Effect of an Acute Intranasal Aerosol Dose of PH94B on Social and Performance Anxiety in Women With Social Anxiety Disorder
Abstract
Objective
Although social anxiety disorder is a common and sometimes disabling condition, there are no approved treatments that can be used on an as-needed basis. The authors examined the acute use of PH94B, an intranasally administered neurosteroidal aerosol, for the acute management of the symptoms of social anxiety disorder.
Method
The authors conducted a phase 2, multicenter, randomized, double-blind, placebo-controlled, single-dose study of PH94B. Ninety-one women 19–60 years of age with generalized social anxiety disorder received placebo intranasal spray (single-blind) 15 minutes before laboratory-simulated public speaking and social interaction challenges. Patients who experienced significant distress during at least one challenge returned 1 week later to receive either intranasal PH94B or placebo aerosol spray (double-blind) before repeat challenges.
Results
Patients who received PH94B during the second set of challenges had a significantly greater decrease in mean Subjective Units of Distress scores during the public speaking and social interaction challenges compared with the first set of challenges, than did patients who received placebo for both sets of challenges. A significantly greater proportion of the PH94B group were much or very much improved from the first to the second sets of challenges compared with the placebo group (75% and 37%, respectively). The side effects of PH94B were benign.
Conclusions
PH94B may be a novel, effective, and well-tolerated acute treatment for performance and social anxiety in women with social anxiety disorder.
Social anxiety disorder is a common and sometimes disabling condition involving excessive fear and avoidance of situations in which individuals feel scrutinized or evaluated by others (1). DSM-IV recognized a generalized subtype of social anxiety disorder in which affected individuals experience anxiety and avoidance in most interpersonal situations. Generalized social anxiety disorder leads to compromised academic, social, and vocational functioning and often predisposes to depression and substance abuse (2).
Several treatment options have emerged for generalized social anxiety disorder. The selective serotonin reuptake inhibitors paroxetine, sertraline, and fluvoxamine (sustained-release), along with the serotonin norepinephrine reuptake inhibitor venlafaxine (extended-release), have been approved by the U.S. Food and Drug Administration for social anxiety disorder, based on 12-week placebo-controlled trials in generalized social anxiety disorder (3–6). Certain monoamine oxidase inhibitors and benzodiazepines have shown efficacy in similar trials (7, 8). Cognitive-behavioral therapy has also been shown to be effective for social anxiety disorder in 12-week trials (9).
Since many individuals with social anxiety disorder, including those with generalized social anxiety disorder, experience distress in events that occur infrequently and can be anticipated (social gatherings, public speaking), a treatment that can be used on an as-needed basis could be highly useful. Also, many patients benefit only partially from treatments that have been found effective for generalized social anxiety disorder (10) and might still use an additional as-needed therapy. While there are no approved treatments for social or performance anxiety that work acutely on an as-needed basis, anecdotal evidence suggests that beta-blockers, benzodiazepines, and alcoholic beverages have some efficacy in this regard. However, beta-blockers such as propranolol target the autonomic arousal that occurs during an anxious episode, which has been found to be more characteristic of individuals with pure performance anxiety than those with generalized social anxiety disorder (11). Also, benzodiazepines administered acutely, at least in doses that do not diminish mental acuity, seem, anecdotally, to be more helpful for the anticipatory anxiety than the anxiety and arousal experienced during the event.
PH94B
With the aim of filling this treatment gap, we studied PH94B in patients with generalized social anxiety disorder. PH94B (3β-androsta-4,16-dien-3-ol) is an investigational drug for the acute treatment of social anxiety disorder. Chemically, PH94B is a synthetic neuroactive steroid that lacks affinity for steroidal hormone receptors. Nanomolar quantities of PH94B have been shown to induce inward currents in isolated human vomeronasal receptor cells (half maximal effective concentration [EC50]=0.2 µM) and significantly increased cytosolic calcium (12, 13). PH94B spray administered intranasally to human volunteers produces dose-dependent and reversible depolarization of the human nasal chemosensory epithelium (effective dose [ED50]=4×10–1 µg), followed by decreases in heart rate, respiratory rate, electrodermal activity frequency, and eye blink frequency and increases in EEG alpha activity and body temperature. These rapid effects were ∼30% higher in female than male volunteers, and a number of female participants reported feeling distinctly calmer and less nervous. Similar results were obtained in a phase 1 dose escalation study.
Nasal Chemosensory Systems
In mammals, the hypothalamic-limbic areas of the brain receive direct afferent neural inputs from peripheral nasal chemosensory neurons of the olfactory organs (15, 16). In humans, as in other mammals, there is a subgroup of nasal chemosensory (olfactory) receptor neurons concentrated in the vomeronasal organ (17, 18). Sensory transduction of external chemical cues in olfactory neurons triggers sensory inputs that reach the hypothalamus and the limbic amygdala through an oligosynaptic (rapid) neural path (olfactory nerves) (19). Pheromones (20) are species-specific external chemical messengers that in mammals bind to nasal chemosensory receptors in the vomeronasal organ (21). The actions of pheromones on nasal chemosensory neurons differ significantly across species and are critical in triggering specific social and reproductive behaviors.
In the early 1990s, researchers discovered a family of synthetic neuroactive steroids that they called pherines (initially vomeropherins). Pherines selectively target human nasal chemosensory receptors, similarly to naturally occurring pheromones (22, 23, 25), and broadcast chemosensory information via the olfactory nerves to specific brain areas (the cingulate gyrus, the hypothalamus, the limbic amygdala, the anterior gyrus, and the prefrontal cortex), which are different from the brain areas activated by olfactory stimuli (17, 25). Brain activation by the pherine PH94B does not produce olfactory awareness and can directly modulate brain autonomic, psychophysiological, and neuroendocrine responses without systemic uptake and distribution (13).
Here we report a phase 2 trial of PH94B in the acute treatment of social anxiety disorder. Because initial work suggested stronger effects for PH94B in women, this trial enrolled only female patients. The PH94B dose chosen for this study was based on pharmacology studies in vitro (using patch-recorded human receptor cells) and in vivo (with human volunteers), where the effective dose range of PH94B was 0.8–1.6 μg. This dose range also effectively relieved symptoms in a subsequent bioequivalence study.
Method
This was a two-phase, randomized, double-blind, placebo-controlled, parallel, single-dose (administered twice) study in women diagnosed with generalized social anxiety disorder.
Participants
The study design included 90 women 18–65 years of age diagnosed with generalized social anxiety disorder according to DSM-IV criteria. Other important inclusion criteria were having a score ≥60 on the Liebowitz Social Anxiety Scale (26), being a nonsmoker, being physically healthy, being able to give written informed consent, using adequate birth control if of childbearing potential, being free of other psychotropic medications and major psychiatric conditions, having a score <18 on the 17-item Hamilton Depression Rating Scale (HAM-D) (27), and having no nasal pathology (ascertained by examination). Participants were excluded if they had other primary psychiatric diagnoses, had recently used psychotropic medication or abused drugs or alcohol, or were undergoing other treatment for social anxiety. Participants who reported less than moderate to severe anxiety during the placebo challenge were also excluded from further participation.
The study was conducted at three sites: the Medical Research Network in New York City, Capital Clinical Research Associates in Rockville, Md., and the Carracci Medical Group in Mexico City. The first 40 participants were studied in Mexico City; the results were then unblinded for an interim review of efficacy and safety. The study was subsequently completed at all three sites. Participants were recruited from existing databases and through local media advertisements.
The study and consent form were approved by the Western Institutional Review Board. All participants provided written informed consent after receiving a complete description of the study.
Procedures
At visit 1, the diagnosis of social anxiety disorder was confirmed by the Mini-International Neuropsychiatric Interview (28), and a medical evaluation was performed. At visit 2, after all inclusion and exclusion criteria were rechecked, participants underwent two challenges. In the first, the participant was given, single-blind, an intranasal administration of placebo (two sprays in each nostril). Fifteen minutes later, the participant was instructed to pick a topic for a speech and asked for a subjective anxiety rating (resting phase). She was then allowed 2 minutes to mentally prepare, and subjective anxiety ratings were solicited at each minute (anticipation phase). Next, she was required to give a 5-minute speech, without the use of notes, to an audience of three unfamiliar clinic staff (public speaking challenge); subjective anxiety ratings were solicited just before the speech and then at each minute during the speech (performance phase). After a 30-minute break, the participant underwent the second challenge, which consisted of a second single-blind intranasal placebo administration, a 15-minute waiting period, and then a social interaction challenge (interacting with three role-players in a mock party situation) that followed the same format as the speech paradigm.
Participants who reported moderate to severe anxiety or distress (a score ≥75 on at least one Subjective Units of Distress Scale rating [29]) during the 5-minute performance phase of the visit 2 public speaking or social challenge were invited back for visit 3. Those who had lower scores were excluded from the protocol and given open clinical care. At visit 3, the procedures for the public speaking and social interaction challenges were repeated, but this time patients were randomly assigned to receive, on a double-blind basis, pretreatment with either PH94B or placebo for both challenges. Each PH94B spray (50 µL) delivered 0.4 µg of PH94B; with two sprays in each nostril at each challenge, the total dose administered was 1.6 µg of PH94B.
A week later, all patients returned for a follow-up (visit 4), during which concomitant medications were recorded, a repeat medical evaluation was performed, and clinical ratings were made.
The primary efficacy measures were the changes in average subjective anxiety ratings on the Subjective Units of Distress Scale during the 5-minute performance phases of the public speaking and social interaction challenges from visit 2 to 3, and the physician’s evaluation of overall clinical improvement from visit 2 to 3, using the improvement item of the Clinical Global Impressions Scale (CGI) (30).
Outcome Measures
Subjective Units of Distress Scale.
The Subjective Units of Distress Scale, used at visits 2 and 3, was scored 0–100 (operationalized for the participants in this study as 0=no anxiety, 30=mild anxiety, 60=moderate anxiety, 90=severe anxiety). It is a standard instrument for rating social and performance anxiety in patients with social anxiety disorder during role playing situations (9, 29, 31).
Clinical Global Impressions Scale.
The CGI was administered at visit 1 (severity) and visit 3 (change from visit 2). Participants with CGI improvement scores of 1 (very much improved) or 2 (much improved) at visit 3 were considered “responders.”
Liebowitz Social Anxiety Scale.
This 24-item investigator-rated scale for social anxiety disorder was administered at visits 1 and 4. It rates anxiety and avoidance in performance and social situations for the past week on a 0–3 scale, with higher scores indicating greater severity. It has demonstrated good reliability, validity, and sensitivity to change (32).
Hamilton Depression Rating Scale.
The HAM-D was administered at visits 1 and 4 (27).
The first two authors provided training at all three sites on administration of the Subjective Units of Distress Scale queries, the study rating scales, and the study protocol. The ChiMatrix Digital Data Capture System was used to record all study procedures and ratings. This digital data capture system contains built-in algorithms to highlight discrepancies between different scales, giving the rater the option of retaining or adjusting an investigator-rated measure, such as the CGI, while the patient is still present. An audit trail documents all such queries to raters and their responses. As a patient-rated outcome, Subjective Units of Distress scores were not subject to such adjustment.
Data Analysis
Efficacy was assessed by evaluating whether there was a statistically significant treatment effect of PH94B over placebo in Subjective Units of Distress scores from visit 2 to 3 for the public speaking and social interaction performance phases and by comparing the CGI improvement item response rate (percent of participants receiving a score of 1 or 2 in each group at visit 3).
Change in Liebowitz Social Anxiety Scale score from visit 1 to visit 4 and changes in average resting and anticipatory Subjective Units of Distress scores for the public speaking and social interaction challenges were also evaluated.
A separate longitudinal linear mixed-effects model was used to model each Subjective Units of Distress response endpoint. Model estimates of the effect of treatment on change in Subjective Units of Distress scores for the social interaction challenge and for the public speaking challenge were based on the model-estimated interaction of treatment and visit. A random subject effect was estimated in the model, resulting in a compound-symmetry covariance structure (using PROC MIXED in SAS, version 9.3 for Windows 7). Since subjects were nested within investigator, and treatment effect is estimated within subject, no estimate of investigator effect was undertaken in the model.
For the CGI improvement endpoint at visit 3, results were analyzed with a cumulative logistic regression model with proportional odds (using PROC LOGISTIC).
An interim data analysis had previously been conducted when the first 40 patients completed the study. No adjustment for multiplicity was specified in the original trial design, so the present analysis is retrospective. No adjustment was made for the multiplicity of the endpoints, with efficacy contingent on statistical significance of all three primary outcome measures.
Results
Of 183 participants screened, 85 were excluded and 98 were randomly assigned to receive PH94B or placebo at visit 3 (Figure 1). Seven allocated participants were eliminated from the final data analyses, five because of inadequate clinical site training of a rater and patient documentation at the Mexico City site, one because of doubts about the patient’s clinical authenticity at the New York City site, and one because of an error in randomization documentation at the Rockville site. All were eliminated on a blind basis prior to data analysis. Two participants who failed to return for visit 4 were considered early terminators but were included in the data analyses because they had completed ratings at the first three visits.
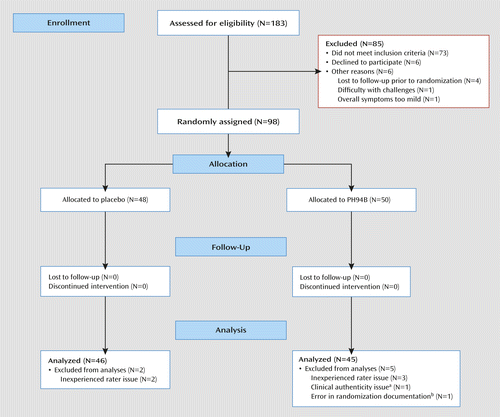
a The patient presented with social anxiety disorder and met study criteria, but because she appeared very outgoing and social during the study, the investigators believed that she had been misdiagnosed.
b At audit, the investigators could not verify whether the patient had received PH94B or placebo.
Ninety-one participants were included in the final data analysis (15 from the New York City site, 15 from the Rockville site, and 61 from the Mexico City site). The participants’ mean age was 33.2 years (SD=10.9, range=19–60), and their mean educational level was 13.7 years (SD=3.2). At baseline, the mean HAM-D score was 6.3 (SD=3.7), the mean CGI severity score was 5.2 (SD=0.9), and the mean Liebowitz Social Anxiety Scale score was 94.6 (SD=18.3). There were no differences between treatment groups on any of these measures. Patients tended to have long-standing single episodes of marked social anxiety disorder.
Primary Efficacy
During the performance phase of the public speaking challenge, the decrease in anxiety from visit 2 to 3 (Table 1) for the PH94B group was 12.68 Subjective Units of Distress Scale units greater than for the placebo group (95% CI=4.72, 20.64; t=3.16, df=89, p=0.002). During the performance phase of the social interaction challenge, the decrease in anxiety from visit 2 to 3 for the PH94B group was 11.66 Subjective Units of Distress Scale units greater than for the placebo group (95% CI=3.00, 20.33; t=2.67, df=89, p=0.009). Effect sizes (Cohen’s d) for the public speaking and social interaction challenges were 0.66 and 0.56, respectively. For the CGI improvement endpoint at visit 3, the cumulative logistic regression model with proportional odds indicated a statistically significant treatment group slope (p<0.001). Overall, comparing visit 3 to visit 2, 34 participants (75.6%) in the PH94B group were much or very much improved on the CGI improvement item and considered responders, compared with 17 participants (37%) in the placebo group (χ2=19.32, df=1, p<0.001).
| Visit 2a (Baseline) | Visit 3 (Treatment) | |||||||
|---|---|---|---|---|---|---|---|---|
| PH94B Group (N=45) | Placebo Group (N=46) | PH94B Group (N=45) | Placebo Group (N=46) | |||||
| Challenge and Phase | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Public speaking | ||||||||
| Resting | 54.89 | 29.3 | 55.22 | 23.2 | 46.22 | 28.1 | 50.22 | 29.1 |
| Anticipation | 61.03 | 24.9 | 66.03 | 23.1 | 47.72 | 26.2 | 60.87 | 26.1 |
| Performance | 79.23 | 13.2 | 80.69 | 13.7 | 52.55 | 22.9 | 66.68 | 19.1 |
| Social interaction | ||||||||
| Resting | 45.60 | 27.5 | 49.78 | 29.6 | 33.33 | 24.0 | 39.22 | 28.2 |
| Anticipation | 51.22 | 26.7 | 52.45 | 29.0 | 34.02 | 23.4 | 40.20 | 26.3 |
| Performance | 52.79 | 26.3 | 54.16 | 27.4 | 34.54 | 24.2 | 47.58 | 24.4 |
Further exploratory analyses indicated no significant differences between treatment groups on resting, anticipation, or performance phase measures when both groups received placebo during the visit 2 public speaking and social interaction challenges (Figures 2 and 3).
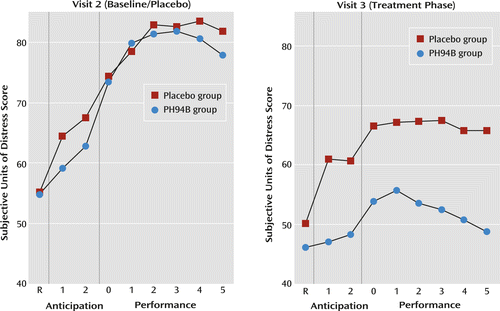
a At visit 2, both groups received placebo. R=resting.
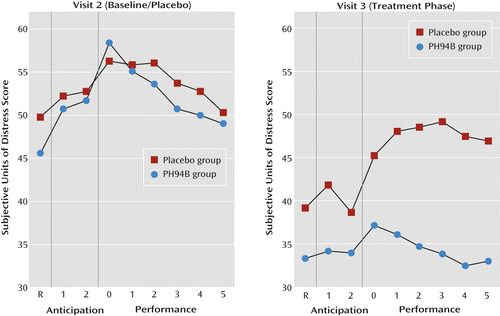
a At visit 2, both groups received placebo. R=resting.
There were no differences between groups on the change from visit 2 to 3 in subjective anxiety during the resting phase for the public speaking or social interaction challenges (Table 1, Figures 2 and 3).
During the anticipation phase of the public speaking challenge, the mean improvement from visit 2 to 3 in the PH94B group (13.3 subjective anxiety units) exceeded that of the placebo group (5.2 subjective anxiety units) (t=2.02, p=0.047) (Table 1, Figure 2). During anticipation of social interaction, the improvement from visit 2 to 3 for the PH94B group (17.2 subjective anxiety units) did not differ significantly from that of the placebo group (12.2 subjective anxiety units) (Table 1, Figure 3).
There were no significant differences between groups in change in Liebowitz Social Anxiety Scale score from visit 1 (mean=94.6, SD=18.3) to visit 4 (mean=86.8, SD=21.4).
Sensitivity
A sensitivity analysis on the primary outcome measures was conducted to assess the influence of the seven participants who were excluded from the final efficacy analyses. The overall trend of all the tests remained the same when the excluded participants were included in the analysis. Differences between PH94B and placebo treatment remained strong for the public speaking challenge (p=0.01) and the CGI improvement score (p=0.001), although the change for the social interaction challenge fell short of significance (p=0.054).
Safety and Tolerability
Participants were asked about adverse events at each visit. Mild or moderate adverse events were infrequent and did not differ significantly between the PH94B and placebo groups (Table 2). Headache and gastrointestinal discomfort were the most commonly reported adverse events for the PH94B group, and nasal soreness, sore mouth or teeth, and headache for the placebo group. No severe or serious adverse events were reported, and no participants were withdrawn prematurely because of adverse events. No adverse effects of PH94B were observed in ECG readings, laboratory values, or vital signs.
| PH94B Group (N=45) | Placebo Group (N=46) | |||
|---|---|---|---|---|
| System | N | % | N | % |
| Ear nose throat | ||||
| Itchy ear canals | 0 | 0.0 | 1 | 2.2 |
| Dry mouth | 1 | 2.2 | 0 | 0.0 |
| Nasal soreness | 0 | 0.0 | 3 | 6.5 |
| Sore mouth or teeth | 0 | 0.0 | 2 | 4.3 |
| Cold sore | 0 | 0.0 | 1 | 2.2 |
| Central nervous system | ||||
| Headache | 3 | 6.6 | 3 | 6.5 |
| Migraine | 1 | 2.2 | 0 | 0.0 |
| Increased hot flashes | 1 | 2.2 | 0 | 0.0 |
| Insomnia | 1 | 2.2 | 0 | 0.0 |
| Gastrointestinal | ||||
| General malaise | 1 | 2.2 | 1 | 2.2 |
| Upset stomach | 1 | 2.2 | 0 | 0.0 |
| Heartburn | 1 | 2.2 | 0 | 0.0 |
| Abdominal tenderness | 1 | 2.2 | 0 | 0.0 |
| Nausea | 1 | 2.2 | 0 | 0.0 |
| Genitourinary | ||||
| Urinary infection | 1 | 2.2 | 0 | 0.0 |
| Urinary discomfort | 0 | 0.0 | 1 | 2.2 |
| Missed period | 0 | 0.0 | 1 | 2.2 |
| Musculoskeletal | ||||
| Low back pain | 0 | 0.0 | 2 | 4.3 |
Discussion
While the mechanism of action for PH94B has not been fully elucidated, when administered intranasally, it binds to peripheral nasal chemosensory receptors, which in turn trigger rapid (neural) modulation of basal forebrain areas (hypothalamus, limbic amygdala) and rapid relief (in minutes) of social anxiety symptoms. PH94B has a high affinity for a subgroup of 7-transmembrane G-protein coupled nasal chemosensory receptors. In vitro pharmacology studies show that nanogram quantities of PH94B can induce concentration-dependent and reversible inward (depolarizing) currents and increase calcium cytosol in human nasal chemosensory neurons. The neural connectivity of human nasal chemosensory neurons to basal forebrain areas shown in physiology, pharmacology, and brain imaging studies provides a short latency path for the modulation of vital CNS structures involved in the pathophysiology of social anxiety disorder (33). The onset of efficacy of PH94B nasal spray is rapid because it bypasses the circulation and the blood-brain barrier. Interestingly, a neurosteroidal mechanism of action has also been suggested, by the finding that highly diluted extracts of the plant Gelsemium sempervirens may have anxiolytic effects in mice (34).
The rapid-acting nature of PH94B required several unique design features for this trial. Twelve-week placebo-controlled parallel group trials have been standard in social anxiety disorder, with an onset of drug action seen in 4–6 weeks. For those trials, the Liebowitz Social Anxiety Scale, which measures social and performance anxiety over the past week in the patient’s natural setting, served as the principal outcome measure. In the present trial, patients gave speeches and engaged in social interactions in the clinic setting, with anxiety assessed on a minute-by-minute basis. The Liebowitz Social Anxiety Scale was not suitable for this task, and the patient-rated Subjective Units of Distress Scale was used as a principal outcome measure.
Assessing patients in the clinic has certain advantages, allowing standardization of challenges and fine-grained temporal measurements. Although it remains to be studied, drug effects seen in this setting should also generalize to everyday life, since patients in this trial were helped by PH94B to cope with very high levels of anxiety during the clinic simulations. If so, then patients with social anxiety disorder could pretreat themselves with PH94B on an as-needed basis in their daily lives just before entering performance or social situations in which they anticipate disturbing levels of anxiety. A next step in studying PH94B will be to test it for as-needed use as a monotherapy in patients with social anxiety disorder in their natural settings. Future trials should also assess PH94B as an as-needed adjunct for patients already using another medication or a psychotherapeutic approach for social anxiety disorder.
Several limitations of this study should be mentioned. For one thing, the findings will need to be replicated to be considered established. Also, PH94B still needs to be tested in patients going about their daily lives. In such a study, repeat dosing over an extended period would need to be examined, both for repeated efficacy and for signs of toxicity or abuse potential. In addition, efficacy in men remains to be examined.
While the mechanism of action of PH94B is not yet fully understood, this has also been true of many important psychiatric therapies when first introduced. Continued positive findings for PH94B would suggest a novel mechanism of drug action via human nasal chemosensory receptors. If so, this could lead to the ability to treat psychopathological states with nanomolar doses of drugs that do not even enter the systemic circulation and may represent a distinct advance in psychotherapeutics.
1 : Social phobia: review of a neglected anxiety disorder. Arch Gen Psychiatry 1985; 42:729–736Crossref, Medline, Google Scholar
2 : Social phobia: comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry 1992; 49:282–288Crossref, Medline, Google Scholar
3 : Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA 1998; 280:708–713Crossref, Medline, Google Scholar
4 : Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry 2001; 158:275–281Link, Google Scholar
5 : A double-blind placebo-controlled study of controlled release fluvoxamine for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol 2004; 24:49–55Crossref, Medline, Google Scholar
6 : Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry 2005; 62:190–198Crossref, Medline, Google Scholar
7 : Phenelzine vs atenolol in social phobia: a placebo-controlled comparison. Arch Gen Psychiatry 1992; 49:290–300Crossref, Medline, Google Scholar
8 : Treatment of social phobia with clonazepam and placebo. J Clin Psychopharmacol 1993; 13:423–428Crossref, Medline, Google Scholar
9 : Cognitive-behavioral therapy for social anxiety disorder: current status and future directions. Biol Psychiatry 2002; 51:101–108Crossref, Medline, Google Scholar
10 : Therapeutic strategies for the patient with treatment resistant anxiety. J Clin Psychiatry 1993; 54(suppl):69–74Medline, Google Scholar
11 : Responses of “generalized” and “discrete” social anxiety during public speaking. J Anxiety Disord 1993; 7:207–221Crossref, Google Scholar
12 : Patch recorded isolated adult human vomeronasal organ cells are electrically excitable and respond to skin steroidal substances: androsta-4,16-dien-3-one and estra-1,3,5(10),16-tetraen-3-ol, in Abstracts of the Seventeenth Annual Meeting of the Association for Chemoreception Sciences (AChemS XVII). Chem Senses 1995; 20:745Google Scholar
13 : The human vomeronasal system. Psychoneuroendocrinology 1994; 19:673–686Crossref, Medline, Google Scholar
14 Grosser BI, Monti L, Jennings-White C: Rapid anti-anxiety effects in women of picogram quantities of a 19-carbon steroid. Presented at the 28th CINP World Congress of Neuropsychopharmacology, Stockholm, June 3–7, 2012Google Scholar
15 : A proposed mechanism of emotion. Arch Neurol Psychiatry 1937; 4:725–743Crossref, Google Scholar
16 : The limbic system (“visceral brain”) and emotional behavior. AMA Arch Neurol Psychiatry 1955; 73:130–134Crossref, Medline, Google Scholar
17 : The human vomeronasal system: a review. Ann N Y Acad Sci 1998; 855:373–389Crossref, Medline, Google Scholar
18 : Structure and function of the human vomeronasal organ, in Handbook of Olfaction and Gustation. Edited by Doty R. New York, Marcel Dekker, 1995, 793–820Google Scholar
19 : Neurobiology, 4th ed. New York, Oxford University Press, 1994Google Scholar
20 : “Pheromones”: a new term for a class of biologically active substances. Nature 1959; 183:55–56Crossref, Medline, Google Scholar
21 : The organization and function of the vomeronasal system. Annu Rev Neurosci 1987; 10:325–362Crossref, Medline, Google Scholar
22 : Effect of putative pheromones on the electrical activity of the human vomeronasal organ and olfactory epithelium. J Steroid Biochem Mol Biol 1991; 39(4B):573–582Crossref, Medline, Google Scholar
23 : Modulation of serum testosterone and autonomic function through stimulation of the male human vomeronasal organ (VNO) with pregna-4,20-diene-3,6-dione. J Steroid Biochem Mol Biol 1998; 65:237–242Crossref, Medline, Google Scholar
24 : Behavioral and electrophysiological effects of androstadienone, a human pheromone. Psychoneuroendocrinology 2000; 25:289–299Crossref, Medline, Google Scholar
25 : Blind smell: brain activation induced by an undetected air-borne chemical. Brain 1999; 122:209–217Crossref, Medline, Google Scholar
26 Liebowitz MR: Social phobia, in Modern Problems of Pharmacopsychiatry: Anxiety. Edited by Klein DF, Liebowitz MR, Gorman JM, Fyer AF. Unionville, Conn, Karger, 1987, pp 141–173Google Scholar
27 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
28 : The Mini-International Neuropsychiatric Interview (MINI), a short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 1997; 12:224–231Crossref, Google Scholar
29 : The Practice of Behavior Therapy, 2nd ed. New York, Pergamon Press, 1973Google Scholar
30 Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
31 : Cognitive behavioral group therapy vs phenelzine for social phobia: 12-week outcome. Arch Gen Psychiatry 1998; 55:1133–1141Crossref, Medline, Google Scholar
32 : The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med 2001; 31:1025–1035Crossref, Medline, Google Scholar
33 : The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35:169–191Crossref, Medline, Google Scholar
34 : Dose-effect study of Gelsemium sempervirens in high dilutions on anxiety-related responses in mice. Psychopharmacology (Berl) 2010; 210:533–545Crossref, Medline, Google Scholar



