Perinatal Maternal Depressive Symptoms as an Issue for Population Health
Abstract
The importance of maternal depression for child outcomes is well established, and impairments in psychosocial function and parenting are as severe in women with high subsyndromal levels of depressive symptoms as they are in women with clinical depression. The author conducted a systematic review that explored the association between maternal depressive symptoms and child neurodevelopmental outcomes, including in neuroimaging studies. The results strongly suggest that the influences of maternal depressive symptoms operate across a continuum to influence child outcomes, implying that maternal depression may appropriately be considered an issue of population health. This conclusion is strengthened by recent findings that reveal distinct influences of positive maternal mental health on parenting and child outcomes.
[AJP AT 175: Remembering Our Past As We Envision Our Future
April 1851: Fleetwood Churchill, “On the Mental Disorders of Pregnancy and Childbed”
“Women affected with any degree of mental derangement during pregnancy are more disposed than others to puerperal mania. But the serious character of these attacks is even deepened by the fact, abundantly established, that the evil is not limited to the mother. Not only may organic diseases of the body be transmitted to the infant, but a predisposition to insanity, thus multiplying the distress in a most alarming ratio.” (Am J Psychiatry 1851; 7:297–317)]
Clinicians in obstetrics, neonatology, and pediatrics are increasingly sensitive to the importance of maternal mental health as a major influence on the development and health of the offspring. The World Health Organization (http://www.who.int/pmnch), the International Monetary Fund (http://www.imf.org/external/np/exr/facts/mdg.htm), and other international organizations position maternal depression as an issue of global significance. A report from the London School of Economics (LSE) (1) estimated the annual costs of maternal mental health problems in the United Kingdom at 8.1 billion pounds. In addition to the suffering of affected women, peripartum maternal depression influences a wide range of outcomes for offspring, including nutritional status, infant growth, physical health (including common pediatric autoimmune disorders), executive functions, socioemotional development, and academic achievement, as well as, of course, the risk for psychopathology (2–9). The economic importance of these findings is reflected in the LSE study (1), which estimated that 72% of the costs associated with maternal mental health problems derive from services for the child. This estimate does not include the diminished human capital as a result of poorer offspring academic achievement (8), which greatly increases the cost to society.
Maternal depression is amenable to treatment and thus is a modifiable risk factor for poor child outcomes (10–12). Successful treatment of maternal depression reduces the risk for childhood behavioral problems (12). However, effective public health policy demands a full understanding of the impact of maternal mental health for both the mother and the child. Public health analyses of maternal mental health largely focus on women with clinical levels of depressive symptoms. Likewise, routine screening practices and clinical services commonly focus on women with “probable” clinical depression based on validated cutoffs with tools such as the Edinburgh Postnatal Depression Scale (EPDS) (13) and the Center for Epidemiologic Studies Depression Scale (CES-D) (14). About 10%–20% of women in economically developed countries score above clinical cutoffs, depending on the region and socioeconomic status (15–17), with even higher rates among certain middle- and low-income countries where risk factors abound (3, 15).
Screening for maternal depression is without question an important clinical practice. Services concentrate on probable cases of maternal depression, and the use of cutoffs is understandable, since EPDS and CES-D scores predict severity. While these efforts represent an important advance for women and their children, there is compelling evidence for the idea that the consequences of maternal mental health for maternal psychosocial function as well as child health and capacity are not limited to the offspring of clinically depressed mothers. This point is well established with respect to maternal psychosocial function (18, 19) and parenting (20).
This review emphasizes more recent studies of child neurodevelopmental outcomes, including neuroimaging studies, that report effects of maternal depressive symptoms examined as a continuous variable and imply that the influence of maternal depressive symptoms operates across a continuum and is an issue for population health. This point is buttressed by findings of associations of positive maternal mood states with both parenting and child outcomes independent of symptoms of depression or anxiety.
Depressive Symptoms And Maternal Psychosocial Function
A study of the National Institute of Mental Health’s Epidemiologic Catchment Area Program (18) revealed that levels of household strain, social irritability, and financial strain as well as limitations in physical or job functioning, restricted activity days, bed days, and poor health status were significantly higher in individuals with clinical levels of depressive symptoms than in those with no disorder. With the exception of the health status ratings, individuals with high subsyndromal levels of depressive symptoms did not differ significantly from those with clinical depression. Impairments of psychosocial function among individuals in the general population with high subsyndromal levels of depressive symptoms are comparable to those of individuals with clinical depression. The scenario is comparable for peripartum women. Weinberg et al. (21) documented the functioning of mothers at 3 months postpartum and found that, compared with mothers with no depression, mothers with high subclinical levels of symptoms showed significantly more negative and less positive affect, poorer maternal self-esteem and adaptation, and more anxiety, and indeed, they did not differ significantly on these measures from clinically depressed mothers or nondepressed mothers with a previous history of depression. Likewise, Goodman and Tully (19) found that levels of anxiety and stress and of distress related to interpersonal and emotional function were comparable between mothers with clinical depression and those with high subclinical levels of depressive symptoms.
These findings reveal an impressive impact of high subclinical levels of depressive symptoms on maternal health and function. The importance of these findings for public health are apparent given that while 10%−15% of mothers reveal clinical depression, a substantially greater proportion experience high subsyndromal levels of depressive symptoms (Figure 1). These data reinforce the idea that subclinical depression is a significant public health issue because of its prevalence in the population and because individuals with subsyndromal symptoms are both less likely to seek treatment and to be eligible for clinical services, as they do not meet cutoffs for “probable” depression on routine screening assessments (19).
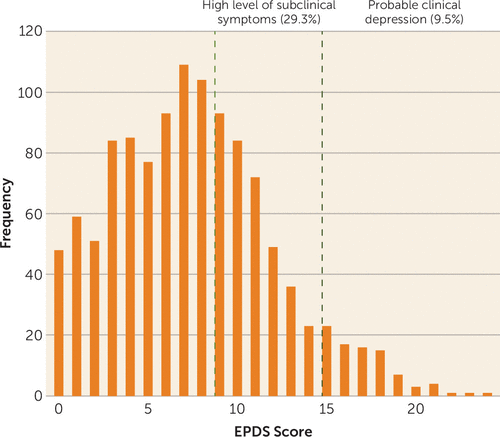
FIGURE 1. Distribution of Maternal Scores on the Edinburgh Postnatal Depression Scale (EPDS) in the Growing Up in Singapore Towards Healthy Outcomes Cohort (N=1,143) at 26 Weeks of Gestationa
a Probable clinical depression and high subclinical levels of depressive symptoms are based on established cutoffs (scores of 15 or more and of 9 to 13, respectively. The mean score was 7.5 (SD=4.5). The estimated portion of “probable cases” in this study is comparable to those of studies in North American and Western Europe.
Maternal Depressive Symptoms And Parenting
The influence of depression on maternal psychosocial function includes effects on parenting, which are considered to be a mechanism for the intergenerational transmission of the risk for later depression in the offspring (22). Symptoms of depression are associated with socioemotional problems that create difficulties in parenting (23), especially with younger children (20). The influence of maternal depressive symptoms extends to infant care and includes effects on rates of breastfeeding and quality of nutrition provided to the child (2). An extensive meta-analysis (20) revealed that the strength of the association between depression and parenting behavior did not differ between studies in which participants were clinically diagnosed with depression and community samples that used self-report scales of depressive symptoms. The author concluded that the results “suggest that the parenting difficulties associated with depressive disorder may not be specific to depressive syndrome and may characterize the difficulties of mothers with sub-syndromal affective conditions” (20, p. 585). This conclusion is consistent with the evidence for comparable interpersonal and socioemotional difficulties in mothers with clinical and subsyndromal levels of depressive symptoms. Moreover, personality factors such as neuroticism, suspicion, greater emotional reactivity, and inattention are correlated with increased levels of depressive symptoms and are also associated with a poorer quality of maternal care (24). The influence of maternal depressive symptoms on parenting is thus not unique to clinical cases of depression but rather operates across a continuum to influence the neurodevelopment of the offspring.
Maternal Depressive Symptoms And Offspring Neurodevelopment
Literature Review
There is compelling evidence for the intergenerational effect of depression: offspring of mothers with a history of depression are at significantly greater risk for depression and other forms of psychopathology than are offspring of never-depressed mothers (9). Studies of twin children and their parents (25) as well as studies of children conceived through in vitro fertilization and their parents (26) underscore the importance of environmental factors for the intergenerational transmission of depression, with evidence for the importance of depression-related effects on parenting as a mediational mechanism (22).
The broad relation between maternal depressive symptoms and psychosocial function, including parenting, suggests a continuous effect of maternal depressive symptoms on child neurodevelopmental outcomes. This issue was examined through a systematic review of the relevant literature, based on a search of MEDLINE on Ovid (1996 to December 2017) for studies with human subjects, published in English, using the following keywords: child, child development, depression, development, maternal, maternal depression. The resulting 124 articles were scanned for evidence of the use of maternal depressive symptom scores as a continuous variable, thus allowing for analysis of effects of symptoms on child outcomes across a continuum. (The extracted articles are summarized in an annotated bibliography in the online supplement.)
The term “maternal depressive symptoms” is used in reporting the results of studies in which “depression” was quantified by scores on an inventory of depressive symptoms, and “clinical depression” is used in studies that used confirmatory diagnostic criteria. The present review, admittedly, focuses almost exclusively on maternal depression as an index for maternal mental health. There is nevertheless clear evidence for comparable effects for symptoms of maternal anxiety (27–29), some examples of which are cited below. The reasons for the focus on depressive symptoms are that 1) the absence of established clinical cutoffs for relevant anxiety symptom scales precludes an analysis of categorical versus continuous effects and 2) position papers in the global health community on the topic of maternal mental health focus almost exclusively on maternal depression.
A review of the relevant studies (see the online supplement) reveals that maternal depressive symptoms operate across a continuum to influence child neurodevelopment. This conclusion is consistent with the idea that depressive disorders should be conceptualized as a “fluid continuum” rather than as discrete syndromes (30). This point is further underscored by recent findings on the effects of “positive maternal mental health” on parenting and child socioemotional and cognitive development.
Research Findings
There is intergenerational transmission of the risk for depression from parents, especially the mother, to offspring (9, 31). Imaging studies focusing on neural systems implicated in affective disorders reveal significant differences between the offspring of mothers with and without a history of clinical depression. The unaffected adolescent offspring of mothers clinically diagnosed with major depressive disorder differ from offspring of mothers without mental disorders on measures of hippocampal volume (32), the neural circuitry associated with reward processing (33), amygdala volume (34), and cortical thickness (35). Children whose mothers showed persistently high levels of postnatal depressive symptoms, comparable to those exhibited during chronic mild depression, show amygdala enlargement (35), typical of individuals at risk for mood disorders.
More recent studies reveal that the maternal influence on the neurodevelopment of the offspring is not limited to instances where maternal depressive symptoms exceed clinical thresholds. These studies examined neural development as a function of maternal depressive symptoms across the continuum. The reports (see the online supplement) emerge from a number of community-based samples using number of depressive symptoms as a continuous variable. For example, in the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort, brain structure and connectivity were assessed as a function of antenatal maternal depressive symptoms using the EPDS. Validated cutoffs for probable depression on the EPDS (13) are symptom scores of 13 or more for the postpartum period and 15 or more for the antenatal period, with the latter adjusted for overlap between specific symptoms and the normal conditions of pregnancy, such as sleep or appetite. The mean number of antenatal depressive symptoms on the EPDS scale in the GUSTO study was 7.5 (SD=4.5), with only 9.5% of women with scores above the antenatal EPDS cutoff (Figure 1). Nevertheless, in studies in which offspring underwent imaging shortly after birth, the number of antenatal depressive symptoms was associated with alterations in amygdala microstructure at birth, as determined using diffusion tensor imaging (36) (Figure 2A) as well as at 6 months of age with functional connectivity of the amygdala with a range of prefrontal regions, including the insula (39, 40). Individual differences in amygdala structure at birth predicted later behavioral problems, as did differences in the insular cortex (40, 41). The association of prenatal maternal depressive symptoms with amygdala structure persisted through to 4.5 years of age.
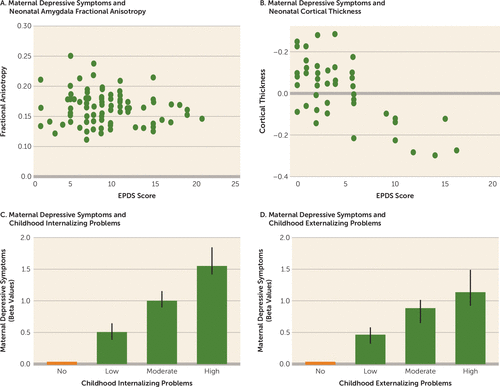
FIGURE 2. Associations of Levels of Maternal Depressive Symptoms With Imaging Findings in Neonates and With Internalizing and Externalizing Problems in Childrena
a Panel A (adapted from reference 36) is a scatterplot of fractional anisotropy from diffusion tensor imaging of the amygdala as a function of antenatal maternal depressive symptoms assessed with the Edinburgh Postnatal Depression Scale (EPDS). Panel B (adapted from reference 37) is a scatterplot of cortical thickness as a function of antenatal maternal depressive symptoms assessed with the EPDS. Panels C and D (adapted from reference 38) depict the association between internalizing and externalizing problems as a function of maternal perinatal depressive symptom trajectories. Error bars indicate 95% confidence intervals.
The association between maternal depressive symptoms and fetal neurodevelopment in the GUSTO cohort was observed across the normal range of antenatal depressive symptoms. This same finding was apparent in a study with a U.S. sample (42) in which depressive symptoms were evaluated using the CES-D scale, with a clinical cutoff score of 16. The mean CES-D score in the sample was well below the cutoff score (mean=6.0; SD=4.1; see Figure 2 in reference 42 for associations of cortical thickness across CES-D scores) and revealed significant cortical thinning in children, primarily in the right frontal lobe, that was associated with the number of antenatal maternal depressive symptoms used as a continuous variable. The strongest association was with maternal symptoms at 25 weeks of gestation, with cortical thinning in 19% of the whole cortex and 24% of the frontal lobes, primarily in the right superior medial orbital and frontal pole regions of the prefrontal cortex. The significant association between prenatal maternal depressive symptoms and subsequent child externalizing behavior was mediated by cortical thinning in prefrontal areas of the right hemisphere. Likewise, in a Canadian sample, Lebel et al. (37) (Figure 2B) found that antenatal EPDS scores were negatively correlated with offspring cortical thickness in right inferior frontal and middle temporal regions, an association that survived correction for postpartum EPDS scores. Postpartum EPDS scores were negatively correlated with offspring right superior frontal cortical thickness and with diffusivity in white matter originating from that region after correcting for prenatal EPDS scores. The mean EPDS score for the mothers in the study was 4.7 (SD=4.2) (for the second trimester, when the greatest effect was observed), and the association was apparent across the full range of depressive symptoms. The importance of these findings is underscored by studies revealing reduced cortical thickness as a risk factor for depression (35), suggesting that the intergenerational transmission of the neurodevelopmental risk for depression in offspring is not unique to clinical cases of maternal depression. The same conclusion emerges from the GUSTO study findings (39–41) of altered right amygdala-insula connectivity, which is strongly associated with anxiety disorders (43). In sum, these findings reveal a continuous relation between the quality of maternal mental health and fetal neurodevelopment: the higher the number of maternal depressive symptoms, the greater the neuroanatomical evidence for vulnerability in the offspring.
The association between maternal depressive symptoms and those of adolescent offspring shows a continuous relation across the range of maternal symptoms (44). The same pattern emerges from studies examining the influence of maternal mental health on measures of child behavioral problems. Studies of community samples commonly include mothers with a wide range of variation in self-reported symptoms of depression or anxiety. In contrast, clinical studies examine the offspring of mothers with verified disorders compared with control subjects without disorders. An extensive meta-analysis (6) of the relation between maternal depressive symptoms and child behavioral problems revealed that for internalizing problems, the associations were somewhat stronger in clinical studies, where depression was clinically diagnosed, than in community studies relying on self-reported symptoms. In contrast, there was no such difference between clinical and community studies in effect size for externalizing problems. Likewise, effect sizes were no different between diagnosed depression and self-reported symptoms with measures of child psychopathology.
A number of community-based studies reveal significant associations between maternal mood and child behavioral problems using depressive symptom measures as a continuous rather than a categorical variable (e.g., 45, 46; see also the online supplement). The significant associations observed in these studies imply an effect that cuts across the population. This issue was systematically explored in a detailed analysis of the trajectories of depressive symptoms in a large community sample of mothers and children in Rotterdam, the Netherlands (Generation R) (38). Only 34% of mothers showed no evidence of depressive symptoms over the perinatal period, revealing a distribution comparable to that observed in the GUSTO cohort (see Figure 1). The authors report evidence for a graded increase in the frequency of child behavioral problems in association with the level of maternal depressive symptoms, with a significant increase apparent even between the offspring of mothers with low levels of depressive symptoms compared with offspring of mothers with no symptoms (Figure 2C,D). Likewise, an analysis of data from the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development (47) showed that a continuous measure of maternal depressive symptoms in infancy predicted cognitive function in early primary school. Depressive symptoms were assessed with the CES-D (using a cutoff score of 16); the mean score for the sample was 9.2 (SD=7.2), with ∼15% of the mothers scoring above the cutoff. Self-reported levels of maternal depressive symptoms across the normal range also predicted the developmental change that occurred in emotion regulation: children of mothers reporting fewer symptoms showed improved emotion regulation between ages 4 and 7 years, an effect that was especially pronounced among children with greater physiological reactivity (48). Similarly, antenatal maternal depressive symptoms across the normal range predicted negative emotional reactivity among infants (49). Finally, Goodman et al. (50) examined depressive symptoms in a cohort of mothers at high risk for depression in relation to infant neurodevelopment, using the Brazelton scales. Depressive symptom scores were significantly associated with social interaction, state organization, autonomic system, and irritability scales; however, there was no significant difference between infants whose mothers met diagnostic criteria for depression and those whose mothers had high levels of depressive symptoms but did not meet diagnostic criteria. Comparable findings emerged from the Avon Longitudinal Study of Parents and Children, which examined cognitive abilities and found that maternal depressive symptoms were negatively associated with IQ (51).
The graded effects of maternal depressive symptoms are consistent with extensive studies revealing that self-reported level of maternal distress in nonclinical samples is associated with measures of fetal physiology, including fetal cardiovascular function (see reference 4 for a review). Likewise, in a population-based sample, personality traits of mothers associated with increased depressive symptoms, including anger, impulsivity, detachment, and suspicion, predicted increased symptoms of depression and anxiety in the young adult offspring (52). These studies provide compelling evidence for the importance of maternal depressive symptoms across the continuum for child health and development.
Positive Mental Health
Maternal symptoms of depression or anxiety do not capture the full spectrum of emotional well-being. Mental health is a continuum that includes a sense of well-being not merely defined by the absence of illness or disability (World Health Organization; who.int/features/factfiles/mental_health/en/). Selected items on self-report measures used with peripartum women can reveal multiple dimensions of emotional status, including positive mental health. An exploratory bifactor analysis with data from a community sample with over 1,000 women (53) revealed seven independent factors derived from the EPDS, the Beck Depression Inventory, and the State-Trait Anxiety Index: a general affective symptoms factor, with a high loading for virtually all items; a low self-esteem or self-loathing factor; anxiety; somatic complaints; sadness; and two positive mental health factors, positive mood and positive self. The latter two factors captured dimensions associated with positive emotional well-being (“I feel calm,” “I feel satisfied,” “I make decisions easily,” “I am self-confident,” etc.). These two positive mental health factors, independent of the negative factors, predicted specific outcomes in children at age 4, especially outcomes associated with social communication and sociability. The general affective symptoms factor, which reflects symptoms of both depression and anxiety, better predicted measures of negative emotionality, commonly associated with maternal symptoms of depression or anxiety.
Distinct effects of positive and negative maternal mental health were also observed on measures of parental style (54). The general affective symptoms factor predicted levels of authoritarian and permissive parenting, both of which enhance the risk for later psychopathology. This effect was due to a significant effect on punitive and verbally hostile parenting behaviors. In contrast, the positive maternal mental health factors, but not the general affective symptoms factor, predicted authoritative parenting, which promotes optimal neurodevelopmental outcomes. This effect was due to a main effect of maternal positive mental health on warmth and supportive as well as reasoning and inductive parenting behaviors.
Positive and negative mental health dimensions, although anticorrelated, are distinct constructs (55). Effective interventions may reduce depressive symptoms, but they do little to enhance positive mental well-being, reflecting the independence of these constructs. Positive mental health and mental illness symptoms have different antecedents, including demographic and socioemotional variables (56). The Phua et al. analysis (53) suggests that standardized mental health screening tools in common use in epidemiological studies of peripartum women can be exploited for more comprehensive analysis of emotional well-being and mental health for public health.
Moderators Of Associations Between Maternal Depressive Symptoms And Child Development
The impact of maternal depressive symptoms on neurodevelopmental outcomes varies widely across children (57), and there is evidence for moderation by social context and differential susceptibility in the offspring. The research on this topic has considerable potential to inform public health programs. The study of differential susceptibility as well as contextual influences permits improved identification of high-risk children at the level of the individual child and thus the optimization of prevention programs. The study of contextual moderators also offers insights into effective community-based public health approaches that might buffer children against the influence of maternal depressive symptoms.
Moderation by “Differential Susceptibility”
An extensive review of the relation between perinatal maternal depressive symptoms and socioemotional development in offspring (6) noted that despite the consistent statistical strength of the association, maternal mental health measures generally account for only ∼5% of the child outcome measures. The influence of maternal symptoms of depression, like other forms of childhood adversity, varies across children (58). There is remarkable interindividual variation in the consequences of even extreme forms of adversity, with a substantial portion of individuals seemingly resilient with respect to mental health outcomes (59). Such “differential susceptibility” to adverse environmental conditions is also apparent in the extensive interindividual differences in “biological sensitivity” to context that is apparent among children in response to a normal range of social experience (58).
Genetic factors contribute to differential susceptibility to the environmental conditions of early life. Studies in behavioral genetics reveal that a family history of psychopathology is associated with an increased sensitivity to environmental adversity in offspring (60, 61). Studies focused on candidate genes (e.g., SLC6A4, COMT, DRD4, BDNF, and FKbp5) suggest that individuals carrying specific allelic variants are more sensitive to adverse environmental conditions with respect to a range of mental health outcomes (62, 63). There is also evidence for the importance of child genotype in determining the impact of peripartum maternal depressive symptoms. A polygenic risk score (PRS) reflecting the genetic vulnerability for major depressive disorder moderates the association between antenatal maternal depressive symptoms and amygdala and hippocampal volume in newborns (64). The moderation was apparent using the depression PRS of the infant, but not the mother, which discounts the possibility that the effects occurred as a function of passive genetic transmission. Rather, offspring genotype moderated the influence of adversity, including maternal symptoms of depression, on neural development.
Two studies revealed a similar moderation of the effects of peripartum maternal symptoms of anxiety on neurodevelopmental outcomes as a function of a functional single-nucleotide polymorphism (val158met) in the catechol-O-methyltransferase (COMT) gene, which codes for a protein heavily implicated in catecholamine metabolism, particularly in the cortex. This val158met COMT variant is associated with differences in volume of the temporal cortex (65) and moderates the association between antenatal maternal symptoms of anxiety and child working memory as well as symptoms of attention deficit hyperactivity disorder (66), with stronger associations between maternal anxiety and child behavior in the offspring carrying the val/val (G:G) variant. A COMT haplotype that included the val158met COMT variant moderated the association between antenatal maternal symptoms of anxiety and prefrontal and parietal cortical thickness in neonates (67). Likewise, a variant in the BDNF gene encoding brain-derived neurotrophic factor moderated the association between antenatal maternal symptoms of anxiety and DNA methylation, with a substantially greater number of maternal anxiety–related CpG sites in offspring carrying the met/met alleles (68).
In each of the studies noted above, the measures of maternal depressive symptoms were analyzed as continuous variables, with effects apparent across the full range of symptom scores. These studies reveal that the influence of peripartum maternal emotional well-being is moderated by factors influencing the sensitivity of the child to maternal signals, including child genotype. Child genotype, and accompanying variations in susceptibility, could thus move the curve describing the relation between maternal symptoms and child outcome to the left, or right, underscoring the importance of considering maternal symptoms over a continuum.
The effective identification of vulnerability for psychopathology at the level of the individual child requires the integration of information on clinically relevant environmental conditions, such as maternal depressive symptoms, with measures of variants in gene networks associated with susceptibility (63, 69). Likewise, an understanding of the origins of psychopathology will depend on an ability to identify the biological pathways that link maternal depressive symptoms to child neurodevelopment. While variations in parenting are considered a primary mediator of the effects of postnatal parental depression, the signals that mediate antenatal influences are unknown. The importance of this gap in knowledge is underscored by findings suggesting that antenatal influences of maternal depressive symptoms may actually be more consequential than postnatal effects for certain outcomes (5, 51). Elevated exposure to fetal glucocorticoids was once an attractive candidate. Fetal cortisol levels are associated with neurodevelopmental outcomes (70). However, fetal cortisol levels are not reliably associated with prenatal maternal symptoms of depression or anxiety or with measures of perceived stress (29). An emerging alternative candidate mechanism is that of proinflammatory cytokines, which are linked to major depressive disorder (71). Graham et al. (72) found a significant positive correlation between maternal levels of the proinflammatory cytokine interleukin-6 (IL-6) and right amygdala volume in neonates. Right amygdala volume mediated the association between maternal IL-6 concentrations during pregnancy and impulse control in the offspring at 24 months of age. These findings implicate placental mechanisms, and potentially placental inflammation, as a biological interface between maternal emotional well-being and child neurodevelopment (73).
Moderation by Context
The association between maternal depressive symptoms and child developmental outcomes is also moderated by social context (6, 23, 57, 74). There is a stronger association between maternal depressive symptoms and childhood behavioral problems in families with low socioeconomic status or where the mother is a single parent (6). Contextual factors that determine family resources may render children more affected by moderate levels of maternal depressive symptoms (74). Since parenting is often considered a mediating factor for the association between maternal depression and child outcomes, it is not surprising that these same contextual factors also moderate the relation between maternal depressive symptoms and parenting, with evidence for a stronger association among economically disadvantaged families (20). Further evidence for the importance of familial contextual factors emerges from studies of the association between paternal depressive symptoms and child emotional problems (e.g., 75). Maternal and paternal depressive symptoms are positively correlated, perhaps reflecting assortative mating or phenotypic convergence as well as an influence of paternal well-being on that of the mother. Indeed, the association between paternal depressive symptoms and child outcomes is mediated by measures of the quality of the family environment, which suggests an indirect, “contextual” paternal influence on child mental health (76).
Social influences can also buffer children, diminishing the impact of maternal depressive symptoms, and studies on this effect can potentially inform community-based prevention programs. For example, high neighborhood social capital (e.g., social cohesion) attenuated the association between maternal depressive symptoms and both internalizing and externalizing behavioral problems in adolescents (77). Likewise, high-quality group-based child care programs significantly attenuated the association between maternal depressive symptoms and child outcomes. Among children of mothers with elevated maternal depressive symptoms, early entry to child care was found to reduce the risk for socioemotional problems in comparison to children who remained in maternal care or were cared for by a relative or babysitter (78). A similar study (79) showed that formal, but not informal child care at age 2 modified the association between recurrent maternal depressive symptoms and behavioral problems at age 5. An important caveat in each study is the focus on mothers with generally mild to moderate levels of depressive symptoms. However, high-quality group-based child care might be considered an important public health initiative, especially in lower socioeconomic settings, where there is both an increased rate of maternal depression (17) and evidence for a greater impact of maternal depressive symptoms on child development (6). These studies reflect the degree to which contextual variables can operate to define the impact of maternal mental health on child outcomes, enhancing (e.g., low socioeconomic status) or diminishing (e.g., high social capital) the influence of maternal depressive symptoms.
Conclusions
A review of studies in which measures of maternal depressive symptoms were analyzed as continuous variables reveals effects that are apparent across the full range of values for maternal mental health. The influence of peripartum maternal depressive symptoms is moderated by factors that influence the sensitivity of the child to maternal signals, including child genotype. Likewise, contextual factors that determine the quality of family function can moderate the impact of maternal symptoms on neurodevelopmental outcomes in the child. Taken together, these findings suggest that a certain portion of children may be more affected by problems of maternal mental health, such as children in economically disadvantaged settings. Hence, the impact of maternal depressive symptoms on offspring neurodevelopment is not simply a function of the severity of maternal problems, which further argues for the importance of maternal depressive symptoms across the spectrum of severity. The importance of this consideration is underscored by findings that suggest that emotional problems in women after childbirth are actually more the rule than the exception, with up to 80% of women reporting at least some levels of some symptoms (80). While this review focused on the topic of maternal depression, the evidence for the influence of perinatal maternal anxiety on child development, including imaging studies of neurodevelopment (e.g., 67, 81), is as compelling as that for maternal symptoms of depression. While research on maternal depression has served as a critical fulcrum for policy and guidelines in mother-child health, we need to expand the emphasis on maternal mental health to include multiple dimensions of perinatal emotional well-being, including common sources of distress (4) as well as positive emotional states (53). The discussion must be broadened to include a consideration of the quality of maternal emotional well-being across the continuum as an issue for population health. And the importance of the issue transcends concerns for traditional mental health outcomes in the offspring. Maternal mental health predicts offspring academic achievement (8), a classic measure of “human capital” for economists. The ability of nations to develop knowledge-based economies relies on the “brain health” of the population: brain health thus equates to economic health. The brain health of the next generation is intimately linked to that of mothers—all mothers.
1 : The Costs of Perinatal Maternal Mental Health Problems. London, Centre for Mental Health and London School of Economics, 2014Google Scholar
2 : Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br J Psychiatry 2013; 203:417–421Crossref, Medline, Google Scholar
3 : Grand challenges: integrating maternal mental health into maternal and child health programmes. PLoS Med 2013; 10:e1001442Crossref, Medline, Google Scholar
4 : Research review: maternal prenatal distress and poor nutrition: mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry 2013; 54:115–130Crossref, Medline, Google Scholar
5 : Maternal perinatal mental health and offspring academic achievement at age 16: the mediating role of childhood executive function. J Child Psychol Psychiatry 2016; 57:491–501Crossref, Medline, Google Scholar
6 : Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14:1–27Crossref, Medline, Google Scholar
7 : The effects of maternal postnatal depression and child sex on academic performance at age 16 years: a developmental approach. J Child Psychol Psychiatry 2010; 51:1150–1159Crossref, Medline, Google Scholar
8 : Associations of parental depression with child school performance at age 16 years in Sweden. JAMA Psychiatry 2016; 73:239–246Crossref, Medline, Google Scholar
9 : Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163:1001–1008Link, Google Scholar
10 : Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev 2013; (2):CD001134Medline, Google Scholar
11 : Children of depressed parents: a public health opportunity. JAMA Psychiatry 2016; 73:197–198Crossref, Medline, Google Scholar
12 : Treatment of maternal depression in a medication clinical trial and its effect on children. Am J Psychiatry 2015; 172:450–459Link, Google Scholar
13 : Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Women Ment Health 2006; 9:309–315Crossref, Medline, Google Scholar
14 : The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401Crossref, Google Scholar
15 : Maternal depression: a global threat to children’s health, development, and behavior and to human rights. Child Dev Perspect 2009; 3:51–59Crossref, Google Scholar
16 : Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005; 106:1071–1083Crossref, Medline, Google Scholar
17 : Maternal depression in the United States: nationally representative rates and risks. J Womens Health (Larchmt) 2011; 20:1609–1617Crossref, Medline, Google Scholar
18 : Socioeconomic burden of subsyndromal depressive symptoms and major depression in a sample of the general population. Am J Psychiatry 1996; 153:1411–1417Link, Google Scholar
19 : Recurrence of depression during pregnancy: psychosocial and personal functioning correlates. Depress Anxiety 2009; 26:557–567Medline, Google Scholar
20 : Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev 2000; 20:561–592Crossref, Medline, Google Scholar
21 : Subsyndromal depressive symptoms and major depression in postpartum women. Am J Orthopsychiatry 2001; 71:87–97Crossref, Medline, Google Scholar
22 : Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev 1999; 106:458–490Crossref, Medline, Google Scholar
23 : Children of depressed parents: an integrative review. Psychol Bull 1990; 108:50–76Crossref, Medline, Google Scholar
24 : Maternal inattention and impulsivity and parenting behaviors. J Clin Child Adolesc Psychol 2007; 36:455–468Crossref, Medline, Google Scholar
25 : Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. J Child Psychol Psychiatry 2010; 51:734–744Crossref, Medline, Google Scholar
26 : Familial transmission of depression and antisocial behavior symptoms: disentangling the contribution of inherited and environmental factors and testing the mediating role of parenting. Psychol Med 2011; 41:1175–1185Crossref, Medline, Google Scholar
27 : Maternal depression, anxiety, and stress during pregnancy and child outcome: what needs to be done. Best Pract Res Clin Obstet Gynaecol 2014; 28:25–35Crossref, Medline, Google Scholar
28 : The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol 2014; 26:393–403Crossref, Medline, Google Scholar
29 : Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry 2017; 174:319–328Link, Google Scholar
30 : Classification and treatment of sub-threshold depression. Curr Opin Psychiatry 2006; 19:9–13Crossref, Medline, Google Scholar
31 : Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 2013; 70:1312–1319Crossref, Medline, Google Scholar
32 : Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry 2010; 67:270–276Crossref, Medline, Google Scholar
33 : Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 2010; 67:380–387Crossref, Medline, Google Scholar
34 : Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108:14324–14329Crossref, Medline, Google Scholar
35 : A brain-based endophenotype for major depressive disorder. Annu Rev Med 2011; 62:461–474Crossref, Medline, Google Scholar
36 : Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 2013; 74:837–844Crossref, Medline, Google Scholar
37 : Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol Psychiatry 2016; 80:859–868Crossref, Medline, Google Scholar
38 : Trajectories of maternal depressive symptoms predict child problem behaviour: the Generation R study. Psychol Med 2013; 43:13–25Crossref, Medline, Google Scholar
39 : Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 2015; 5:e508Crossref, Medline, Google Scholar
40 : Neonatal neural networks predict children behavioral profiles later in life. Hum Brain Mapp 2017; 38:1362–1373Crossref, Medline, Google Scholar
41 : Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry 2017; 7:e1103Crossref, Medline, Google Scholar
42 : Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry 2015; 77:324–334Crossref, Medline, Google Scholar
43 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
44 : Maternal depressive symptoms and depressive symptoms in adolescents. J Child Psychol Psychiatry 1995; 36:1161–1178Crossref, Medline, Google Scholar
45 : Towards a family process model of maternal and paternal depressive symptoms: exploring multiple relations with child and family functioning. J Child Psychol Psychiatry 2005; 46:479–489Crossref, Medline, Google Scholar
46 : Gender-specific pathways between maternal depressive symptoms, family discord, and adolescent adjustment. Dev Psychol 1997; 33:657–668Crossref, Medline, Google Scholar
47 : Mothers’ depressive symptoms and children’s cognitive and social agency: predicting first-grade cognitive functioning. Dev Psychol 2016; 52:1291–1298Crossref, Medline, Google Scholar
48 : Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children’s physiological regulation. Dev Psychol 2008; 44:1110–1123Crossref, Medline, Google Scholar
49 : Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy 2004; 6:319Crossref, Google Scholar
50 : Deconstructing antenatal depression: what is it that matters for neonatal behavioral functioning? Infant Ment Health J 2011; 32:339–361Crossref, Medline, Google Scholar
51 : The timing of maternal depressive symptoms and child cognitive development: a longitudinal study. J Child Psychol Psychiatry 2012; 53:632–640Crossref, Medline, Google Scholar
52 : Impact of dysfunctional maternal personality traits on risk of offspring depression, anxiety, and self-harm at age 18 years: a population-based longitudinal study. Psychol Med 2017Google Scholar
53 : Positive maternal mental health during pregnancy associated with specific forms of adaptive development in early childhood: evidence from a longitudinal study. Dev Psychopathol 2017; 29:1573–1587Crossref, Medline, Google Scholar
54 : Maternal childhood adversity moderates the association between maternal mental health and parenting: implications for inter-generational transmission of parting styles. Unpublished 2018 manuscriptGoogle Scholar
55 : Evidence for the independence of positive and negative well-being: implications for quality of life assessment. Br J Health Psychol 2003; 8:107–122Crossref, Medline, Google Scholar
56 : Can the 12-item General Health Questionnaire be used to measure positive mental health? Psychol Med 2007; 37:1005–1013Crossref, Medline, Google Scholar
57 : Maternal depressive disorder and contextual risk: contributions to the development of attachment insecurity and behavior problems in toddlerhood. Dev Psychopathol 1998; 10:283–300Crossref, Medline, Google Scholar
58 : Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Dev Psychopathol 2011; 23:7–28Crossref, Medline, Google Scholar
59 : Resilience in the face of adversity: protective factors and resistance to psychiatric disorder. Br J Psychiatry 1985; 147:598–611Crossref, Medline, Google Scholar
60 : Genetic epidemiology in psychiatry: taking both genes and environment seriously. Arch Gen Psychiatry 1995; 52:895–899Crossref, Medline, Google Scholar
61 : Transition from stress sensitivity to a depressive state: longitudinal twin study. Br J Psychiatry 2009; 195:498–503Crossref, Medline, Google Scholar
62 : Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 2006; 7:583–590Crossref, Medline, Google Scholar
63 : Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 2012; 233:102–111Crossref, Medline, Google Scholar
64 : Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb Cortex 2017; 27:3080–3092Crossref, Medline, Google Scholar
65 : Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex 2014; 24:1230–1246Crossref, Medline, Google Scholar
66 : Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS One 2017; 12:e0177506Crossref, Medline, Google Scholar
67 : COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. Am J Psychiatry 2015; 172:163–172Link, Google Scholar
68 : Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism influences the association of the methylome with maternal anxiety and neonatal brain volumes. Dev Psychopathol 2015; 27:137–150Crossref, Medline, Google Scholar
69 : Epigenetics and the biological definition of gene × environment interactions. Child Dev 2010; 81:41–79Crossref, Medline, Google Scholar
70 : Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA 2012; 109:E1312–E1319Crossref, Medline, Google Scholar
71 : The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16:22–34Crossref, Medline, Google Scholar
72 : Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 2018; 83:109–119Crossref, Medline, Google Scholar
73 : The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 2016; 41:207–218Crossref, Medline, Google Scholar
74 : The developmental psychopathology of perinatal depression: implications for psychosocial treatment development and delivery in pregnancy. Can J Psychiatry 2012; 57:530–536Crossref, Medline, Google Scholar
75 : Paternal depression in the postnatal period and child development: a prospective population study. Lancet 2005; 365:2201–2205Crossref, Medline, Google Scholar
76 : Paternal depression in the postnatal period and child development: mediators and moderators. Pediatrics 2015; 135:e339–e347Crossref, Medline, Google Scholar
77 : Does neighborhood social capital buffer the effects of maternal depression on adolescent behavior problems? Am J Community Psychol 2014; 53:275–285Crossref, Medline, Google Scholar
78 : Maternal depressive symptoms and children’s emotional problems: can early child care help children of depressed mothers? JAMA Psychiatry 2013; 70:830–838Crossref, Medline, Google Scholar
79 : Maternal depressive symptoms and child care during toddlerhood relate to child behavior at age 5 years. Pediatrics 2011; 128:e78–e84Crossref, Medline, Google Scholar
80 : Mood disturbance in the early puerperium: a review. Arch Women Ment Health 2003; 6(suppl 2):S33–S42Crossref, Medline, Google Scholar
81 : Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J Am Acad Child Adolesc Psychiatry 2015; 54:313–21.e2Crossref, Medline, Google Scholar



