Continuation of Atypical Antipsychotic Medication During Early Pregnancy and the Risk of Gestational Diabetes
Abstract
Objective:
Some atypical antipsychotics are associated with metabolic side effects, which are risk factors for gestational diabetes. The authors examined the risk of developing gestational diabetes associated with the continuation of treatment with aripiprazole, ziprasidone, quetiapine, risperidone, and olanzapine during pregnancy compared with discontinuation of these antipsychotic drugs.
Method:
Nondiabetic pregnant women who were linked to a live-born infant and enrolled in Medicaid (2000–2010) and who received one or more prescriptions dispensed for an antipsychotic drug during the 3 months before pregnancy were included in the analyses. Among 1,543,334 pregnancies, some expectant mothers at baseline were receiving treatment with aripiprazole (N=1,924), ziprasidone (N=673), quetiapine (N=4,533), risperidone (N=1,824), or olanzapine (N=1,425). For each antipsychotic drug, women with two or more dispensings (“continuers”) were compared with women with no dispensings (“discontinuers”) during the first half of pregnancy. A generalized linear model and propensity-score stratification were used to obtain absolute and relative risks of developing gestational diabetes, with adjustment for confounders.
Results:
Women who continued antipsychotic treatment during pregnancy generally had higher comorbidity and longer baseline antipsychotic use. The crude risk of developing gestational diabetes among continuers compared with discontinuers, respectively, was 4.8% and 4.5% for aripiprazole, 4.2% and 3.8% for ziprasidone, 7.1% and 4.1% for quetiapine, 6.4% and 4.1% for risperidone, and 12.0% and 4.7% for olanzapine. The adjusted relative risks were 0.82 (95% CI=0.50–1.33) for aripiprazole, 0.76 (95% CI=0.29–2.00) for ziprasidone, 1.28 (95% CI=1.01–1.62) for quetiapine, 1.09 (95% CI=0.70–1.70) for risperidone, and 1.61 (95% CI=1.13–2.29) for olanzapine.
Conclusions:
Compared with women who discontinued use of an atypical antipsychotic medication before the start of pregnancy, women who continued treatment with olanzapine or quetiapine had an increased risk of gestational diabetes that may be explained by the metabolic effects associated with these two drugs.
Gestational diabetes mellitus is a complication of pregnancy, defined as carbohydrate intolerance with onset or recognition during pregnancy (1). It can lead to adverse pregnancy outcomes, such as preeclampsia, cesarean delivery, neonatal hypoglycemia, and macrosomia (2). In 2010, the estimated prevalence of gestational diabetes in the United States ranged between 4.6% and 9.2% (3). Up to 50% of women with gestational diabetes develop type 2 diabetes mellitus in the decades following pregnancy (4), a risk more than seven times greater than that among women without gestational diabetes (5). Many risk factors for gestational diabetes are similar to those for type 2 diabetes, including older age, nonwhite race, and obesity (2, 6).
There is a well-recognized association between treatment with some atypical antipsychotic medications and metabolic side effects, including weight gain and diabetes, in the general population (7–11). In 2003, the U.S. Food and Drug Administration required all manufacturers of atypical antipsychotics to add to their labels a warning about the risk for hyperglycemia and diabetes. However, the metabolic safety of antipsychotic drugs for pregnant women, who are already predisposed to insulin resistance (12), is not fully understood. A small number of studies and case reports have suggested that there is an increased risk of developing gestational diabetes with antipsychotic use during pregnancy (13–16); however, no association has been reported in more recent studies (17, 18). Furthermore, although there are differences in the severity of metabolic side effects between antipsychotic drugs (19) and biochemical evidence explaining such differences exists (20), data on the comparative risk of gestational diabetes are scarce (21).
Psychiatric disorders that are treated with antipsychotic medications, such as bipolar disorder, are often recognized during the reproductive age range (22) and have a significant impact on the health and wellness of female patients around the time of pregnancy (23). Despite limited safety information regarding the use of antipsychotic drug treatment during pregnancy, an increasing number of women of reproductive age are treated with antipsychotics in the United States (24–26). For some women, treatment continuation during pregnancy is necessary to prevent the sequelae of untreated mental illness (4), whereas for others, clinicians must weigh the risks and benefits of continuing treatment and may consider either discontinuation or switching to an alternative treatment. Understanding the potential risk of developing gestational diabetes and how this risk may vary by the type of antipsychotic used is an important consideration for patients and clinicians. To assess the risk of gestational diabetes associated with a particular drug, previous studies compared pregnant women who were treated with antipsychotics during pregnancy with women who were not (15–18). Such a design is prone to confounding by indication, because women who do not take antipsychotic medications around the time of pregnancy differ in many ways from women who require antipsychotic treatment. These differences, such as having a healthier lifestyle and diet patterns, might affect the risk of developing gestational diabetes. In a nationwide cohort of pregnant women who were all treated with antipsychotic medications before the start of pregnancy, we compared the risk of developing gestational diabetes between women who continued antipsychotic treatment during pregnancy and women who discontinued treatment before the start of pregnancy.
Method
Data Source and Study Population
The Medicaid Analytic eXtract (27) is a person-level, nationwide claims database that contains information on demographic characteristics, hospitalizations, outpatient visits, and pharmacy dispensing records. We created a cohort from this Medicaid database comprising pregnant women linked to their live-born infants (2000–2010) (27). This database has been successfully used in studies of medication safety in pregnancy (28–30). Women were required to have had continuous Medicaid coverage from 3 months before the date of their last menstrual period to 1 month after the date of delivery. Additionally, they were required not to have had other insurance benefits, which could lead to incomplete ascertainment of claims.
The study cohort consisted of women who during the 3 months before their last menstrual period filled a prescription for one of the five most frequently used atypical antipsychotics: aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone. Women with preexisting diabetes were excluded, because they are not at risk for developing gestational diabetes. To identify these women, we modified an algorithm developed and validated by Andrade et al. (31) (for a detailed description of the algorithm, see Figure S1 in the data supplement accompanying the online version of this article).
Outcome Definition
Based on the algorithm by Andrade et al. (31), we classified a woman as having gestational diabetes if she had two or more diagnosis codes for any diabetes between 141 days after her last menstrual period and delivery and she had a glucose tolerance test or a gestational diabetes diagnosis within the same time frame. The original algorithm had a positive predictive value of 88% in identifying a diagnosis of gestational diabetes in claims data. We compared the results with and without considering metformin as an antidiabetic medication, since it is sometimes used to treat polycystic ovarian syndrome. The results were identical, and thus metformin was included.
Exposure Definition
We examined medication exposure that occurred during the first 140 days of pregnancy (for further details, see Figure S1 in the online data supplement). Women who had two or more additional prescriptions dispensed during the first 140 days of their pregnancy for the same antipsychotic medication they received before pregnancy were classified as “continuers.” Women who had no prescriptions dispensed for an antipsychotic medication during the first 140 days of pregnancy were classified as “discontinuers.” To estimate the relationship between the risk for developing gestational diabetes and receipt of antipsychotic drugs at lower doses, we conducted dose-response analyses, including women with only one dispensing during the first 140 days of pregnancy. We excluded women with dispensings for a drug during pregnancy that was different from the one they received before the start of pregnancy and women who received dispensings for more than one type of antipsychotic drug during the first 140 days of pregnancy. As a result, the five exposure groups (aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone) were mutually exclusive. Furthermore, we combined the users of individual medications to form three “risk-stratified” groups on the basis of the drugs’ weight-gain potential and associated risk of diabetes outside of pregnancy (7). Aripiprazole and ziprasidone comprised the low-risk group, quetiapine and risperidone formed the medium-risk group, and olanzapine constituted the high-risk group.
Covariates
Covariates for confounding adjustment were assessed from 3 months before to 3 months after the last menstrual period. The covariates were selected on the basis of clinical plausibility as confounders or proxies of confounders for the association between antipsychotic continuation and gestational diabetes and included demographic data (age, race, and Medicaid eligibility type), psychiatric diagnoses (anxiety disorders, attention deficit hyperactivity disorder, bipolar disorder, depression, schizophrenia or other psychoses, and other psychiatric disorders), comorbidity (pain disorders, hypertension, obesity, and dyslipidemia), other medication use (anticonvulsants, antidepressants, anxiolytics, benzodiazepines, mood stabilizers other than antipsychotics, opioids, other hypnotics, stimulants, and antihypertensives), history of gestational diabetes, and the duration of antipsychotic treatment received during the 3 months before the last menstrual period. To capture health service use as a general marker of the extent of comorbid illness, we quantified the number of different generic drugs received and the number of emergency department visits during the 3 months before the last menstrual period.
Analyses
Individual drugs.
Analyses were conducted separately for each of the five antipsychotic drugs (aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone). We first examined the characteristics of the continuers and discontinuers of each antipsychotic. The unadjusted risk differences per 100 women (RD100) and relative risks with corresponding 95% confidence intervals were estimated by using generalized linear regression models with identity (for RD100) or log link (for relative risks). Propensity-score stratification was used to adjust for confounding (32). The propensity score was the predicted probability of continuing the treatment as opposed to discontinuing the treatment after the last menstrual period, estimated by using logistic regression with all of the covariates mentioned above. After trimming patients in the nonoverlapping parts of the propensity-score distribution (33), we created 50 strata on the basis of the distribution of the propensity score among the continuers. Weighted generalized linear models were used to estimate the adjusted RD100 and relative risks, along with 95% confidence intervals, with the discontinuers weighted on the basis of the distribution of the continuers across the strata. To address potential residual confounding, we added to the outcome model covariates with a standardized difference that remained >0.1 after propensity-score weighting and examined whether this changed the interpretation of the results from the model without additional covariates.
We explored dose-response relationships between the risk of developing gestational diabetes and the cumulative dose of each antipsychotic during the 140-day exposure window. Restricted cubic splines were used to allow for nonlinear relationships, with adjustment for known and suspected risk factors for gestational diabetes, including age, nonwhite race, obesity, diagnosis of schizophrenia or bipolar disorder, and the duration of antipsychotic use during the 3-month baseline period (34).
Risk-stratified drug groups.
Due to the small number of patients in the drug-specific analyses, several additional analyses were conducted at the risk-stratified drug group level. First, we restricted the analyses to women with a recorded diagnosis of the approved indications for antipsychotics (schizophrenia, bipolar disorder, or depression), with the rationale that some antipsychotics are used off label for nonpsychiatric conditions, such as insomnia, at different doses (35) and that the different usage of these drugs may be associated with different baseline risks for gestational diabetes. Second, we extended the baseline period to 6 or 12 months before the last menstrual period among the subsets of women who had Medicaid eligibility during this time in order to assess whether a longer baseline period allowed for better confounding control. For the same reason, we used the high-dimensional propensity-score algorithm to empirically identify 50 additional covariates that may serve as proxies of unmeasured confounders and used them in the propensity-score model alongside the predefined covariates (36).
Effect of missing data on obesity.
Because obesity is one of the most salient risk factors for gestational diabetes but is incompletely captured in claims data, we conducted a bias analysis to examine the extent to which adjustment for confounding by unmeasured or poorly measured obesity would change the observed associations (37). The prevalence estimate of overweight or obese women was obtained from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics (38). Informed by the literature, we assumed that women who were classified as overweight or obese would have four times the risk for gestational diabetes compared with women not classified as overweight or obese (39), and we examined the potential bias over a range of obesity prevalence differences (0%−25%) between continuers and discontinuers.
All analyses were performed with R (R Core Team [https://www.r-project.org]) and SAS 9.4 (SAS Institute, Cary, N.C.).
Results
Among 1,543,334 linked pregnancies in the Medicaid Analytic eXtract, we identified 1,924 women who met our inclusion criteria with a filled prescription for aripiprazole during the 3 months before their last menstrual period, 673 with a filled prescription for ziprasidone, 4,533 with a filled prescription for quetiapine, 1,824 with a filled prescription for risperidone, and 1,425 with a filled prescription for olanzapine. In the first exclusion step, the proportions excluded with preexisting diabetes were 4.9% for aripiprazole, 6.5% for ziprasidone, 4.6% for quetiapine, 5.3% for risperidone, and 4.5% for olanzapine (for further details, see Figure S2 in the online data supplement). Depending on the drug, 19.7%–34.0% of women continued treatment during the first half of pregnancy (Table 1). Across most of the antipsychotic groups, continuers were slightly older and in some groups showed slightly greater use of other psychotropic medications. Continuers in all groups except quetiapine and risperidone were more likely to have a diagnosis of obesity, and continuers in all groups had received treatment with antipsychotics for a longer duration before their last menstrual period (for further details, see Table S1 in the online data supplement). After propensity-score weighting, most of the patient characteristics were well balanced between the continuers and discontinuers, with the exception of a few salient covariates, such as obesity and bipolar disorder, which remained slightly unbalanced between olanzapine continuers and discontinuers (Table 1) (also see Table S2 in the online data supplement).
| Aripiprazole | Ziprasidone | Quetiapine | Risperidone | Olanzapine | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Continuers (N=416) | Discontinuers (N=1,421) | Continuers (N=140) | Discontinuers (N=431) | Continuers (N=1,542) | Discontinuers (N=2,951) | Continuers (N=343) | Discontinuers (N=1,449) | Continuers (N=375) | Discontinuers (N=978) | |||||||||||||||||||||
| Characteristic | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD |
| Age (years)b | 24.8 | 7.2 | 24.4 | 6.7 | 25.0 | 6.4 | 25.0 | 5.4 | 26.8 | 6.4 | 26.5 | 6.3 | 25.3 | 7.4 | 25.5 | 7.5 | 28.5 | 6.9 | 27.1 | 6.4 | ||||||||||
| Race | ||||||||||||||||||||||||||||||
| Caucasian | 66.6 | 68.6 | 67.9 | 68.2 | 72.4 | 72.5 | 49.3 | 50.3 | 51.2 | 52.6 | ||||||||||||||||||||
| African American | 19.7 | 19.2 | 20.7 | 21.0 | 15.4 | 16.0 | 30.3 | 30.9 | 24.5 | 23.4 | ||||||||||||||||||||
| Other | 13.7 | 12.3 | 11.4 | 10.8 | 12.1 | 11.5 | 20.4 | 18.8 | 24.3 | 24.1 | ||||||||||||||||||||
| Mental health diagnosis | ||||||||||||||||||||||||||||||
| Attention deficit hyperactivity disorder | 9.4 | 9.2 | 10.7 | 10.0 | 8.3 | 8.3 | 14.9 | 15.0 | 4.5 | 5.3 | ||||||||||||||||||||
| Bipolar disorder | 44.5 | 45.0 | 44.3 | 45.2 | 35.9 | 35.3 | 25.1 | 25.6 | 31.7c | 41.9c | ||||||||||||||||||||
| Schizophrenia/other psychoses | 10.1 | 10.7 | 17.1 | 17.8 | 7.4 | 8.2 | 17.8 | 18.7 | 22.9 | 24.1 | ||||||||||||||||||||
| Depression | 39.2 | 38.0 | 41.4 | 39.8 | 42.9 | 43.7 | 44.3 | 45.6 | 38.7 | 39.3 | ||||||||||||||||||||
| Anxiety disorders | 24.8 | 25.9 | 22.1 | 22.6 | 28.7 | 29.5 | 19.8 | 19.3 | 20.8 | 24.1 | ||||||||||||||||||||
| Comorbidity and other psychotropic use | ||||||||||||||||||||||||||||||
| Previous gestational diabetesd | 4.8 | 4.7 | 2.1 | 1.6 | 4.9 | 5.2 | 3.2 | 2.8 | 2.9 | 1.5 | ||||||||||||||||||||
| Obesity | 5.3 | 4.6 | 3.6 | 4.5 | 2.3 | 2.2 | 2.3 | 1.7 | 2.4c | 0.7c | ||||||||||||||||||||
| Antidepressants | 71.6 | 71.8 | 73.6 | 72.4 | 75.6 | 75.5 | 72.6 | 72.2 | 70.7 | 71.2 | ||||||||||||||||||||
| Benzodiazepines | 33.7 | 35.2 | 39.3 | 40.5 | 39.4 | 38.9 | 23.6 | 24.7 | 29.1 | 31.3 | ||||||||||||||||||||
| Mood stabilizerse | 29.6 | 28.9 | 31.4 | 35.4 | 31.8 | 31.3 | 28.6 | 32.1 | 21.3c | 26.5c | ||||||||||||||||||||
| Opioids | 35.8 | 38.1 | 40.7 | 40.3 | 46.7 | 47.1 | 27.4 | 27.7 | 32.8 | 34.1 | ||||||||||||||||||||
| Antipsychotic use in the 90 days before the last menstrual period | ||||||||||||||||||||||||||||||
| Exposed ≤30 days | 20.2 | 20.0 | 18.6 | 17.3 | 19.3 | 19.3 | 27.7 | 28.0 | 20.5 | 21.0 | ||||||||||||||||||||
| Exposed >30 days, ≤60 days | 30.8 | 31.2 | 23.6 | 25.8 | 20.2 | 20.4 | 28.9 | 29.9 | 25.9 | 23.6 | ||||||||||||||||||||
| Exposed >60 days | 49.0 | 48.8 | 57.9 | 56.9 | 60.5 | 60.2 | 43.4 | 42.1 | 53.6 | 55.4 | ||||||||||||||||||||
TABLE 1. Selected Patient Characteristics of Discontinuers of Each Atypical Antipsychotic Medication (Weighted by Propensity Score)a
The absolute risk for gestational diabetes ranged from 4.2% to 12.0% among continuers and from 3.8% to 4.7% among discontinuers (Table 2 and Figure 1), depending on the drug assessed. The unadjusted relative risk for gestational diabetes associated with continuing treatment during the first 140 days of pregnancy was 1.06 (95% confidence interval [CI]=0.65, 1.72) for aripiprazole, 1.12 (95% CI=0.48, 2.61) for ziprasidone, 1.75 (95% CI=1.36, 2.24) for quetiapine, 1.56 (95% CI=0.98, 2.49) for risperidone, and 2.55 (95% CI=1.73, 3.74) for olanzapine (Table 2). There was evidence for an elevated risk for gestational diabetes after confounding adjustment for olanzapine (adjusted relative risk=1.61 [95% CI=1.13, 2.29]) and quetiapine (adjusted relative risk=1.28 [95% CI=1.01, 1.62]) but not for aripiprazole (adjusted relative risk=0.82 [95% CI=0.50, 1.33]), ziprasidone (adjusted relative risk=0.76 [95% CI=0.29, 2.00]), and risperidone (adjusted relative risk=1.09 [95% CI=0.70, 1.70]). In a dose-response analysis, the risk increased with increasing cumulative dose of olanzapine until approximately 700 mg and plateaued thereafter (Figure 2). No clear trajectory was observed for the other antipsychotics considering the width of the confidence band.
| Unadjusted | Adjusted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication and Risk Group | Nb | Number of Women | Risk (%) | RD100 | 95% CI | Relative Risk | 95% CI | Risk (%) | RD100 | 95% CI | Relative Risk | 95% CI |
| Aripiprazole | ||||||||||||
| Continuers | 419 | 20 | 4.8 | 0.3 | –2.0, 2.6 | 1.06 | 0.65, 1.72 | 4.6 | –1.0 | –3.4, 1.3 | 0.82 | 0.50, 1.33 |
| Discontinuers | 1,505 | 68 | 4.5 | 5.6 | ||||||||
| Ziprasidone | ||||||||||||
| Continuers | 167 | c | 4.2 | 0.4 | –3.0, 3.9 | 1.12 | 0.48, 2.61 | 3.6 | –1.1 | –4.8, 2.6 | 0.76 | 0.29, 2.00 |
| Discontinuers | 506 | 19 | 3.8 | 4.7 | ||||||||
| Quetiapine | ||||||||||||
| Continuers | 1,543 | 110 | 7.1 | 3.1 | 1.6, 4.5 | 1.75 | 1.36, 2.24 | 7.1 | 1.6 | 0.0, 3.1 | 1.28 | 1.01, 1.62 |
| Discontinuers | 2,990 | 122 | 4.1 | 5.6 | ||||||||
| Risperidone | ||||||||||||
| Continuers | 359 | 23 | 6.4 | 2.3 | –0.4, 5.0 | 1.56 | 0.98, 2.49 | 6.7 | 0.6 | –2.4, 3.5 | 1.09 | 0.70, 1.70 |
| Discontinuers | 1,465 | 60 | 4.1 | 6.1 | ||||||||
| Olanzapine | ||||||||||||
| Continuers | 384 | 46 | 12.0 | 7.3 | 3.8, 10.8 | 2.55 | 1.73, 3.74 | 11.7 | 4.4 | 0.8, 8.1 | 1.61 | 1.13, 2.29 |
| Discontinuers | 1,041 | 49 | 4.7 | 7.3 | ||||||||
| Low-risk groupd | ||||||||||||
| Continuers | 586 | 27 | 4.6 | 0.3 | –1.6, 2.2 | 1.07 | 0.70, 1.62 | 4.5 | –0.4 | –2.4, 1.5 | 0.91 | 0.60, 1.39 |
| Discontinuers | 2,011 | 87 | 4.3 | 4.9 | ||||||||
| Medium-risk groupe | ||||||||||||
| Continuers | 1,902 | 133 | 7.0 | 2.9 | 1.6, 4.2 | 1.71 | 1.38, 2.13 | 7.0 | 1.9 | 0.6, 3.2 | 1.37 | 1.12, 1.69 |
| Discontinuers | 4,455 | 182 | 4.1 | 5.1 | ||||||||
| High-risk groupf | ||||||||||||
| Continuers | 384 | 46 | 12.0% | 7.3 | 3.8, 10.8 | 2.55 | 1.73, 3.74 | 11.7% | 4.4 | 0.8, 8.1 | 1.61 | 1.13, 2.29 |
| Discontinuers | 1,041 | 49 | 4.7% | 7.3% | ||||||||
TABLE 2. Unadjusted and Adjusted Risk of Gestational Diabetes Among Continuers and Discontinuers of Each Antipsychotic Medication and Risk Groupa
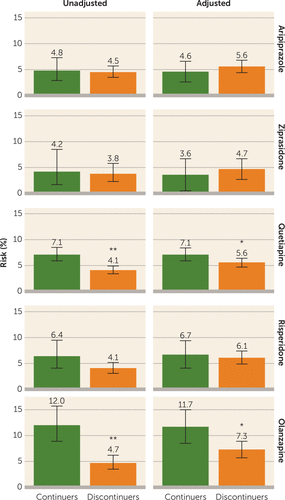
FIGURE 1. Absolute Risks of Gestational Diabetes for Unadjusted and Adjusted Analyses Among Women Who Continued and Discontinued Antipsychotic Treatment During Early Pregnancya
a The values at the top of each bar graph indicate the unadjusted and adjusted absolute risks.
*p<0.05. **p<0.0001.
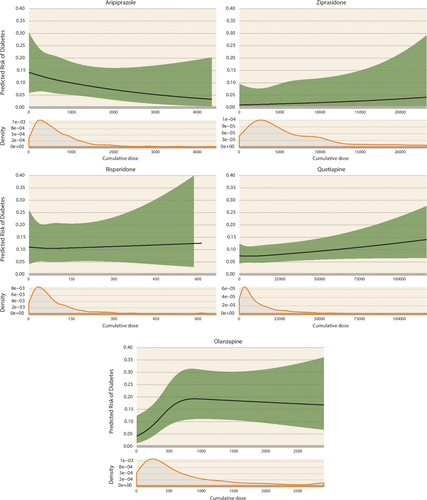
FIGURE 2. Dose-Response Analyses Between the Cumulative Dose of Antipsychotic Exposure During the First 20 Weeks of Pregnancy and the Risk of Gestational Diabetesa
a The upper panels show restricted cubic spline curves with three knots at the 25th, 50th, and 75th percentiles of the cumulative dose (mg) during the first 20 weeks of pregnancy (the last menstrual period to 140 days after the last menstrual period), with adjustment for age, race, obesity, diagnosis of schizophrenia or bipolar disorder, and the duration of treatment during the 3 months before the last menstrual period. The lower panels show the density curve indicating the distribution of the cumulative dose among the users of each antipsychotic medication who had one or more prescriptions dispensed during the first 20 weeks of pregnancy. To stabilize the dose-response curve at the extreme ranges, the maximum possible cumulative dose during the 140-day exposure window was limited to the daily maximum dose multiplied by 140 days for each antipsychotic drug (mg). To convert to a daily dose, the cumulative dose can be divided by the duration of the exposure window (140 days).
The adjusted relative risks in the low-risk (aripiprazole, ziprasidone), medium-risk (risperidone, quetiapine), and high-risk (olanzapine) groups were 0.91 (95% CI=0.60, 1.39), 1.37 (95% CI=1.12, 1.69), and 1.61 (95% CI=1.13, 2.29), respectively. Additional results for the group-level analyses are presented in Figure 3 (for further details, see Table S3 in the online data supplement). Across the different analyses, the risk for gestational diabetes appeared to be elevated among continuers compared with discontinuers in the high-risk and medium-risk groups but not in the low-risk group. However, the effects were less precisely estimated in some of the analyses due to the reduced sample size.
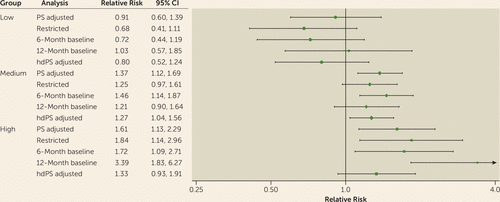
FIGURE 3. Forest Plot of the Results From Additional Analyses Based on the Risk-Stratified Groupsa
a The propensity-score (PS)–adjusted analysis was conducted to determine the adjusted relative risk from the PS stratification in the main analysis. The restricted analysis was limited to women with a baseline diagnosis of schizophrenia, bipolar disorder, or depression 6 months or 12 months before the last menstrual period (i.e., the analysis determined results from extending the 3-month baseline to either 6 months or 12 months). The high-dimensional PS (hdPS) analysis was conducted to determine stratification adjustment by using 50 additional confounder proxy variables in the PS estimation.
The bias analyses illustrated that with an overall obesity prevalence of 62% among women observed in the Massachusetts General Hospital registry who were treated with atypical antipsychotics, the absolute difference in the prevalence of obesity between continuers and discontinuers would have to be more than 25% for the observed relative risk of 1.61 among women treated with olanzapine to be completely attributable to residual confounding (Figure 4A) (also see Table S4 in the online data supplement). To put this into context, a 25% difference would mean that 80% of continuers and 55% of discontinuers would be obese patients, with all other covariates balanced between the two groups. Among women treated with quetiapine, the difference would have to be greater than 20% (Figure 4B) (also see Table S4 in the online data supplement). If, in contrast, there were more obese women among discontinuers than among continuers, the obesity-adjusted relative risks would be higher than what we observed for both olanzapine and quetiapine. However, if we assume the relative risks to be closer to the lower bounds of each confidence interval, confounding due to smaller differences in obesity could explain the increased relative risk.
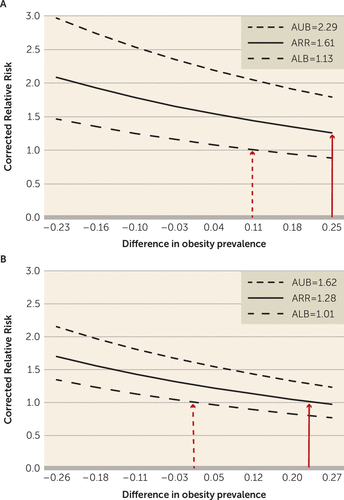
FIGURE 4. The Potential Effect of Obesity as an Unmeasured Confounder on the Observed Relative Risk Among Women Who Received Treatment With Olanzapine or Quetiapinea
a Difference in the obesity prevalence (x-axis) was calculated as PC1–PC0. Assuming that obese women have four times the risk of gestational diabetes compared with nonobese women, the corrected or true relative risk (y-axis) was obtained by dividing the apparent relative risk (ARR) before adjustment for obesity with the bias factor on the right side of the following formula:

Dotted lines above and below the solid lines correspond to the upper and lower bounds, respectively, of the confidence intervals for the point estimate. The solid red arrows show that a >25% difference in the obesity prevalence among women who received treatment with olanzapine, or a >20% difference in the obesity prevalence among women who received treatment with quetiapine, was required to explain the observed effect (ARR) by confounding only. The dotted red arrows show that when the uncertainty of the estimated observed effect was taken into account, a smaller difference in the obesity prevalence could account for the observed effect. ALB=apparent lower bound; AUB=apparent upper bound, PC1=obesity prevalence among the group exposed to the medication; PC0=obesity prevalence among the group unexposed to the medication; and RRCD=strength of association between obesity and the risk of gestational diabetes.
Discussion
Among pregnant women who were treated with an atypical antipsychotic before the start of pregnancy, continuation of treatment throughout the first half of pregnancy was associated with a moderate increased risk for gestational diabetes with olanzapine and quetiapine. We did not observe evidence of an increased risk among women who continued treatment with aripiprazole, ziprasidone, or risperidone. Multiple analyses consistently showed a stronger association with olanzapine. Furthermore, there was evidence of a cumulative dose-response relationship with olanzapine.
It is important to consider alternative explanations for these findings, as warranted for any observational study. The main concern pertaining to this study is potential residual confounding by factors not captured comprehensively in the data, particularly obesity. Through formal bias analyses, we demonstrated that the imbalance in the obesity prevalence between treatment continuers and discontinuers would have to be very high (i.e., 20%−25%) after accounting for all other covariates to fully explain the observed risk. Although this possibility cannot be excluded, it seems unlikely given that all of the women were treated with an atypical antipsychotic before the start of pregnancy and we accounted for a broad range of proxy variables. The prevalence of preexisting diabetes and risk factors tended to be higher with ziprasidone and lower with olanzapine, which suggests that the prescribing of antipsychotics may be somewhat selective with respect to such factors. Alternatively, this finding may be explained by depletion of the most susceptible women, who could have developed diabetes after initiation of olanzapine and before their last menstrual period and were excluded from our cohort, leaving for inclusion women with a lower baseline risk who continued treatment. Despite having fewer documented baseline risk factors for developing gestational diabetes, olanzapine continuers had the highest absolute risk for gestational diabetes compared with women who continued treatment with antipsychotics that are less likely to cause significant weight gain. Our findings are consistent with prior knowledge that olanzapine induces the most weight gain among the five study drugs (aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone) (7, 21), which provides a plausible mechanism for the observed elevation in risk among women who continued treatment with this medication. Although quetiapine was associated with a small increased risk for gestational diabetes in this study, risperidone, which is similarly associated with modest weight gain (21), was not. Use of low-dose quetiapine for insomnia is well known, and women without psychiatric disorders who are treated with quetiapine for insomnia before the start of pregnancy may be more likely to discontinue after becoming pregnant. While this possible scenario could have resulted in confounding in the main quetiapine analysis, restricting the analysis to women with a diagnosis of a psychiatric disorder showed nearly identical results. Further research is needed to confirm this finding.
Only a few studies have investigated the association between antipsychotic exposure during pregnancy and the risk of gestational diabetes, and even fewer have considered specific drugs. By using the Swedish National registries, Reis and Källén (16) observed an increase in the risk for gestational diabetes (odds ratio=1.78 [95% CI=1.04, 3.01]) among women who self-reported any antipsychotic use during early pregnancy, and Bodén et al. (15) reported that women who were treated with olanzapine or clozapine during pregnancy had a higher risk for gestational diabetes (odds ratio=1.94 [95% CI=0.97, 3.91]) compared with women who were not treated with either drug. In a high-dimensional propensity-score-matched cohort comprising Canadian women, Vigod et al. (17) did not observe an increased risk for gestational diabetes among women who were treated with any of the antipsychotics assessed (relative risk=1.10 [95% CI=0.77, 1.57]) or in a specific analysis of women who were treated with olanzapine (N=166). Unlike in our study, the three aforementioned studies included women who were unexposed to antipsychotics as a reference group, a population less comparable in terms of health status and illness severity than discontinuers, and these studies did not exclude women with preexisting diabetes. The higher relative risks in the Swedish studies may be partly due to the fact that they adjusted for only a small number of confounders. The absolute risk among the unexposed women in the Vigod et al. (17) study was higher than the absolute risk among the discontinuers in our study (6.2% compared with 4%−5%), which implies significant difference in the baseline risk among the two study populations and potentially why we observed different results. In a recent study (18), women who were treated with antipsychotics during early pregnancy were compared with women with psychiatric disorders who were not treated with antipsychotics to increase the comparability of the groups, under the assumption that other psychotropic medications do not affect the risk of gestational diabetes. However, the sample size was insufficient to consider individual antipsychotics, and specific antipsychotics may have differential effects on the risk of gestational diabetes.
Our study has several strengths. The study population was taken from the nationwide Medicaid program, which finances close to 50% of all pregnancies in the United States (40). Moreover, Medicaid finances 80% of all antipsychotic prescriptions and 36% of all treatment costs for gestational diabetes in the United States (41, 42). To define exposure, we used automated dispensing records, which are free of recall bias, and a validated outcome definition. To our knowledge, this study is one of the largest studies of women who continued antipsychotic treatment during pregnancy, and we were able to investigate individual drug effects rather than a drug-class effect.
However, our study is not without some limitations. It is possible that residual confounding due to unmeasured or poorly measured factors, such as lifestyle factors, occurred. Yet comparing treatment continuers with discontinuers rather than with persons unexposed to antipsychotics alleviates this concern because discontinuers are likely to be more similar to continuers than to a group unexposed to antipsychotics. In addition to conducting a formal bias analysis, we showed that adjusting for a large number of empirically identified confounders that may serve as proxies for unmeasured or poorly measured confounders does not change the findings. We could not fully adjust for the duration of antipsychotic exposure, which may have extended many years before recording was conducted in our database. However, adjusting for the treatment duration during the year before pregnancy provided consistent results. We do not have data on the reasons for discontinuation of treatment, which may be associated with illness severity or indication for antipsychotic treatment not recorded in our data. However, illness severity seems unlikely to explain the observed associations, since we observed an increased risk only for gestational diabetes with use of selected antipsychotics. A pharmacy-dispensing record does not guarantee the actual intake of a specific drug. However, by requiring at least two prescriptions during the first 20 weeks of pregnancy, we were fairly confident that the continuers in our study actually took the prescribed medication.
Conclusions
In a large cohort of women without preexisting diabetes who were treated with antipsychotics before pregnancy, we observed an increased risk for developing gestational diabetes among women who continued to use olanzapine or quetiapine during the first 20 weeks of pregnancy compared with women who discontinued treatment. There was a positive dose-response relationship between the use of olanzapine and risk for gestational diabetes. We did not observe a difference in the risk for gestational diabetes when comparing women who continued treatment with aripiprazole, ziprasidone, and risperidone with women who discontinued treatment with these antipsychotics. Further studies are needed in order to understand the potential effect of switching antipsychotic agents during pregnancy on the risk for gestational diabetes. Such information would aid treatment decisions pertaining to women for whom treatment discontinuation is not an option. In conclusion, although the risk for gestational diabetes is an important consideration in the selection of a specific drug, other dimensions of antipsychotic treatment, including the benefit of continuing a specific treatment and the risk of efficacy loss due to changes in treatment, should be taken into account in the treatment decisions for pregnant women.
1 Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014; 103:341–363Crossref, Medline, Google Scholar
2 : Practice bulletin no 137: gestational diabetes mellitus. Obstet Gynecol 2013; 122:406–416Crossref, Medline, Google Scholar
3 : Prevalence estimates of gestational diabetes mellitus in the United States–Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014; 11:E104Crossref, Medline, Google Scholar
4 : Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007): use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111:1001–1020Medline, Google Scholar
5 : Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009; 373:1773–1779Crossref, Medline, Google Scholar
6 : Gestational diabetes: the need for a common ground. Lancet 2009; 373:1789–1797Crossref, Medline, Google Scholar
7 : Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 2014; 9:e94112Crossref, Medline, Google Scholar
8 : Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 2013; 70:1067–1075Crossref, Medline, Google Scholar
9 : Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics 2011; 128:1135–1141Crossref, Medline, Google Scholar
10 : Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596–601Crossref, Medline, Google Scholar
11 : Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord 2002; 70:19–26Crossref, Medline, Google Scholar
12 : What is gestational diabetes? Diabetes Care 2007; 30(suppl 2):S105–S111Crossref, Medline, Google Scholar
13 : Atypical antipsychotic use in pregnancy: a retrospective review. Arch Women Ment Health 2009; 12:53–57Crossref, Medline, Google Scholar
14 : Pregnancy exposure to second-generation antipsychotics and the risk of gestational diabetes. Expert Opin Drug Saf 2014; 13:1583–1590Crossref, Medline, Google Scholar
15 : Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry 2012; 69:715–721Crossref, Medline, Google Scholar
16 : Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol 2008; 28:279–288Crossref, Medline, Google Scholar
17 : Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. BMJ 2015; 350:h2298Crossref, Medline, Google Scholar
18 : Use of atypical antipsychotics in pregnancy and maternal gestational diabetes. J Psychiatr Res 2017; 95:84–90Crossref, Medline, Google Scholar
19 Long-term treatment with atypical antipsychotics and the risk of weight gain: a literature analysis. Drug Saf 2006; 29:303–319Crossref, Medline, Google Scholar
20 : Contributing factors to weight gain during long-term treatment with second-generation antipsychotics: a systematic appraisal and clinical implications. Obes Rev 2009; 10:527–542Crossref, Medline, Google Scholar
21 : Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19(suppl 1):1–93Crossref, Medline, Google Scholar
22 : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
23 : Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry 2008; 65:805–815Crossref, Medline, Google Scholar
24 : Prevalence and trends in the use of antipsychotic medications during pregnancy in the US, 2001–2007: a population-based study of 585,615 deliveries. Arch Women Ment Health 2013; 16:149–157Crossref, Medline, Google Scholar
25 : Increasing use of atypical antipsychotics and anticonvulsants during pregnancy. Pharmacoepidemiol Drug Saf 2013; 22:794–801Crossref, Medline, Google Scholar
26 : Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatr Serv 2017; 68:1112–1119Link, Google Scholar
27 : Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One 2013; 8:e67405Crossref, Medline, Google Scholar
28 : Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407Crossref, Medline, Google Scholar
29 : Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA 2015; 313:2142–2151Crossref, Medline, Google Scholar
30 : Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73:938–946Crossref, Medline, Google Scholar
31 : Validation of algorithms to ascertain clinical conditions and medical procedures used during pregnancy. Pharmacoepidemiol Drug Saf 2011; 20:1168–1176Crossref, Medline, Google Scholar
32 : A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017; 28:249–257Crossref, Medline, Google Scholar
33 : Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006; 98:253–259Crossref, Medline, Google Scholar
34 Li R, Hertzmark E, Louie M, et al: The SAS LGTPHCURV9 Macro. Cary, NC, SAS, 2011Google Scholar
35 Maglione M, Ruelaz MA, Hu J, et al: Off-label use of atypical antipsychotics: an update, comparative effectiveness review no 43. Rockville, Md, Agency for Healthcare Research and Quality, 2011. http://www.effectivehealthcare.ahrq.gov/reports/final.cfmGoogle Scholar
36 : High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009; 20:512–522Crossref, Medline, Google Scholar
37 : Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006; 15:291–303Crossref, Medline, Google Scholar
38 : Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry 2015; 76:986–989Crossref, Medline, Google Scholar
39 : Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007; 30:2070–2076Crossref, Medline, Google Scholar
40 Pandit S: Issue Brief, 2012–Maternal and Child Health Update. Washington, DC, National Governors Association, 2013Google Scholar
41 : Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manag 2009; 12:165–174Crossref, Medline, Google Scholar
42 : Mental health policy and psychotropic drugs. Milbank Q 2005; 83:271–298Crossref, Medline, Google Scholar



