Cannabis, Psychosis, and Mortality: A Cohort Study of 50,373 Swedish Men
Abstract
Objective:
The authors assessed 1) the overall risk of death among cannabis users compared with nonusers and the extent to which psychosis affects excess mortality; 2) mortality among persons with psychotic disorders and the extent to which cannabis use affects excess mortality; and 3) the interaction effect of cannabis use and diagnosis of psychotic disorders on mortality.
Method:
This was a longitudinal study of 50,373 Swedish male military conscripts (ages 18–19) who were followed in the National Cause of Death Register up to around age 60. Cox proportional hazard modeling was used to assess risk of death in relation to baseline cannabis use and diagnosis of psychotic disorders.
Results:
Subjects with a baseline history of heavy cannabis use had a significantly higher risk of death (hazard ratio=1.4, 95% CI=1.1, 1.8) than those without such a history. The authors found an excess mortality among subjects with psychotic disorders, but the level did not differ between those with a history of cannabis use (ever users: hazard ratio=3.8, 95% CI=2.8, 5.0; heavy users: hazard ratio=3.8, 95% CI=2.6, 6.2) and those without such a history (hazard ratio=3.7, 95% CI=3.1, 44). No interaction was observed between cannabis use and diagnosis of psychotic disorders with regard to mortality.
Conclusions:
The results suggest that individuals with an early history of heavy use of cannabis are at a higher risk of death than those with a history of no use of cannabis. Although the authors adjusted for several confounders at baseline, the results should be interpreted with caution because of a lack of information on confounders in the period after conscription.
Cannabis abuse is associated with adverse effects on aspects of adolescent mental health, including cognitive and psychosocial functioning (1, 2). Also, cannabis smoke has many elements in common with tobacco smoke, and effects in relation to cancer, cardiovascular disease, and pulmonary disease have been documented (3, 4). Although these medical conditions are in turn associated with excess mortality in the general population (5), a systematic review by Calabria et al. (6) did not find sufficient evidence to conclude that the all-cause mortality of cannabis users is increased compared with that of nonusers.
Calabria et al. (6) found only two long-term cohort studies dealing with all-cause mortality (7, 8). One of the longest of these studies, by Andréasson et al. in 1990 (7), was a Swedish study of male military conscripts (the present cohort), in which subjects were followed to around age 33. Calabria et al. concluded that there is a need for long-term cohort studies that follow individuals into old age, when the health-related detrimental effects of cannabis use, such as cancer, pulmonary disease, and coronary heart disease, are more likely to emerge.
Cannabis use is also associated with nonaffective psychosis (9). The mortality rate is two to three times higher among persons with such a psychosis, including schizophrenia, than in the general population (10, 11). With regard to premature deaths, about 40% are explained by suicide or have other unnatural causes, while about 60% have natural causes, such as cardiovascular and pulmonary disease (10, 12). Although the evidence for an association between cannabis and nonaffective psychosis is robust (1, 9), it is still unclear whether individuals with nonaffective psychosis with a history of cannabis use have excess mortality compared with those without a history of cannabis use.
Use of cannabis among individuals with nonaffective psychosis increases the risks of persistent psychotic experiences (13), poor medication adherence, and relapses (14), all of which can hypothetically lead to greater numbers of accidents and suicide attempts. Use of cannabis, like tobacco smoking and alcohol consumption, could therefore lead to an increased risk of premature death (3, 15), although this has not yet been addressed in long-term studies (7).
Since presence of a psychotic disorder does increase the risk of death, and possibly so also does cannabis use, it is of interest to find out whether there is an interaction effect of the two on risk of death. The interaction of cannabis use and psychotic disorder on mortality rates can be interpreted as either the psychotic disorder affecting the association between cannabis use and mortality, or cannabis use affecting the association between the psychotic disorder and mortality. Both can of course operate simultaneously.
To address these issues, we performed an additional follow-up of the 1990 study by Andréasson et al. (7), extending the follow-up period to around age 60, when the detrimental somatic effects of cannabis use are more likely to be apparent. The Swedish conscript surveys are, to date, the longest follow-up studies of subjects with data on cannabis use.
Our aims in this study were to assess 1) the overall risk of death until around age 60 among cannabis users compared with nonusers, as well as the extent to which a psychosis diagnosis during the follow-up affects excess mortality; 2) the overall risk of death among persons with psychotic disorders compared with those without psychotic disorders, as well as the extent to which cannabis use affects excess mortality; and 3) the possible interaction effect of cannabis use and diagnosis of psychotic disorders on mortality.
Method
Subjects
The cohort consisted of 50,373 Swedish men conscripted in 1969 and 1970 for compulsory military training. Over 93% of the men were 18 or 19 years old. Only 2%−3% of men were exempted from conscription, mainly because of a severe mental or physical handicap or a congenital disorder. All of the men completed two nonanonymous questionnaires at the time of conscription. The first covered social background, upbringing conditions, friendships, relationships, attitudes, and adjustment to school and work. The second concerned use of alcohol, tobacco, and other substances. Details of the survey and the methods of the follow-up have been described elsewhere (16–18).
All conscripts were assessed by a psychologist after a structured interview. Those presenting with psychiatric symptoms were referred to a psychiatrist, and any psychiatric disorder found was diagnosed according to ICD-8 criteria. Permission to use the conscription database for research purposes and to perform relevant record linkages was granted by the Stockholm Regional Ethical Review Board.
Exposure
Information on cannabis use was obtained from the survey at the time of conscription. In questionnaires, conscripts were asked whether they had ever used drugs, which drugs they had used, the first drug they used, the drug they most commonly used, their frequency of use, and which specific drugs (from a list of possible drugs) they had used.
Information on level of cannabis use was obtained through questions on the number of occasions the subject had used cannabis: never, once, 2–4 times, 5−10 times, 11–50 times, or >50 times. Because of the small numbers of deaths among subjects with a psychotic disorder, we compared outcomes for those having ever used cannabis (thereby putting all subjects who reported any cannabis use into a single category) with persons who had never used cannabis, and also outcomes for those reporting moderate use (once or 2–4, 5–10, or 11–50 times) and those with the highest level of use (>50 times) with those who had never used cannabis.
Follow-Up Data
The cohort of Swedish conscripts was followed from 1969 until 2011. Statistics Sweden maintains records of the unique personal ID numbers of persons in Sweden. We used the personal ID numbers of the Swedish conscripts to link the cohort to data in the registers described below.
The National Cause of Death Register.
This register records all deaths among people registered in Sweden, and it is more than 99% complete. Causes of death are coded according to ICD (ICD-8 for 1965–1986, ICD-9 for 1987–1996, and ICD-10 for 1997–2011) and are divided into the following diagnostic groups: infections: ICD-8 and ICD-9 codes 001–139 and ICD-10 codes A00–B99; cancer: ICD-8 and ICD-9 codes 140–239 and ICD-10 codes C00–D48; endocrine: ICD-8 and ICD-9 codes 240–279 and ICD-10 codes E00–E58; nervous system: ICD-8 and ICD-9 codes 320–389 and ICD-10 codes G00–G99; cardiovascular: ICD-8 and ICD-9 codes 390–429 and ICD-10 codes I20–I52; cerebrovascular: ICD-8 and ICD-9 codes 430–458 and ICD-10 codes I60–I69; respiratory: ICD-8 and ICD-9 codes 460–519 and ICD-10 codes J00–J99; gastrointestinal: ICD-8 and ICD-9 codes 520–579 and ICD-10 codes K00–K99; injuries/accidents: ICD-8 and ICD-9 codes 800–929 and ICD-10 codes V00–V99, W00–W99, and X40–X49; suicide: ICD-8 and ICD-9 codes 950–959 and ICD-10 codes X60–X84; injury undetermined whether accidentally or purposely inflicted: ICD-8 and ICD-9 codes 980–989 and ICD-10 codes Y10–Y34; and other causes: ICD codes not included in the categories above.
The Swedish National Inpatient Register.
The Swedish National Inpatient Register, which covers all inpatient admissions for psychiatric care in Sweden, was used to record the date of first admission to inpatient care for a psychotic episode during the follow-up (1970 until 2011). Diagnoses of psychotic disorders were coded according to the Swedish versions of ICD as follows: schizophrenia: ICD-8 code 295 (excluding 295.50, 295.70); ICD-9 code 295 (excluding 295F, 295H); and ICD-10 code F20; other nonaffective psychoses: ICD-8 code 294.30 for psychosis associated with other physical conditions/drug or poison intoxication; ICD-9 code 292 for drug psychosis; ICD-10 codes F125 and F127 for psychotic disorder due to use of cannabinoids; ICD-8 and ICD-9 code 298 for reactive psychosis; ICD-9 code 293 for transient organic psychotic conditions; ICD-10 code F23 for acute and transient psychotic disorders; ICD-8 code 299.99 for unspecified psychosis; ICD-10 code F28 for other nonorganic psychotic disorders; ICD-10 code F29 for unspecified nonorganic psychosis; ICD-8 and ICD-9 code 297 for paranoia status; and ICD-10 code F22 for persistent delusional disorders.
Subjects with a diagnosis of a psychotic disorder at conscription were excluded from the study.
Migration.
Statistics Sweden maintains a national Register of the Total Population based on information from the Swedish Tax Administration. The register includes data on immigration and emigration since 1966. It has national coverage and is considered to be of high quality.
Outcomes
Overall risk of death among cannabis users compared with nonusers.
Deceased cohort members were identified during the follow-up through the National Cause of Death Register. Overall mortality in the cohort was assessed according to information on the level of cannabis use obtained from the survey at conscription.
Overall risk of death among persons with a diagnosis of a psychotic disorder.
Overall mortality among persons with a diagnosis of a psychotic disorder in the cohort was assessed, through the National Cause of Death Register, according to whether the subjects received a diagnosis of a psychotic disorder in the Inpatient Register during the follow-up. Psychotic disorder was defined as any diagnosis of schizophrenia or other nonaffective psychosis, contingent on the subject seeking and obtaining mental health care. We excluded those who had received a diagnosis of a psychotic disorder at conscription.
Stratified Analyses
First, we assessed the overall risk of death among all subjects with a history of cannabis use compared with those without such a history in the whole cohort, stratified according to whether the subjects received a diagnosis of a psychotic disorder during follow-up (two main categories: subjects without a diagnosis of a psychotic disorder, subjects with a diagnosis of a psychotic disorder). Because of the small numbers of deaths, we compared outcomes for heavy users (more than 50 times) and moderate users (between one and 50 times) with those who had never used cannabis.
Second, we performed a stratified analysis of subjects with a diagnosis of a psychotic disorder, and subjects with the specific diagnosis of schizophrenia, compared with those without such diagnoses, stratified by the three main categories of cannabis users (never users, ever users, heavy users).
Third, since alcohol intake and tobacco smoking are associated with an increased risk of premature death in the general population, we performed stratified analyses that excluded subjects with a history of risky use of alcohol or tobacco smoking at conscription.
Covariates
We selected the covariates listed below on the basis of prior research indicating that they are likely to be associated with our outcome (mortality) and with our exposures (cannabis use, diagnosis of psychosis) (1–3, 5–7, 10–12, 17, 19–25). Data on the covariates were obtained from the conscription survey and the psychological assessments at the time of conscription.
Contact with juvenile authorities.
In the conscription questionnaire, subjects were asked whether they had been in contact with juvenile authorities. Responses were categorized as several times, sometimes, or never.
Run away from home.
Questionnaire information was available on whether the subjects had run away from home during childhood, with responses categorized as two or more times, once, or never.
Truancy.
Data on truancy were based on self-reported information from the questionnaire, categorized as once a week, once a month, once per term, or occasionally.
Smoking.
Data on smoking were based on the questionnaire, categorized as >20 cigarettes/day, 11–20 cigarettes/day, 6–10 cigarettes/day, 1–5 cigarettes/day, or nonsmoking.
Solvent abuse.
Data on solvent abuse were obtained from the questionnaire, categorized as >10 times, 2–10 times, once, or never.
Risky use of alcohol.
Information on risky use of alcohol was obtained from responses to questions related to consumption of alcohol: none versus at least one of the following indicators: consumption of at least 250 g 100% alcohol/week; have taken an eye-opener during a hangover; have been apprehended for drunkenness; have reported being drunk often.
Intravenous drug use.
Use of intravenous drugs was based on information from the questionnaires, categorized as several times, once, or never.
Use of other drugs.
Information on the use of other drugs was obtained from the questionnaire, and the following types of drugs were specified: phenmetrazine (a stimulant), amphetamine, LSD, morphine, pentobarbital, and opium. Use of other drugs was categorized as ever used versus never used.
Psychiatric diagnosis at conscription.
Psychiatric diagnoses at conscription were made by a psychiatrist and categorized in this study as any versus none.
Parents divorced.
Data on whether subjects had grown up with divorced parents were obtained from the questionnaire, categorized as yes or no.
Statistical Analysis
Of all 50,373 conscripts, information on cannabis use was missing for 4,998 (9.9%). Thus, 45,375 subjects were included in the final analysis. Table S1 in the data supplement that accompanies the online edition of this article summarizes the baseline characteristics of the initial cohort of 50,373.
Model selection.
Model selection was performed using the R bestglm package (https://cran.r-project.org/web/packages/bestglm/index.html). This package uses the lexicographical method suggested by Morgan and Tatar (26) for general linear models to perform an exhaustive search by first choosing the best model for each number of covariates and then the best of all selected models. The optimality criterion used was the Akaike information criterion. A covariate was included in the model search if it met the criteria for being a confounder (27). The covariates included in each analysis were selected according to outcomes (overall risk of death among cannabis users compared with nonusers, and overall risk of death among persons with a diagnosis of a psychotic disorder compared with those without) and stratified analyses.
Cox proportional hazards model.
We used Cox proportional hazards modeling to estimate hazard ratios for 1) the overall risk of death among cannabis users compared with nonusers, 2) the overall risk of death among persons with a diagnosis of a psychotic disorder compared with those without, and 3) stratified analyses. Crude and adjusted hazard ratios and 95% confidence intervals were computed. The assumption of proportional hazards was investigated by studying graphs of the log-cumulative hazard functions and the Schoenfeld residuals. The date of first emigration was used as a censoring point. Day of death was used as the primary endpoint.
Interaction.
To test for any interaction effect of psychosis and cannabis use on mortality, we added the interaction between psychosis and cannabis use to the model. We first performed an additive interaction (biological interaction), since it is the most appropriate indicator of interaction in psychiatric epidemiology (24, 28, 29). We used three measures to estimate additive interaction between cannabis use and diagnosis of a psychotic disorder with regard to mortality: relative excess risk due to interaction, attributable proportion due to interaction, and the synergy index. We calculated 95% confidence intervals for all three measures. A confidence interval including zero in the relative excess risk due to interaction and in the attributable proportion due to interaction indicates no interaction, as does a confidence interval including 1 in the synergy index (30). We calculated the three measures of interaction with confidence intervals by using a SAS program from Lundberg et al. (31). Multiplicative interaction between cannabis use and diagnosis of a psychotic disorder with regard to mortality was also tested. The accepted threshold for statistical significance was 0.05.
Causes of death.
In the analysis of causes of death by reported cannabis use, chi-square tests were used to test the differences between never users and moderate users (once to 50 times), and between never users and heavy users (>50 times). Linear hypothesis testing was performed to calculate the p value for trend for a dose-response relationship.
The analyses were performed in SAS, version 9.1, and R, version 3.0.2.
Results
Overall Risk Of Death Among Cannabis Users
In the cohort of 45,375 individuals, 3,918 (8.6%) died during the 42 years of follow-up. Of those who died, 651 (17%) had reported cannabis use at conscription.
Figure 1 presents the survival curves by age at death according to category of cannabis use (never users, ever users, and heavy users). Ever users as well as heavy users had an earlier age at death than nonusers. The difference was statistically significant for both categories of users compared with never users (log-rank test, p<0.001).
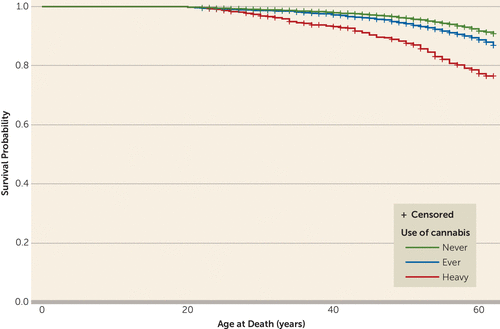
FIGURE 1. Survival Curves: Age at Death, by Baseline Level of Cannabis Use, in a Male Swedish Cohort: Never Users Compared With Ever Users and Heavy Usersa
a The figure shows product-limit survival estimates. Cannabis use was self-reported in a questionnaire at conscription (ages 18–19). “Ever users” are those who reported having used cannabis one to 50 times, and “heavy users” are those who reported having used cannabis more than 50 times. The difference was statistically significant for both categories of users compared with never users (log-rank test, p<0.001).
Table 1 lists hazard ratios for mortality for all subjects by level of cannabis use. Compared with never users, all categories of users had an increased hazard ratio for mortality. After adjustment for confounders, the association persisted only for heavy users (hazard ratio=1.4, 95% CI=1.1, 1.8).
| Level of Cannabis Use | N | Deaths (N) | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioa | 95% CI |
|---|---|---|---|---|---|---|
| Never users | 40,359 | 3,267 | 1 | 1 | ||
| Once | 1,106 | 97 | 1.1 | 0.9, 1.3 | 0.9 | 0.7, 1.4 |
| 2–4 | 1,468 | 161 | 1.4 | 1.2, 1.6 | 1.0 | 0.8, 1.2 |
| 5–10 | 852 | 111 | 1.7 | 1.4, 2.0 | 1.1 | 0.9, 1.5 |
| 11–50 | 723 | 91 | 1.6 | 1.3, 1.9 | 0.9 | 0.7, 1.2 |
| >50 | 867 | 191 | 3.0 | 2.6, 3.5 | 1.4 | 1.1, 1.8 |
TABLE 1. Overall Mortality in a Male Swedish Cohort, by Level of Cannabis Use
Table S2 in the online data supplement lists causes of death in the cohort according to reported cannabis use. The only cause-of-death category for which there was a dose-dependent relationship was “injury undetermined whether accidentally or purposely inflicted” (p for trend, <0.01).
Risk of death stratified by psychosis.
Table 2 lists hazard ratios for mortality for all subjects by level of cannabis use compared with nonusers, stratified according to whether the subjects received a diagnosis of a psychotic disorder during follow-up. Subjects with a history of cannabis use had a greater risk of death compared with those without such a history, independent of any diagnosis of a psychotic disorder during follow-up. After adjustment for confounders, the association persisted only for heavy users (subjects without psychosis: hazard ratio=1.2, 95% CI=1.0, 1.5; subjects with psychosis: hazard ratio=1.8, 95% CI=1.1, 2.9).
| All Subjects | Subjects Without Psychosis | Subjects With Psychosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabis Use Group | Deaths (N) | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioa | 95% CI | Deaths (N) | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioa | 95% CI | Deaths (N) | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratiob | 95% CI |
| Never used cannabis (N=40,359) | 3,267 | 1 | 1 | 3,101 | 1 | 1 | 166 | 1 | 1 | ||||||
| Used cannabis 1 to 50 times (N=4,149) | 460 | 1.4 | 1.3, 1.5 | 0.9 | 0.8, 1.1 | 429 | 1.4 | 1.2, 1.5 | 0.9 | 0.8, 1.1 | 31 | 1.4 | 1.0, 2.1 | 1.2 | 0.7, 1.8 |
| Used cannabis >50 times (N=867) | 191 | 3.0 | 2.6, 3.5 | 1.4 | 1.1, 1.8 | 162 | 2.8 | 2.4, 3.3 | 1.2 | 1.0, 1.5 | 29 | 2.0 | 1.4, 3.0 | 1.8 | 1.1, 2.9 |
TABLE 2. Mortality Among Subjects With a History of Cannabis Use in a Male Swedish Cohort, by Whether the Subjects Received a Diagnosis of Psychotic Disorders During Follow-Up
Because of the potential confounding effects of alcohol and tobacco use on risk of death, we performed analyses that excluded subjects who reported risky use of alcohol and tobacco smoking at conscription. As shown in Table S3 in the online data supplement, ever users of cannabis had an increased hazard ratio for mortality (hazard ratio=1.6, 95% CI=1.5, 1.8), and the associations for ever users of cannabis persisted after we excluded individuals with a history of risky use of alcohol and tobacco smoking at conscription (hazard ratio=1.4, 95% CI=1.1, 1.9).
Mortality Among Persons With Psychotic Disorders
Among all 45,375 subjects, a total of 683 (1.5%) had diagnoses of psychotic disorders during the 42 years of follow-up. Among these subjects, a total of 226 (33%) died during follow-up.
Risk of death stratified by cannabis use.
Table 3 lists the hazard ratios for risk of mortality among individuals with a diagnosis of psychosis at follow-up. We found a threefold to fourfold greater risk of mortality among those with a diagnosis of psychosis, but the risk did not differ between cannabis users and nonusers (ever users: hazard ratio=3.8, 95% CI=2.8, 5.0; heavy users: hazard ratio=3.8, 95% CI=2.6, 6.2; nonusers: hazard ratio=3.7, 95% CI=3.1, 44). We also examined whether cannabis use affected excess mortality among subjects with the specific diagnosis of schizophrenia, but this proved not to be the case (data not shown).
| Total | Never Users | Ever Users (1–50 times) | Heavy Users (>50 times) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Group | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioa | 95% CI | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratiob | 95% CI | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioc | 95% CI | Crude Hazard Ratio | 95% CI | Adjusted Hazard Ratioc | 95% CI |
| Subjects without psychosis (N=44,691) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Subjects with psychosis (N=683) | 4.7 | 4.0, 5.3 | 3.8 | 3.2, 4.3 | 4.4 | 3.8, 5.1 | 3.7 | 3.1, 4.4 | 4.6 | 3.5, 6.0 | 3.8 | 2.8, 5.0 | 4.6 | 2.1, 4.7 | 3.8 | 2.6, 6.2 |
TABLE 3. Mortality Among Subjects in a Male Swedish Cohort Who Received Diagnoses of Psychotic Disorders During Follow-Up, by Cannabis Use Category
The Interaction Effect of Cannabis Use and Diagnosis of Psychotic Disorders on Mortality
Among the 226 deceased subjects with diagnoses of psychotic disorders, 72 (32%) had used cannabis and 27 (12%) had been heavy users at the time of conscription.
Table 4 summarizes the results of the interaction analyses. No significant interaction was found between cannabis use and diagnosis of psychotic disorders with regard to an increased crude risk of death (relative excess risk due to interaction, 3.0, 95% CI=−0.4, 6.5). We found a borderline significant attributable proportion due to interaction and synergy index between cannabis use and diagnosis of psychotic disorders, but the effect disappeared after adjustment for confounders (attributable proportion due to interaction, 0.13, 95% CI=−0.2, 0.4; synergy index, 1.2, 95% CI=0.7, 2.0). We also assessed the possibility of a multiplicative interaction between cannabis use and diagnosis of psychotic disorders with regard to mortality, but we did not find a significant interaction of this kind (data not shown).
| Relative Excess Risk Due to Interaction | Attributable Proportion Due to Interaction | Synergy Index | |||||
|---|---|---|---|---|---|---|---|
| Diagnostic and Cannabis Use Categories | Deaths (N) | Interaction Effect | 95% CI | Interaction Effect | 95% CI | Interaction Effect | 95% CI |
| No psychotic disorder and no cannabis use (N=39,809) | 3,101 | 0 | 0 | 1 | |||
| Psychotic disorder and ever use of cannabisa (N=133) | 73 | ||||||
| Crude hazard ratio | 3.0 | –0.4, 6.5 | 0.3 | 0.06, 0.58 | 1.5 | 1.0, 2.4 | |
| Adjusted hazard ratiob | 0.6 | –1.2, 2.6 | 0.13 | –0.2, 0.4 | 1.2 | 0.7, 2.0 | |
| Psychotic disorder and heavy use of cannabisc (N=56) | 27 | ||||||
| Crude hazard ratio | 3.0 | –0.4, 6.4 | 0.3 | 0.06, 0.58 | 1.5 | 1.0, 2.4 | |
| Adjusted hazard ratiob | 0.6 | –1.2, 2.5 | 0.13 | –0.2, 0.5 | 1.2 | 0.7, 2.0 | |
TABLE 4. Interaction Between Cannabis Use and Diagnosis of Psychotic Disorder With Regard to Mortality in a Male Swedish Cohort
Discussion
In this long-term follow-up of Swedish male conscripts, we found that those with a baseline history of heavy cannabis use had a significantly higher risk of death (40%) over the 42-year follow-up period than those without a history of cannabis use. The association persisted after controlling for several possible confounders.
Our findings may seem surprising in light of a previous study of this cohort (7) in which cannabis use was not found to be associated with an increased risk of death. However, in this longer-term follow-up, the cohort members had reached an age where the detrimental somatic effects of cannabis use were more likely to be apparent.
Even though our study shows that subjects with an early history of cannabis use had a significantly higher risk of death even after excluding those with an early history of tobacco smoking and risky use of alcohol, its results should be interpreted with caution. In particular, there is a lack of information about cannabis use, tobacco smoking, and risky use of alcohol for the period after conscription.
It is possible that heavy users of cannabis at baseline continued using cannabis during follow-up, and that this had the strongest effect on mortality at greater ages. The cumulative evidence does suggest that early initiation of cannabis use increases the chances of becoming a daily or nearly daily user (32, 33). About 10% of persons who ever use cannabis, and one-third to one-half of those who use it daily, will become dependent (34) and continue using it despite experiencing problems (32, 34).
Persons at the greatest risk of cannabis dependence have a history of poor academic achievement, deviant behavior in childhood and adolescence, rebelliousness, and poor parental relationships (1). Although we adjusted for several of these confounders at baseline, it is possible that adverse events in adulthood associated with cannabis use affect the results, which should therefore be interpreted with caution.
We found that the group of subjects with a baseline history of moderate or heavy cannabis use had a significantly higher percentage of deaths due to “injury undetermined whether accidentally or purposely inflicted” than the group without a baseline history of cannabis use. Although the number of persons is small, it is still possible that cannabis use might trigger impulsive acts, ultimately leading to death. Cannabis users have been found to have higher rates of hospital admission for injuries from all causes (1) and of fatal traffic collisions (19) compared with nonusers.
However, we did not find an increased risk of suicide among cannabis users, as was the case in a previous study using the same longitudinal population as our own (22). A review by Serafini et al. (35) showed that the association between cannabis use and suicidal risk is not robust in the literature and is also inconsistent in relation to completed suicide (22, 36, 37).
The relation between cannabis use and cancer has been investigated in a number of reviews (4, 20). Evidence for a link is conflicting, but there is reason to suspect that cannabis use can cause some forms of cancer, including lung cancer. In relation to cardiovascular fatalities related to cannabis use, research findings suggest that cannabis may cause death in individuals with existing vulnerability (38, 39), but more evidence is needed before any firm conclusions can be drawn.
Although our previous study of this cohort (16) indicated that schizophrenia patients with a history of cannabis use receive a significantly larger amount of inpatient care, both psychiatric and somatic, we did not find that a history of cannabis use increased the risk of death in subjects with psychotic disorders, and we did not find an interaction effect between cannabis use and diagnosis of psychotic disorders with regard to mortality. We are limited in this respect in that we did not have data regarding the treatment of psychotic disorders or substance abuse in the inpatient register. The introduction of second-generation antipsychotics in the 1990s may have decreased the risk of death among individuals with psychotic disorders who use cannabis. This has been suggested in a review by Wobrock et al. (40), in which second-generation antipsychotic agents were shown to have greater efficacy than first-generation antipsychotic agents in reducing substance use in patients with schizophrenia.
Medication is not the only factor that can affect the association between cannabis use and risk of death among subjects with diagnoses of psychotic disorders. Other factors, such as psychological and social support and type of care, may also have an impact (23).
Our study has several limitations, and the results should be interpreted with caution. First, since our information on cannabis use is based solely on a survey at conscription, the study fails to account for differences in lifetime use of cannabis. Nevertheless, performing a long-term longitudinal study does make it possible to conduct analyses of risk of death up to old age in relation to background factors. The Swedish conscript survey provides for the longest follow-up to date of subjects with data on cannabis use. Replication in other long-term cohorts with data on cannabis use would be valuable, although such cohorts, as shown by Calabria et al. (6) and Degenhardt et al. (41), are rare.
Second, the causal pathway between cannabis use and risk of death is complicated. Even though cannabis use was assessed at baseline, we do not know the extent to which subjects continued use into adulthood. It is possible that other risk factors after baseline influenced our results, such as risky behavior and use of tobacco, alcohol, and other substances.
Third, the National Cause of Death Register does not record the deaths of persons who emigrate from Sweden. However, we found that subjects with a history of cannabis use had a slightly higher rate of emigration than those without such a history (data not shown), so the effect of unrecorded deaths of emigrants would result in an underestimation of the true effect of cannabis use on mortality in this cohort.
Fourth, identification of diagnoses of psychosis was limited to persons in inpatient care. Jörgensen et al. (42) found that using data only from inpatient care results in lower estimates of incidence rates compared with using both inpatient and outpatient data. This applies especially since the 1990s, when a higher proportion of patients with psychosis have been treated as outpatients rather than inpatients. However, Jörgensen et al. (35) also showed that 75% of all persons identified with a nonaffective psychosis do appear in inpatient care when a longer time frame is used or when older cohorts are included. Thus, the facts that most of our subjects were treated before the major period of deinstitutionalization in Sweden and that our study has a long observation period suggest that we are likely to have captured a large majority of patients with psychosis in the cohort.
Cannabis use has also been associated with affective disorders. However, the evidence for causal links between cannabis use and affective disorders is less convincing (9, 43, 44). In another study using the same longitudinal population as this study, we found no association between baseline cannabis use and subsequent risk of affective disorders after adjusting for confounders (45). Thus, we decided to focus in this study on nonaffective psychosis.
Fifth, we did not have data on the type of treatment received and possible comorbid substance abuse later in life. Thus, we do not know the extent to which the introduction of second-generation antipsychotics during the 1990s influenced our results on the risk of death among subjects with psychotic disorders. Second-generation antipsychotics have been shown to improve effectiveness in the treatment of schizophrenia and comorbid substance abuse (40), which might decrease the mortality gap between users and never users of cannabis with psychosis.
Regarding the validity of diagnoses in Sweden’s National Inpatient Register, a number of studies have demonstrated the adequate validity of major psychiatric diagnoses, including schizophrenia, in epidemiology (46, 47).
In summary, we report on what is to date the longest follow-up of subjects with data on cannabis use and mortality. Study limitations notwithstanding, we found that subjects with a baseline history of heavy use of cannabis have an increased risk of death over the course of follow-up. We did not find that a history of cannabis use affects the excess mortality among subjects with psychotic disorders, and we did not find an interaction effect between cannabis use and diagnosis of psychotic disorders with regard to mortality.
1 : Adverse health effects of non-medical cannabis use. Lancet 2009; 374:1383–1391Crossref, Medline, Google Scholar
2 : The adverse health effects of chronic cannabis use. Drug Test Anal 2014; 6:39–45Crossref, Medline, Google Scholar
3 : Comparing cannabis with tobacco. BMJ 2003; 326:942–943Crossref, Medline, Google Scholar
4 : The association between marijuana smoking and lung cancer: a systematic review. Arch Intern Med 2006; 166:1359–1367Crossref, Medline, Google Scholar
5 : Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128Crossref, Medline, Google Scholar
6 : Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev 2010; 29:318–330Crossref, Medline, Google Scholar
7 : Cannabis and mortality among young men: a longitudinal study of Swedish conscripts. Scand J Soc Med 1990; 18:9–15Crossref, Medline, Google Scholar
8 : Marijuana use and mortality. Am J Public Health 1997; 87:585–590Crossref, Medline, Google Scholar
9 : Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328Crossref, Medline, Google Scholar
10 : 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009; 374:620–627Crossref, Medline, Google Scholar
11 : Mortality in schizophrenia. Pharmacoepidemiol Drug Saf 2007; 16:1308–1312Crossref, Medline, Google Scholar
12 : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Medline, Google Scholar
13 : Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 2011; 342:d738Crossref, Medline, Google Scholar
14 : Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry 2008; 193:357–363Crossref, Medline, Google Scholar
15 : Association of mental disorders in early adulthood and later psychiatric hospital admissions and mortality in a cohort study of more than 1 million men. Arch Gen Psychiatry 2012; 69:823–831Crossref, Medline, Google Scholar
16 : Prognosis of schizophrenia in persons with and without a history of cannabis use. Psychol Med 2014; 44:2513–2521.Crossref, Medline, Google Scholar
17 : Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002; 325:1199Crossref, Medline, Google Scholar
18 : Function of male youths during military service: a follow-up study of a youth clientèle. Acta Psychiatr Scand Suppl 1980; 282:1–60Medline, Google Scholar
19 : Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ 2012; 344:e536Crossref, Medline, Google Scholar
20 : Epidemiologic review of marijuana use and cancer risk. Alcohol 2005; 35:265–275Crossref, Medline, Google Scholar
21 : Alcohol and cannabis use and mortality in people with schizophrenia and related psychotic disorders. J Psychiatr Res 2012; 46:987–993Crossref, Medline, Google Scholar
22 : Cannabis and suicide: longitudinal study. Br J Psychiatry 2009; 195:492–497Crossref, Medline, Google Scholar
23 : Schizophrenia. Lancet 2009; 374:635–645Crossref, Medline, Google Scholar
24 : Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 2008; 34:1066–1082Crossref, Medline, Google Scholar
25 : Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382:1575–1586Crossref, Medline, Google Scholar
26 : Calculation of the residual sum of squares for all possible regressions. Technometrics 1972; 14:317–325Crossref, Google Scholar
27 Greenland S, Lash TL: Bias analysis, in Modern Epidemiology, 3rd ed. Edited by Rothman KJ, Greenland S, Lash TL. Lippincott Williams and Wilkins, 2012, pp 345–380Google Scholar
28 : Gene-environment interactions and depression. JAMA 2009; 302:1860–1861Crossref, Medline, Google Scholar
29 : Interpretation of interactions: guide for the perplexed. Br J Psychiatry 2010; 197:170–171Crossref, Medline, Google Scholar
30 : Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 2011; 26:433–438Crossref, Medline, Google Scholar
31 : A SAS program calculating three measures of interaction with confidence intervals. Epidemiology 1996; 7:655–656Medline, Google Scholar
32 : Early onset cannabis use and psychosocial adjustment in young adults. Addiction 1997; 92:279–296Crossref, Medline, Google Scholar
33 : The consequences in young adulthood of adolescent drug involvement: an overview. Arch Gen Psychiatry 1986; 43:746–754Crossref, Medline, Google Scholar
34 : Are the adverse consequences of cannabis use age-dependent? Addiction 2002; 97:1083–1086Crossref, Medline, Google Scholar
35 : Can cannabis increase the suicide risk in psychosis? A critical review. Curr Pharm Des 2012; 18:5165–5187Crossref, Medline, Google Scholar
36 : Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control 2013; 24:1811–1820Crossref, Medline, Google Scholar
37 : Mortality following treatment for cannabis use disorders: predictors and causes. J Subst Abuse Treat 2013; 44:400–406Crossref, Medline, Google Scholar
38 : An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am Heart J 2008; 155:465–470Crossref, Medline, Google Scholar
39 : Acute cardiovascular fatalities following cannabis use. Forensic Sci Int 2001; 124:200–203Crossref, Medline, Google Scholar
40 : Pharmacotherapy of schizophrenia with comorbid substance use disorder: reviewing the evidence and clinical recommendations. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1375–1385Crossref, Medline, Google Scholar
41 : The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 2013; 8:e76635Crossref, Medline, Google Scholar
42 : The Stockholm Non-Affective Psychoses Study (SNAPS): the importance of including out-patient data in incidence studies. Acta Psychiatr Scand 2010; 121:389–392Crossref, Medline, Google Scholar
43 : Exploring the association between cannabis use and depression. Addiction 2003; 98:1493–1504Crossref, Medline, Google Scholar
44 : The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med 2014; 44:797–810Crossref, Medline, Google Scholar
45 : Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry 2012; 12:112Crossref, Medline, Google Scholar
46 : Young cases of schizophrenia identified in a national inpatient register: are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol 2002; 37:527–531Crossref, Medline, Google Scholar
47 : Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry 2005; 59:457–464Crossref, Medline, Google Scholar



