanzapine plus samidorphan at 10 mg/day was seen to be an effective dosage, with a weight gain pattern similar to the 20 mg/day dosage and superior to the 5 mg/day dosage. As overall adverse events were higher at 20 mg/day, olanzapine plus samidorphan at 10 mg/day was selected as the dosage to advance into phase 3 testing. Administration of olanzapine plus samidorphan at 10 mg/day resulted in greater effects on antipsychotic efficacy and mitigation of weight gain compared with olanzapine plus samidorphan at 5 mg/day. Furthermore, olanzapine plus samidorphan at 20 mg/day did not confer additional benefit (compared with olanzapine plus samidorphan at 10 mg/day) and had a higher rate of adverse events compared with the other dosages; hence, the combination of olanzapine and samidorphan at 10 mg/day was selected for further investigation in phase 3 studies.
The use of atypical antipsychotics in clinical practice has improved outcomes for patients with schizophrenia; however, extrapyramidal symptoms, weight gain, and metabolic syndrome continue to be significant concerns. High rates of obesity, cardiovascular disease, and type 2 diabetes are seen in patients with schizophrenia (25) and contribute to poor quality of life and high morbidity and mortality in this patient population (26). Additionally, metabolic effects of antipsychotics pose a significant challenge because of their negative impact on long-term treatment adherence, which leads to poorer psychiatric treatment outcomes (27).
Although several mechanisms of metabolic syndrome have been extensively explored both centrally and peripherally, weight gain and metabolic dysfunction associated with antipsychotic use are not fully understood (7, 19, 28, 29). Several potential new treatment options, such as metformin (30–32), topiramate (33), and mifepristone (34, 35), have been examined for their ability to mitigate antipsychotic-induced weight gain (36). Of these potential treatments, a common limitation is that patients have gained a significant amount of weight and/or have developed metabolic dysfunction prior to treatment initiation. The combination of olanzapine and samidorphan assessed in this study represents a new mechanistic approach toward addressing olanzapine-induced weight gain, whereby treatment is initiated before significant weight gain and metabolic dysfunction have occurred.
One limitation of the study is that the design included a 1-week open-label olanzapine lead-in period, which was incorporated to identify patients who could not tolerate olanzapine and with the aim of enhancing the ability to detect weight gain, because it is known that patients who exhibit early weight gain with olanzapine treatment are likely to gain weight overall (18). Thus, it is possible that the effects of samidorphan were masked because of the olanzapine lead-in phase, as metabolic effects of olanzapine are robust and occur within a few days (37) and even after one dose (38). An additional limitation is that only patients with stable symptoms were included, and therefore efficacy (assessed by the PANSS) was not fully explored. In addition, the inclusion of potentially nonfasting blood samples makes interpretation of the metabolic parameters difficult. Analysis was based on the collection of precise repeated measures of weight that may be difficult to replicate in non-research settings. Likewise, because of the small subsample size of patients experiencing ≥7% and ≥10% body weight gain, the statistical separation between olanzapine plus samidorphan and olanzapine plus placebo should be interpreted with caution, as this study was not powered to look for these differences in categorical weight gain. Another potential limitation of the study is that the dosage of olanzapine was not predefined throughout the study but was selected and titrated by the investigators on the basis of individual patient needs and clinical condition. The sample was predominantly male and did not include patients with acute psychosis, who constitute an essential population in the treatment and management of schizophrenia. Lastly, the trial duration was relatively short compared with the time course of olanzapine-induced weight gain.
Conclusions
The antipsychotic efficacy of olanzapine plus samidorphan was comparable to that of olanzapine plus placebo, but with clinically meaningful and statistically significant mitigation of weight gain. A consistent and durable mitigation of olanzapine-induced weight gain was observed in patients who were switched from olanzapine plus placebo to olanzapine plus samidorphan. Treatment with the combination of olanzapine and samidorphan was generally well tolerated, and its adverse event profile was similar to that of olanzapine plus placebo, but with higher rates of somnolence, sedation, dizziness, and constipation. The findings from this study identified 10 mg of samidorphan as the daily dose to further assess the mitigation of olanzapine-induced weight gain, and they support the continued development of olanzapine plus samidorphan in a phase 3 program.
1 : Effectiveness and tolerability of schizophrenia treatment in Central and Eastern Europe: results after 1 year from a prospective, observational study (IC-SOHO). Int J Psychiatry Clin Pract 2006; 10:78–90Crossref, Medline, Google Scholar
2 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223Crossref, Medline, Google Scholar
3 : Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008; 371:1085–1097Crossref, Medline, Google Scholar
4 : Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 1996; 14:111–123Crossref, Medline, Google Scholar
5 : Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology (Berl) 1996; 124:159–167Crossref, Medline, Google Scholar
6 : A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol 2003; 23:582–594Crossref, Medline, Google Scholar
7 : Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011; 8:114–126Crossref, Medline, Google Scholar
8 : The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull 2010; 36:71–93Crossref, Medline, Google Scholar
9 Lilly E: Zyprexa (Olanzapine) US Prescribing Information. 2017. http://pi.lilly.com/us/zyprexa-pi.pdfGoogle Scholar
10 : Mice lacking δ-opioid receptors resist the development of diet-induced obesity. FASEB J 2012; 26:3483–3492Crossref, Medline, Google Scholar
11 : Kappa-opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J 2010; 24:1151–1159Crossref, Medline, Google Scholar
12 : Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 2005; 54:3510–3516Crossref, Medline, Google Scholar
13 : Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent mu-receptor antagonist. J Clin Psychopharmacol 2015; 35:242–249Crossref, Medline, Google Scholar
14 : Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett 2009; 19:2289–2294Crossref, Medline, Google Scholar
15 : In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment for major depressive disorder. J Pharmacol Exp Ther 2018; 367:267–281Crossref, Medline, Google Scholar
16 Todtenkopf MS, O’Neill KS, Kelly SM, et al: The novel opioid receptor modulator RDC-0313 (ALKS 33) reduces olanzapine-induced weight gain in female rats. Poster presented at the 40th annual meeting of the Society for Neuroscience, San Diego, November 16, 2010Google Scholar
17 : A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res 2018; 195:245–251Crossref, Medline, Google Scholar
18 : Early evaluation of patient risk for substantial weight gain during olanzapine treatment for schizophrenia, schizophreniform, or schizoaffective disorder. BMC Psychiatry 2008; 8:78Crossref, Medline, Google Scholar
19 : Antipsychotic drugs and obesity. Trends Mol Med 2011; 17:97–107Crossref, Medline, Google Scholar
20 : Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol 2005; 25:255–258Crossref, Medline, Google Scholar
21 : Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis. Br J Clin Pharmacol 2011; 71:377–382Crossref, Medline, Google Scholar
22 : Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry 2008; 165:352–358Link, Google Scholar
23 : A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int J Eat Disord 2013; 46:239–245Crossref, Medline, Google Scholar
24 : Effects of the opioid system modulator, samidorphan, on measures of alcohol consumption and patient-reported outcomes in adults with alcohol dependence. Alcohol Clin Exp Res 2018; 42:2011–2021Crossref, Medline, Google Scholar
25 : Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86:15–22Crossref, Medline, Google Scholar
26 : Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J Clin Psychiatry 2009; 70(suppl 3):5–11Crossref, Medline, Google Scholar
27 : The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009; 70(suppl 4):1–46Crossref, Medline, Google Scholar
28 : Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19(suppl 1):1–93Crossref, Medline, Google Scholar
29 : Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 2010; 17:460–466Crossref, Medline, Google Scholar
30 : A randomized, placebo-controlled trial of metformin for the treatment of overweight induced by antipsychotic medication in young people with autism spectrum disorder: open-label extension. J Am Acad Child Adolesc Psychiatry 2017; 56:849–856.e6Crossref, Medline, Google Scholar
31 : Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiatry 2016; 21:1537–1544Crossref, Medline, Google Scholar
32 : Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol 2015; 35:499–509Crossref, Medline, Google Scholar
33 : Efficacy and safety of adjunctive topiramate for schizophrenia: a meta-analysis of randomized controlled trials. Acta Psychiatr Scand 2016; 134:385–398Crossref, Medline, Google Scholar
34 : The efficacy of mifepristone in the reduction and prevention of olanzapine-induced weight gain in rats. Behav Brain Res 2006; 171:225–229Crossref, Medline, Google Scholar
35 : Mifepristone treatment of olanzapine-induced weight gain in healthy men. Adv Ther 2009; 26:959–969Crossref, Medline, Google Scholar
36 : Efficacy of adjunctive treatments added to olanzapine or clozapine for weight control in patients with schizophrenia: a systematic review and meta-analysis. Sci World J 2015; 2015:970730Crossref, Google Scholar
37 : Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol 2008; 28:601–607Crossref, Medline, Google Scholar
38 : Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002; 59:337–345Crossref, Medline, Google Scholar



