Antidepressant Outcomes Predicted by Genetic Variation in Corticotropin-Releasing Hormone Binding Protein
Abstract
Objective:
Genetic variation within the hypothalamic-pituitary-adrenal (HPA) axis has been linked to risk for depression and antidepressant response. However, these associations have yet to produce clinical gains that inform treatment decisions. The authors investigated whether variation within HPA axis genes predicts antidepressant outcomes within two large clinical trials.
Method:
The test sample comprised 636 patients from the International Study to Predict Optimized Treatment in Depression (iSPOT-D) who completed baseline and 8-week follow-up visits and for whom complete genotyping data were available. The authors tested the relationship between genotype at 16 candidate HPA axis single-nucleotide polymorphisms (SNPs) and treatment outcomes for three commonly used antidepressants (escitalopram, sertraline, and extended-release venlafaxine), using multivariable linear and logistic regression with Bonferroni correction. Response and remission were defined using the Hamilton Depression Rating Scale. Findings were then validated using the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study of outcome predictors in treatment-naive patients with major depression.
Results:
The authors found that the rs28365143 variant within the corticotropin-releasing hormone binding protein (CRHBP) gene predicted antidepressant outcomes for remission, response, and symptom change. Patients homozygous for the G allele of rs28365143 had greater remission rates, response rates, and symptom reductions. These effects were specific to drug class. Patients homozygous for the G allele responded significantly better to the selective serotonin reuptake inhibitors escitalopram and sertraline than did A allele carriers. In contrast, rs28365143 genotype was not associated with treatment outcomes for the serotonin norepinephrine reuptake inhibitor venlafaxine. When patients were stratified by race, the overall effect of genotype on treatment response remained. In the validation sample, the GG genotype was again associated with favorable antidepressant outcomes, with comparable effect sizes.
Conclusions:
These findings suggest that a specific CRHBP SNP, rs28365143, may have a role in predicting which patients will improve with antidepressants and which type of antidepressant may be most effective. The results add to the foundational knowledge needed to advance a precision approach to personalized antidepressant choices.
Disturbances of the hypothalamic-pituitary-adrenal (HPA) axis, a major component of the mammalian response to stress, have been consistently documented in major depressive disorder. Both corticotropin-releasing hormone (CRH), a key regulator of the axis, and cortisol, its end product, play critical roles in coordinating the endocrine, behavioral, autonomic, and immune responses to stress (1). Plasma concentrations of cortisol have been linked not only to risk for depression but also to illness course and treatment outcome in major depression (2, 3). Although genetic variation within HPA axis genes (including those for CRH and its receptors) has been linked to depression risk and prognosis, it remains unknown whether genetic variation within the HPA axis contributes to a differential prediction of outcomes for specific types of antidepressants. In this study, we sought to determine whether individual variation within five HPA axis genes was predictive of antidepressant response, and if so, whether this prediction was related to antidepressant type.
Dysregulation of the HPA axis was one of the earliest reported biological characteristics of major depression (4, 5), and it appears to be a central feature of the pathophysiology of depression. Depressed individuals have been shown to exhibit higher salivary and plasma cortisol concentrations (2, 6) as well as impaired cortisol suppression by dexamethasone and altered 24-hour cortisol amplitude (2, 7). In addition, high levels of morning cortisol have been linked to an elevated risk for depression in adolescents (8, 9). Unsurprisingly, upstream regulators of cortisol, including CRH and its receptors and binding protein, have also been implicated in depression. Elevated CRH concentrations have been repeatedly reported in the CSF of depressed individuals (10, 11) and individuals who died by suicide (12). Disturbances in the HPA axis have also been postulated to correspond with illness course, as HPA axis dysfunction appears to worsen with the number of successive depressive episodes a patient experiences (13, 14). Yet other studies have reported either no difference between cortisol levels in depressed and comparison patients or reduced cortisol levels in depressed individuals (15, 16). Thus, the picture regarding HPA dysfunction, and cortisol dysfunction in particular, appears to be a mixed one, varying as a function of type of measurement, time of measurement, and patient characteristics (for a review, see reference 17).
Given the potential role of CRH and cortisol in the pathophysiology of depression, variation in the genes regulating this pathway has been explored as a potential risk factor for depression. CRH binds to two membrane-bound receptors (CRHR1 and CRHR2) and forms a complex with corticotropin-releasing hormone binding protein (CRHBP) both in the periphery and in the CNS. The formation of this complex regulates the amount of free CRH, which in turn controls direct CNS response to CRH and indirectly affects the amount of cortisol released by the adrenal glands. Cortisol then binds to the glucocorticoid receptor NR3C1 throughout the cortex and the rest of the body, regulating downstream biological and behavioral responses to stress (18). Cortisol system variation that has been found to be associated with depression diagnosis includes two SNPs within CRHBP (rs7728378 and rs1875999) (19), as well as one SNP in the CRHR1 gene (rs110402) that is thought to mediate the relationship between childhood trauma and the development of depression (20). Another variant in CRHR1, rs4076452, was found to correlate significantly with depression severity rating (as well as psychosis in depression) (21). In addition, one CRHR2 SNP (CRHR2s183) has been associated with unipolar depression, although this association was not replicated in a separate cohort (22). A constellation of SNPs within the glucocorticoid receptor NR3C1 was also found to be associated with both cortisol levels and psychosis in major depression (21), providing further evidence that the HPA axis plays a key role in depressive symptomatology.
HPA axis dysregulation may also be associated with recovery from depression. Treatments for major depression, including pharmacotherapy, may (at least partially) begin to normalize these HPA axis abnormalities (3, 23, 24), suggesting that the normalization process itself may be related to treatment outcome. Moreover, it is possible that baseline HPA axis function (potentially influenced by variation in genotype) may interact with pharmacotherapy to affect the probability of treatment response, independently of treatment-dependent changes in HPA axis function. Recent research appears to support this hypothesis: two loci within CRHR1 (the SNP rs242941, as well as a three-SNP haplotype), together with loci within CRHR2 (rs2270007) and CRHBP (rs10473984), have all been associated with treatment response to various antidepressants (25–28). However, it is unknown whether additional variants within this system contribute to general or class-specific antidepressant response in depression. In sum, it appears that genetic variation is related to both HPA axis function and depression treatment response, but the precise interaction between genetics and treatment response has yet to be fully explored.
Our primary goal in this study was to establish whether variation in five CRH and cortisol-related genes (CRH, CRHR1, CRHR2, CRHBP, and NR3C1) contributes to symptom outcomes following acute antidepressant treatment in major depression. We first assessed whether variation in these genes predicts symptom reductions across three commonly prescribed antidepressants (escitalopram, sertraline, and extended-release venlafaxine), irrespective of antidepressant type. Next, we assessed whether CRH and cortisol-related genotype predicted outcomes based on antidepressant type—selective serotonin reuptake inhibitors (SSRIs) (escitalopram and sertraline) versus serotonin-norepinephrine reuptake inhibitors (SNRIs) (venlafaxine). The ultimate goal is to develop treatment-specific response probabilities based on genetic variation in order to guide treatment decisions in a personalized manner.
Method
Trial Design
The International Study to Predict Optimized Treatment in Depression (iSPOT-D) is a multisite randomized practical clinical trial designed to identify predictors of antidepressant efficacy in major depressive disorder. A total of 1,008 adults with major depression were assessed across 17 sites in the United States, the Netherlands, Australia, New Zealand, and South Africa between December 2008 and January 2012 (29).
Participant Inclusion and Exclusion Criteria
Participants met primary inclusion criteria, including a DSM-IV diagnosis of nonpsychotic major depressive disorder as assessed by the Mini International Neuropsychiatric Interview, version 5.0, a score ≥16 on the Hamilton Depression Rating Scale (HAM-D), and age between 18 and 65 years. Participants were excluded if they had a positive urine toxicology screen, a comorbid psychotic disorder, posttraumatic stress disorder, an axis II personality disorder, or any medical condition that might interfere with assessments or medication safety. Additional details are provided in the protocol report (29).
Treatment
Participants, who either were antidepressant naive or underwent a washout period of at least 1 week, were randomly assigned to receive escitalopram, sertraline, or extended-release venlafaxine, using a block design by PhaseForward’s validated web-based interactive response technology (29). Research personnel involved in subsequent study assessments were blind to randomization. After randomization, medications were adjusted by treating clinicians according to clinical judgment and recommended dosage ranges. Treatment for concomitant medical conditions was permitted and recorded (for sample details, see reference 30).
Genotyping
Genotype extraction was performed using the Puregene DNA method (Qiagen, Valencia, Calif.) on EDTA-treated blood. Genotyping was done using the Illumina VeraCode Golden Gate SNP genotyping platform (Illumina, Hayward, Calif.) by Covance, Inc. (Seattle). Sixteen candidate SNPs from four HPA axis genes, along with 663 other candidate loci in genes that have been implicated previously in depression, were selected for genotyping. To specifically investigate the role of the HPA axis in depression, the present study focuses only on the 16 HPA axis SNPs within the genes CRH, CRHBP, CRHR1, CRHR2, and NR3C1.
We also screened for deviation from Hardy-Weinberg equilibrium at each of the 16 SNPs of interest (Table 1). Because our sample included Caucasian and non-Caucasian groups, recorded using standard World Health Organization formats, we also calculated the p values for deviation from Hardy-Weinberg equilibrium for the two largest racial subgroups in our sample, white and black participants. Although three SNPs deviated from Hardy-Weinberg equilibrium in the entire sample, none deviated significantly in either ethnic subgroup.
| Gene | SNP | Chr:pos | Major/Minor Allele | Minor Allele Frequency (%) | H-W p | H-W p: White Participants | H-W p: Black Participants |
|---|---|---|---|---|---|---|---|
| CRH | rs3176921 | 8:66179144 | A/G | 17.2 | 2.2×10−8 | 0.40 | 0.06 |
| rs5030875 | 8:66181831 | A/C | 5.3b | 0.95 | 0.69 | 0.76 | |
| CRHBP | rs10055255 | 5:76968168 | T/A | 46.7 | 0.0013 | 0.26 | 0.26 |
| rs28365143 | 5:76952261 | G/A | 7.4b | 0.36 | 0.40 | 0.40 | |
| CRHR1 | rs110402 | 17:45802681 | C/T | 46.3 | 0.19 | 0.61 | 0.61 |
| rs1876828 | 17:45834159 | C/T | 18.5 | 0.13 | 0.96 | 0.96 | |
| rs242924 | 17:45808001 | T/G | 46.1 | 0.05 | 0.40 | 0.56 | |
| rs242939 | 17:45818213 | T/C | 9.7b | 0.41 | 0.21 | 0.73 | |
| rs4076452 | 17:45778528 | G/C | 16.0b | 0.96 | 0.93 | 0.71 | |
| rs6472257 | 8:66179945 | C/T/G | 11.9 (G)b | 0.50 | 0.60 | 0.91 | |
| CRHR2 | rs2267712 | 7:30672618 | C/A | 17.2 | 0.46 | 0.95 | 0.57 |
| rs2270007 | 7:30660356 | C/G | 17.9 | 0.031 | 0.52 | 0.81 | |
| rs2284216 | 7:30672345 | G/T | 11.0b | 0.50 | 0.93 | 0.64 | |
| rs4723003 | 7:30686125 | C/T | 10.7b | 0.58 | 0.81 | 0.39 | |
| NR3C1 | rs6918 | 5:143278056 | A/C | 13.8b | 0.40 | 0.17 | 0.59 |
| rs2963156 | 5:143378931 | C/T | 19.2 | 0.52 | 0.94 | 0.92 |
TABLE 1. Candidate SNP Information in a Study of Antidepressant Outcomes and Genetic Variation in Corticotropin-Releasing Hormone Binding Proteina
Sample Characteristics
Of the 1,008 patients randomly assigned to antidepressant treatment, complete genotype data were available for 900. Of these, 636 also completed the 8-week posttreatment follow-up visit. The 636 patients who were genotyped (i.e., had genotype information at >50% of the candidate SNPs) and completed 8 weeks of treatment constituted the sample for the present analysis. (For details, see the CONSORT flow diagram in Figure S1 in the data supplement that accompanies the online edition of this article.)
The 636 patients with complete genotype data did not differ significantly in demographic, clinical, and medication dosage characteristics from the initially randomized sample of 900 patients with complete genotype information (Table 2) or from the full randomized sample of 1,008. Allelic frequency of all 16 SNPs also did not differ between those who completed the study and the full sample (see Table S1 in the online data supplement).
| Variable | Participants Who Completed the Study (N=636) | All Genotyped Participants (N=900) | |||
|---|---|---|---|---|---|
| N | % | N | % | pa | |
| Female | 359 | 56.4 | 508 | 56.4 | 1.00 |
| Race | 0.97 | ||||
| White | 382 | 60.1 | 423 | 47.0 | |
| Black | 97 | 15.2 | 105 | 11.7 | |
| Asian | 43 | 6.7 | 51 | 5.7 | |
| Other | 89 | 14.0 | 104 | 11.6 | |
| Missing | 25 | 3.9 | 217 | 24.1 | |
| Antidepressant | 1.00 | ||||
| Escitalopram | 206 | 32.4 | 299 | 33.2 | |
| Sertraline | 225 | 35.4 | 304 | 33.8 | |
| Venlafaxine (extended release) | 205 | 32.2 | 297 | 33.0 | |
| Mean | SD | Mean | SD | pa | |
| Antidepressant dosage (mg/day) | |||||
| Escitalopram | 12.0 | 6.0 | 12.1 | 7.0 | 0.83 |
| Sertraline | 61.0 | 33.2 | 59.8 | 33.1 | 0.69 |
| Venlafaxine (extended release) | 80.7 | 41.5 | 78.9 | 42.0 | 0.66 |
| Equivalent dosageb (mg/day) | |||||
| Escitalopram | 89.6 | 44.9 | 90.6 | 52.4 | 0.83 |
| Sertraline | 91.6 | 49.7 | 89.7 | 49.6 | 0.69 |
| Venlafaxine (extended release) | 80.7 | 41.5 | 78.9 | 42.0 | 0.66 |
| Age at baseline visit (years) | 38.9 | 12.8 | 38.1 | 12.7 | 0.22 |
| Age at illness onset (years) | 23.3 | 12.2 | 23.1 | 12.1 | 0.80 |
| Duration of illness (years) | 15.1 | 12.6 | 14.4 | 12.2 | 0.30 |
| Hamilton Depression Rating Scalec | |||||
| Baseline score | 21.8 | 4.07 | 21.84 | 4.1 | 0.71 |
| Posttreatment score | 9.7 | 6.36 | |||
TABLE 2. Characteristics of Participants Who Completed the Study Compared With the Full Genotyped Sample in a Study of Antidepressant Outcomes and Genetic Variation in Corticotropin-Releasing Hormone Binding Protein
Treatment Outcome Measures
The primary outcome measures used in this study were consistent with the protocol for the iSPOT-D study (29). Acute outcomes were assessed after 8 weeks of clinician-monitored antidepressant treatment using the clinician-rated symptom change assessed by the 17-item HAM-D. In the present study, we assessed percent reduction in HAM-D score, absolute score reduction, response (defined as a reduction of ≥50% in HAM-D score), and remission (defined as a score ≤7 on the HAM-D).
Statistical Analysis: Treatment Outcome Prediction by Genotype
The first aim of the study was to determine whether the genotype of any of the 16 candidate SNPs predicted the magnitude of symptom improvement after 8 weeks of antidepressant treatment. Linear regression for each SNP was performed with percent reduction in HAM-D score as the primary outcome measure and the candidate SNP (see Table 1) as a predictor. We followed an additive allelic model, with genotype coded as 0, 1, or 2, representing number of alternate alleles in each individual. However, to preserve power in SNPs at which fewer than 20 participants had the homozygous alternate genotype, genotype was coded as a binary variable representing the presence of any alternate alleles. Age and recruitment site were included as covariates in each regression model, because they were significantly associated with our outcomes of interest, as has been reported previously (32). As in that previous study, recruitment sites were grouped by geographic location into seven groups, each with a minimum of 50 participants. Each SNP was then individually regressed on outcome in a regression model including covariates, and all SNPs that significantly predicted outcome above and beyond covariates are reported here. We used the eigenvalue-based method described by Li and Ji (33) to determine the effective number of hypotheses being tested given observed linkage disequilibrium between candidate SNPs; this yielded 13 effective hypotheses and a corrected significance threshold (p) of 0.003938. For the purposes of completeness, we report all SNPs with uncorrected p values below 0.05.
Linear regression was used to assess prediction of continuous measures of symptom change. Because there are small statistical differences between using percent and linear reduction as an outcome measure, all results obtained with percent change as the outcome variable were validated using absolute HAM-D score reduction and controlling for initial score as well.
Following the same approach, we used logistic regression to assess binary treatment outcomes of response and remission, as defined above. In addition to the covariates of age and recruitment site, baseline HAM-D score was also included as a covariate when assessing remission status. A corrected p value of 0.00398 was used as the threshold for statistical significance, with all p values <0.05 reported for completeness. This correction was based on adjustment for the number of SNPs (each was a separate hypothesis) but not for outcome measures, which are metrics derived from the same measure.
Validation Sample
The SNPs that were significantly associated with treatment response in the initial iSPOT-D cohort were then validated using an independent sample of patients from the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) trial (34). To achieve equivalence of treatment arms in the original iSPOT-D and validation PReDICT samples, only PReDICT patients assigned to either of the two pharmacotherapy arms, consisting of treatment with either escitalopram or duloxetine, were included for validation. Although duloxetine was not used in the original iSPOT-D cohort, it belongs to the same medication class (SNRI) as venlafaxine. The full description of this sample has been published previously (34); further details can be found in the Supplementary Methods section in the online data supplement. In this sample, rs28365143 genotype was imputed based on genome-wide markers from the Illumina Omni-Express array data using the 1000 Genomes Project phase 3 reference haplotypes with IMPUTE2 (35) and prephased with SHAPEIT2 (36). Among participants with complete imputed genotypes for rs28365143 (N=141), the best-guess genotype call rate in the full sample was 0.9338.
Validation was evaluated using the primary outcome of percent reduction in HAM-D score and the secondary outcomes of absolute HAM-D reduction, response, and remission. Because of the smaller sample size of the validation cohort, particularly the limited number of participants with the A allele at rs28365143, and the focus on two rather than three medication arms, we focused the validation on the overall effect of SNP on treatment response and not on additional interactions testing differential outcomes based on antidepressant class.
Statistical Analysis: Treatment Outcome Prediction by Genotype and Drug Class
To determine whether the relationship between genotype and treatment outcome was moderated by antidepressant type, treatment arm was introduced as an interaction term to the logistic and linear models mentioned above that met the uncorrected significance threshold of p<0.05 in the original sample. The significance of the interaction between drug type (SSRI versus SNRI) and genotype was reported, along with the main effect for genotype within each medication class. To verify that any significant differences in the SSRI class were not due to a larger sample size for that class, we also conducted regressions to determine the interaction between individual drug (escitalopram, sertraline, venlafaxine) and genotype. The interaction term between genotype and each drug type was then assessed for significance (p<0.05).
As in the previous analysis, the above multivariable linear regressions were conducted with percent change in HAM-D score as the primary outcome variable, followed by multivariable logistic regressions with response and remission as the binary dependent outcome variables. The same set of covariates (age, site, and initial HAM-D score for computation of score reduction and meeting the remission criterion) were used as in the preceding main effect analysis. This interaction was explored only in the original iSPOT-D cohort because of power considerations and the smaller size of the validation (PReDICT) sample.
Statistical Analysis: Stratification by Ethnicity
Given the potential for population stratification to cause false positive associations in genetic association studies, we stratified our original (iSPOT-D) sample by Caucasian versus non-Caucasian participants and examined the association between genotype and outcome for each SNP that was significantly associated with treatment outcome in the entire sample. More specific stratification by ethnicity was not possible because of the small numbers of non-Caucasian participants in our sample belonging to each ethnic subgroup (a total of 254 participants; 97 black, 43 Asian, 114 other). Because of power considerations and the small number of patients with many of the alternate genotypes receiving SNRI treatment, stratification by ethnicity, genotype, and treatment type was not performed. In addition, because of the smaller size of the validation sample (67 Caucasian, 74 other), stratification by ethnicity was only performed in the larger iSPOT-D cohort.
Results
Treatment Outcome Prediction by Genotype
Of the 16 SNPs, only one (rs28365143, within the gene CRHBP) significantly predicted the primary outcome measure (percent HAM-D score reduction) before (p<0.05) and after Bonferroni correction (p<0.003938). Overall, the GG genotype (homozygous reference) was associated with a larger percent reduction in HAM-D score compared with genotypes containing A alleles (AG/AA) (β=−0.12, p=9.9×10−5) (Figure 1). (For results of candidate SNPs that did not meet statistical significance, see Table S2 in the online data supplement.) Similarly, only rs28365143 emerged as a significant predictor of absolute HAM-D score reduction, with the GG genotype again associated with greater score reductions (β=−2.51, p=2.5×10−4).
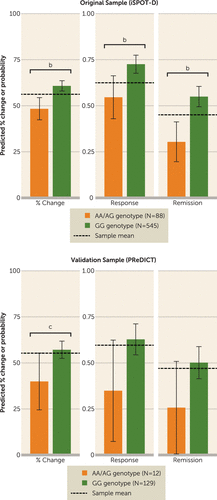
FIGURE 1. CRHBP rs28365143 Genotype and Predicted Reductions in Depressive Symptoms Based on Regression Models in Both the Original and Validation Cohortsa
a Predicted percent reduction in Hamilton Depression Rating Scale (HAM-D) score and probability of response and remission were calculated for each CRHBP rs28365143 genotype using the regression models described in the text. To calculate the expected output for an “average” participant, all other covariates (age, sex, initial HAM-D score, site) were set to the mean value for that variable in the cohort. The p values are those of the beta coefficient in the linear or logistic regression model. iSPOT-D=International Study to Predict Optimized Treatment in Depression; PReDICT=Predictors of Remission in Depression to Individual and Combined Treatments.
b p<0.003938, Bonferroni-corrected threshold for 13 hypotheses.
c p<0.05, uncorrected.
The same associations were observed for the two clinical outcome measures, response and remission, with significant associations between rs28365143 genotype and response (odds ratio=0.46, uncorrected p=0.0017) and remission (odds ratio=0.36, uncorrected p=1.6×10−4). Again, rs28365143 was the only SNP that was significantly associated with outcomes, and these associations withstood Bonferroni correction (Figure 1).
Validation Sample
In the validation sample, participants who were homozygous for the reference allele (GG genotype) also showed greater percent reductions in HAM-D score after 8 weeks of pharmacotherapy with either escitalopram or duloxetine (β=−0.178, p=0.034). When it came to the secondary outcomes of interest—absolute reduction in HAM-D score, response, and remission—the homozygous reference genotype was associated with a favorable effect on antidepressant outcomes, although these results did not reach the threshold for statistical significance, likely because of the smaller size of the validation sample. Notably, however, the size of the effect of the rs28365143 genotype on treatment outcome was equivalent between the original iSPOT-D sample and the validation PReDICT sample (Table 3). Arguably, given the differences in sample sizes, the effect size is a more comparable metric for establishing reproducibility than p value.
| Original Sample (iSPOT-D)(N=636) | Validation Sample (PReDICT)(N=141) | |||
|---|---|---|---|---|
| Measure | Estimate | p | Estimate | p |
| Percent reduction | β=–0.12 | 9.9×10–5 | β=–0.178 | 0.034 |
| Linear reduction | β=–2.51 | 2.5×10–4 | β=–3.578 | 0.031 |
| Response | Odds ratio=0.46 | 0.0017 | Odds ratio=0.31 | 0.069 |
| Remission | Odds ratio=0.36 | 1.6×10–4 | Odds ratio=0.32 | 0.11 |
TABLE 3. Regression Coefficients for the Association of SNP With Antidepressant Treatment Response in the Original iSPOT-D Test Sample and in the PReDICT Validation Samplea
Treatment Outcome Prediction by Genotype and Drug Class
There was additionally a significant interaction between rs28365143 genotype and type of antidepressant (p=0.014) in the original iSPOT-D cohort when predicting percent reduction in HAM-D score. Patients homozygous for the reference allele (GG) had better outcomes (greater percent reductions in HAM-D score) than carriers of the alternate A allele when treated with an SSRI (escitalopram and sertraline arms combined, βSNP=−0.17, p=5.02×10−6). This result held when the escitalopram and sertraline arms were analyzed individually (see Table S3 in the data supplement). While rs28365143 genotype was not a significant predictor of treatment outcome in patients taking the SNRI venlafaxine (βSNP=−0.01, p=0.89), the homozygous reference genotype was linked to a 16%−18% greater reduction in HAM-D score for patients taking SSRIs (escitalopram: βSNP=−0.18, p=5.81×10−4; sertraline: βSNP=−0.16, p=0.0020). The interaction of rs28365143 and type of antidepressant was reproduced when we analyzed absolute reduction in HAM-D score (β=−3.21, p=0.031) and response (β=−1.48, p=0.0066). When it came to remission, while the effect of A allele was significant for both SSRIs combined (βSNP=−1.33, p=4.47×10−5) and each individually (escitalopram: βSNP=−1.30, p=0.0032; sertraline: βSNP=−1.38, p=0.0036), the overall interaction was not significant (β=−1.03, p=0.071). As illustrated in Figure 2, patients homozygous for the reference allele (GG) had consistently better outcomes than carriers of the alternate A allele, irrespective of how outcome was quantified, and specifically when treated with SSRIs. Again, these interactions were not assessed in the validation cohort because of its limited sample size.
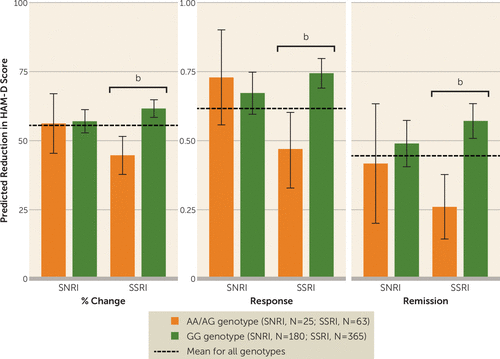
FIGURE 2. Drug Class–Specific Effects of CRHBP rs28365143 Genotype on Response to Pharmacotherapy in the Original iSPOT-D Cohorta
a The graphs illustrate the percent change in score on the Hamilton Depression Rating Scale (HAM-D) and the probabilities of response and remission given the overall regression model with an interaction term between drug class (coded as SSRI or SNRI) and genotype. As in Figure 1, all other covariates (age, sex, initial HAM-D score, site) were set to the mean levels of the entire cohort to isolate the effect of genotype.
b p<0.05.
Stratification by Ethnicity
In both the Caucasian and the non-Caucasian subgroups of the original iSPOT-D cohort, the alternate allele of rs28365143 was again associated with smaller percent reductions in HAM-D score (Caucasian: β=−0.11, p=0.009; non-Caucasian: β=−0.12, p=0.011), and the magnitude of this association was comparable to that of the entire sample (see Table S4 in the data supplement). Because of the reduction in power due to stratification, however, the significance of this association did not withstand correction for multiple hypothesis testing in either subgroup. However, the magnitude and direction of the association between genotype and treatment outcome was comparable to that in the entire sample when the secondary outcomes of linear reduction in HAM-D score, response, and remission were examined.
Discussion
We report on new evidence for the role of the SNP rs28365143, within an intron of the CRHBP gene, in predicting antidepressant treatment outcomes. This predictive relationship was specific to SSRIs and not the SNRI venlafaxine, particularly when looking at Caucasian patients. Patients homozygous for the reference G allele showed greater symptom improvement than patients who were carriers of the alternate A allele, particularly in response to escitalopram and sertraline. These SNP-outcome relationships were reproducible across multiple measures of symptom improvement within the original sample, and the association with the primary outcome measure of percent reduction was replicated in a separate validation cohort.
To our knowledge, these findings are the first to suggest that rs28365143 has a specific and robust role in predicting antidepressant outcomes for multiple SSRIs. The effects for rs28365143 survived stringent correction for multiple hypothesis testing in the full sample but not in the Caucasian subgroup, although the magnitude of the association was the same. Given the strength of this association and the lack of association between treatment outcomes, medication type, and the 15 other HPA-axis SNPs evaluated in this study, these data indicate that rs28365143 may have a specific role in SSRI outcomes. This suggestion is consistent with a previous report of an association between rs28365143 and self-reported symptom improvement (28), although that observation did not withstand correction for multiple testing and did not extend to response and remission rates.
It is notable that the present findings show that rs28365143 is a predictor of remission, given that remission is the pathway to recovery from major depression (37). While the allele frequency of the minor A allele was relatively low in both the original and the validation sample (7.4% and 7.9%, respectively), it is important to consider that the major allele, comprising respectively 92.6% and 92.1% of the samples, was associated with a greater probability of remission. The ability to identify patients who are more likely to remit on an antidepressant, in addition to identifying those not likely to remit, would be an important improvement over current heuristic practices. The observed odds ratio for prediction of remission (0.36) is large enough to illustrate that the findings have potential utility for clinical translation. To test explicitly for clinical translational utility, it would be important to conduct a randomized controlled trial that tests the additional benefit of prospectively guided treatment based on this candidate SNP compared with traditional heuristic prescribing. Investigating the clinical impact of using genetic information to guide antidepressant treatment choices, as well as the sensitivity and specificity of genetic markers and other biomarkers in identifying responders and nonresponders, is a line of inquiry that has been advocated by many others in the field (38–41).
Given the fact that epistatic interactions may interact with different background allele frequencies in different ethnicities, it may be important to consider these results in the context of stratification by ethnicity. Unfortunately, the small number of non-Caucasian participants in the present study made stratification by non-Caucasian ethnicity impractical. While the impact of SNP on outcomes observed in Caucasian and non-Caucasian subgroups was of the same magnitude and direction as the effect that was seen in the overall sample, the majority of these associations did not survive correction for multiple hypothesis testing (excluding remission in the Caucasian sample). Furthermore, well-powered studies are necessary to verify this association between rs28365143 and depression outcomes as well as the interactions with drug class in a variety of different ethnic backgrounds.
Because CRH has been implicated broadly in depression and its treatment, we had anticipated predictive relationships among the 15 other HPA axis SNPs and treatment outcomes. It is possible that treatment-predictive relationships for the other HPA axis SNPs depend also on moderation by other demographic or patient characteristics or by different antidepressant mechanisms of action. In the STAR*D trial, rs10473984 (also within CRHBP) was associated with decreased responsiveness to escitalopram (28). However, we note that this association was specific to African American and Hispanic subgroups of the STAR*D sample, while the present sample was primarily Caucasian. In both Mexican Americans and Han Chinese, three haplotype-defining SNPs (rs1876828, rs242939, and rs242941) have been related to response to desipramine and fluoxetine (25, 26). We did not find such relationships for these three SNPs and response to escitalopram and sertraline. It is of note, however, that both previous studies observed this relationship only in the high-anxiety subgroup of their samples, consisting of 54 participants in the Licinio et al. study (25) and 85 participants in the Liu et al. study (26). The small samples in which this association was observed and the different ethnic background of each of these samples (compared with our predominantly Caucasian sample) makes the lack of replication less surprising. Fluoxetine also belongs to the SSRI class of antidepressants, yet it has a distinct profile of pharmacologic specificity and tolerability. Given this distinct profile, it is possible (although speculative) that distinct CRH SNPs have a specific impact on response to fluoxetine that is separate from the effect of rs28365143 on response to escitalopram and sertraline. This possibility may apply more obviously to desipramine, which belongs to the tricyclic class of antidepressant.
The precise biological role of rs28365143 also warrants further investigation. It is located within an intron of CRHBP, and its function, if any, remains largely unexplored. CRHBP is thought to regulate levels of free CRH available for receptor binding, which in turn affects downstream cortisol release (42). (For further discussion of CRHBP and its roles, see the Extended Discussion section in the online data supplement.) In addition, CRH also has direct effects within the CNS governing response to stress, particularly in the amygdala and the locus ceruleus (43–45). Thus, it is possible that different alleles at this locus or nearby may alter the expression, structure, or function of CRHBP, thus having an impact on downstream effects of CRH and cortisol, direct response to stress, or both. When it comes to identifying the causative allele, it is possible that the true causative SNP may be in linkage disequilibrium with rs28365143. A SNP annotation and proxy search (SNAP) (46) reveals 21 nearby SNPs whose correlations with rs28365143 have r2 values >0.7. Although none of these are expression quantitative trait loci, two of the proxy SNPs (rs41272246 and rs75439203) are classified as “likely to affect transcription factor binding” by RegulomeDB (47). It is possible that variants of CRHBP may be affected differentially by changes in serotonergic and noradrenergic neurotransmission caused by pharmacotherapy for depression. A shift in the balance between the actions of these neurotransmitters and the CNS effects of CRH (in addition to downstream production of cortisol in response to stress caused by differential levels of CRH) may account for the differential treatment response associated with rs28365143 genotype. Further work is required to clarify the causal SNP responsible for this association with treatment response and to clarify the potential biological mechanism for this association, perhaps starting with the effect of rs28365143 on cortisol levels and reactivity.
It is also worth considering the clinical relevance of our finding that the genotype of CRHBP SNP rs28365143 may predict differential response to the SSRIs escitalopram and sertraline, but not necessarily venlafaxine, in the iSPOT-D cohort. While duloxetine belongs to the same class of medication as venlafaxine (both are SNRIs), the PReDICT validation sample was not large enough to test this differential response according to treatment class. Mechanistically, the precise effects of antidepressants at the neurotransmitter level, and the role of cortisol and genetic variation in moderating these effects, remain speculative. While cortisol-moderating genes within the HPA axis may exert their treatment-modifying effects via serotonin and norepinephrine, it is also possible that this effect involves the HPA axis as well (48, 49). For example, Binder et al. (28) found that an allele of another SNP within CRHBP was shown to associate not only with lack of response to escitalopram in major depression but also with exaggerated dexamethasone suppression of cortisol and higher plasma corticotropin levels. Overall, more research is necessary to determine whether CRHBP rs28365143 influences CRH or cortisol levels and to clarify the exact ways in which cortisol, norepinephrine, and serotonin levels interact and are influenced by pharmacologic therapy for major depression. It may be possible in the future to combine genetic information with information about neurotransmitter levels, brain function, and environmental factors to further refine treatment decisions (50).
There are several important limitations to this study. First, the sample was predominantly Caucasian. We addressed this issue to the greatest extent possible by implementing analyses with a stratification of Caucasian versus non-Caucasian participants, which did not affect the magnitude or direction of the association. The findings also replicated in the PReDICT sample, which was more heterogeneous. Seeking to replicate the present results in distinct and homogeneous ethnic and racial subgroups would be an important next step. In addition, future studies could directly examine the prediction of remission in patients stratified by both rs28365143 genotype and treatment type, to examine the drug class–specific nature of this effect and determine whether it is altered by differences in genetic background. The focus on a sample of study completers rather than on an intent-to-treat sample may be an additional limitation, as this could have introduced bias by excluding participants who did not complete the 8-week follow-up visit. This choice was made in order to relate candidate genetic variants to treatment outcomes, consistent with a biomarker trial approach. Notably, HPA axis genotype had no effect on study completion in any of 15 candidate SNPs. In addition, other baseline characteristics, such as age, sex, race, initial depression severity, and age at onset, did not differ between the two groups.
Conclusions
This work supports a potential role for the SNP rs28365143 within CRHBP in predicting response to pharmacotherapy for depression. To our knowledge, this is the first report on a significant role of this SNP in prediction of treatment outcomes in major depression, and also the first study to compare the effects of HPA axis variation across treatment types. Further work is necessary to understand whether the effect of this SNP pertains to other therapies for major depression, such as ECT and cognitive-behavioral therapy.
Overall, pharmacogenomics appears to be a promising avenue for the personalization of psychiatric pharmacotherapy, specifically through targeted genotyping. We are optimistic that this precision approach to antidepressant choices will eventually help improve physicians’ treatment decisions, decrease the number of failed treatment trials and time to remission, and limit disability due to major depression.
1 : Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry 1999; 46:1480–1508Crossref, Medline, Google Scholar
2 : Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology (Berl) 2006; 189:225–235Crossref, Medline, Google Scholar
3 : Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: a longitudinal study. Psychoneuroendocrinology 2012; 37:685–692Crossref, Medline, Google Scholar
4 : Urinary free cortisol excretion in depression. Psychol Med 1976; 6:43–50Crossref, Medline, Google Scholar
5 : Cortisol hypersecretion and cognitive impairment in depression. Arch Gen Psychiatry 1984; 41:279–283Crossref, Medline, Google Scholar
6 : Diurnal neuroendocrine and autonomic function in acute and remitted depressed male patients. Biol Psychiatry 1995; 37:448–456Crossref, Medline, Google Scholar
7 : 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry 2000; 57:755–760Crossref, Medline, Google Scholar
8 : Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiatry 2000; 177:505–510Crossref, Medline, Google Scholar
9 : Serotonin transporter genotype, morning cortisol, and subsequent depression in adolescents. Br J Psychiatry 2009; 195:39–45Crossref, Medline, Google Scholar
10 : Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984; 226:1342–1344Crossref, Medline, Google Scholar
11 : CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 1987; 144:873–877Link, Google Scholar
12 : Elevated CSF CRF in suicide victims. Biol Psychiatry 1989; 25:355–359Crossref, Medline, Google Scholar
13 : Cortisol responses to combined dexamethasone/CRH test in outpatients with a major depressive episode. J Psychiatr Res 2004; 38:553–557Crossref, Medline, Google Scholar
14 : The combined DEX-CRH test in treatment course and long-term outcome of major depression. J Psychiatr Res 2002; 36:287–297Crossref, Medline, Google Scholar
15 : High plasma concentrations of cortisol and thromboxane B2 in patients with depression. Am J Med Sci 1994; 307:228–232Crossref, Medline, Google Scholar
16 : Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res 2001; 104:39–47Crossref, Medline, Google Scholar
17 : HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology 2014; 41:46–62Crossref, Medline, Google Scholar
18 : Transcriptional control of the glucocorticoid receptor: CpG islands, epigenetics, and more. Biochem Pharmacol 2010; 80:1860–1868Crossref, Medline, Google Scholar
19 : The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry 2003; 54:867–872Crossref, Medline, Google Scholar
20 : Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci 2009; 3:41Crossref, Medline, Google Scholar
21 : HPA axis genetic variation, cortisol, and psychosis in major depression. Mol Psychiatry 2014; 19:220–227Crossref, Medline, Google Scholar
22 : Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet 2002; 114:222–226Crossref, Medline, Google Scholar
23 : Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 1993; 150:656–657Link, Google Scholar
24 : Effects of electroconvulsive therapy on the CRH-ACTH-cortisol system in melancholic depression: preliminary findings. Psychopharmacol Bull 1994; 30:489–494Medline, Google Scholar
25 : Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry 2004; 9:1075–1082Crossref, Medline, Google Scholar
26 : Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett 2007; 414:155–158Crossref, Medline, Google Scholar
27 : Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord 2007; 104:83–90Crossref, Medline, Google Scholar
28 : Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry 2010; 67:369–379Crossref, Medline, Google Scholar
29 : International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 2011; 12:4Crossref, Medline, Google Scholar
30 : The International Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res 2015; 61:1–12Crossref, Medline, Google Scholar
31 : Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract 2013; 26:389–396Crossref, Medline, Google Scholar
32 : ABCB1 genetic effects on antidepressant outcomes: a report from the iSPOT-D trial. Am J Psychiatry 2015; 172:751–759Link, Google Scholar
33 : Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005; 95:221–227Crossref, Medline, Google Scholar
34 : Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study. Am J Psychiatry 2017; 174:546–556Link, Google Scholar
35 : A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5:e1000529Crossref, Medline, Google Scholar
36 : A linear complexity phasing method for thousands of genomes. Nat Methods 2011; 9:179–181Crossref, Medline, Google Scholar
37 : Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry 2001; 62(suppl 16):5–9Medline, Google Scholar
38 : Antidepressant pharmacogenetics. Curr Opin Psychiatry 2014; 27:43–51Crossref, Medline, Google Scholar
39 : Concordance between actual and pharmacogenetic predicted desvenlafaxine dose needed to achieve remission in major depressive disorder: a 10-week open-label study. Pharmacogenet Genomics 2017; 27:1–6Crossref, Medline, Google Scholar
40 : Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016; 3:472–480Crossref, Medline, Google Scholar
41 : Does pharmacogenomic testing improve clinical outcomes for major depressive disorder? A systematic review of clinical trials and cost-effectiveness studies. J Clin Psychiatry 2017; 78:720–729Crossref, Medline, Google Scholar
42 : Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain. J Neurochem 1997; 68:2053–2060Crossref, Medline, Google Scholar
43 : Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 1997; 17:6434–6446Crossref, Medline, Google Scholar
44 : Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295Crossref, Medline, Google Scholar
45 : Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci 2004; 24:9703–9713Crossref, Medline, Google Scholar
46 : SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 2008; 24:2938–2939Crossref, Medline, Google Scholar
47 : Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 2012; 22:1790–1797Crossref, Medline, Google Scholar
48 : Serotonin transporter polymorphism, memory, and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry 2007; 12:544–555Crossref, Medline, Google Scholar
49 : Interaction of noradrenaline and cortisol predicts negative intrusive memories in posttraumatic stress disorder. Neurobiol Learn Mem 2014; 112:204–211Crossref, Medline, Google Scholar
50 : Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci USA 2016; 113:11955–11960Crossref, Medline, Google Scholar



