Clinical Outcomes and Genome-Wide Association for a Brain Methylation Site in an Antidepressant Pharmacogenetics Study in Mexican Americans
Abstract
Objective:
The authors compared the effectiveness of fluoxetine and desipramine treatment in a prospective double-blind pharmacogenetics study in first-generation Mexican Americans and examined the role of whole-exome functional gene variations in the patients’ antidepressant response.
Method:
A total of 232 Mexican Americans who met DSM-IV criteria for major depressive disorder were randomly assigned to receive 8 weeks of double-blind treatment with desipramine (50–200 mg/day) or fluoxetine (10–40 mg/day) after a 1-week placebo lead-in period. Outcome measures included the Hamilton Depression Rating Scale (HAM-D), the Hamilton Anxiety Rating Scale, and the Beck Depression Inventory. At week 8, whole-exome genotyping data were obtained for 36 participants who remitted and 29 who did not respond to treatment.
Results:
Compared with desipramine treatment, fluoxetine treatment was associated with a greater reduction in HAM-D score, higher response and remission rates, shorter time to response and remission, and lower incidences of anticholinergic and cardiovascular side effects. Pharmacogenetics analysis showed that exm-rs1321744 achieved exome-wide significance for treatment remission. This variant is located in a brain methylated DNA immunoprecipitation sequencing site, which suggests that it may be involved in epigenetic regulation of neuronal gene expression. This and two other common gene variants provided a highly accurate cross-validated predictive model for treatment remission of major depression (receiver operating characteristic integral=0.95).
Conclusions:
Compared with desipramine, fluoxetine treatment showed a more rapid reduction of HAM-D score and a lower incidence of side effects in a population comprising primarily first-generation Mexican Americans with major depression. This study’s pharmacogenetics approach strongly implicates the role of functional variants in antidepressant treatment response.
Efficacy and side effect data for psychotropic medications in the United States have been investigated primarily in non-Hispanic Caucasian populations. Currently, the Hispanic population is the largest ethnic minority group in the country, representing over 37 million people (1). Within this group, almost 70% are Mexican Americans. Although this population is growing dramatically, there is insufficient research regarding psychiatric diagnosis and treatment in this group (2).
Major depressive disorder is a serious public health problem worldwide, with a lifetime prevalence of 10%–20% in the general population (3). Vega et al. (4) reported that the lifetime prevalence of major depression in U.S.-born Mexican Americans is 14.8%. Although Hispanics have participated in antidepressant treatment studies, it has been difficult to ascertain whether there are any major differences in antidepressant efficacy in that population, for several reasons, including methodological differences among studies, small sample sizes, and the inclusion of several Hispanic subgroups in an attempt to illustrate a “Hispanic response” (5).
A retrospective review (6) suggested that pharmacokinetic factors play a role in the differential sensitivity to tricyclic antidepressants in depressed Puerto Rican American females as compared with Anglo females, resulting in greater efficacy, higher rates of adverse drug reactions, and higher dropout rates in the former group. In an open-label study, nefazodone (7) was found to have similar efficacy but a higher dropout rate in a predominantly Hispanic Caribbean female sample as compared with non-Hispanics from previous nefazodone trials. An open-label study of two selective serotonin reuptake inhibitors (SSRIs) (8) found that although the drugs were comparable in efficacy, Mexican American females had a higher dropout rate even though there were more severe adverse drug reactions reported in the non-Hispanic group. A more recent study of an SSRI reported no differences in efficacy, rates of adverse drug reactions, or dropout rates between Hispanic and non-Hispanic patients with HIV (9).
Three recent meta-analyses on the pharmacogenetics of antidepressants in major depression were not able to show genome-wide significant variations (10–12). The nonsynonymous single-nucleotide polymorphism (SNP) rs6265 in the BDNF (brain-derived neurotrophic factor) gene may have a minor impact on susceptibility to major depression (13) and antidepressant drug response (11); however, the overall conclusion of these meta-analyses was that the results do not support any major effect of any single gene variation in the pharmacogenetics of antidepressants in major depression.
In the present study, we focused on functional SNPs, based on 1) the likely significance of a functional SNP in the BDNF gene (rs6265) in the genetics and pharmacogenetics of major depression; 2) our own recent work proposing a predictive framework for the diagnosis of major depressive disorder using interactions of multiple functional SNPs and environmental factors (14); and 3) a growing body of evidence supporting the involvement of epigenetic mechanisms in major depression and antidepressant action (15–19). Here we present data on the efficacy and adverse drug reaction profiles of desipramine and fluoxetine, two extensively used off-patent antidepressants, and new pharmacogenetic leads that could advance our understanding of genetic variants implicated in antidepressant treatment response.
Method
The study protocol was approved by the University of California Los Angeles and University of Miami institutional review boards and the Australian National University Human Ethics Committee. This was a single-site prospective double-blind 8-week trial with fluoxetine and desipramine conducted in the greater Los Angeles area. All study participants had an initial medical evaluation consisting of a detailed history, physical examination, and blood collection for routine testing and genotyping, followed by two consecutive study phases: a 1-week single-blind placebo lead-in phase to minimize the effect of placebo response, and subsequent random assignment to receive either 10–40 mg/day of fluoxetine or 50–200 mg/day of desipramine, with weekly follow-up visits to assess clinical status. Participants provided written informed consent after receiving a complete description of the study.
Given the proven efficacy of these antidepressant medications, we used a placebo lead-in period followed by active treatment for all patients in order to minimize risk to participants (20, 21).
Participants
All participants met the following inclusion criteria (22): at least three of their four grandparents born in Mexico (23); a DSM-IV diagnosis of current unipolar major depressive episode; a score ≥18 on the 21-item Hamilton Depression Rating Scale (HAM-D) (24), with item 1 (depressed mood) rated ≥2; and age between 18 and 70 years. Exclusion criteria were any axis I disorder other than major depressive disorder or primary anxiety disorders; an active medical illness that could be related to the ongoing depression (e.g., untreated hypothyroidism, myocardial infarction or cerebrovascular incident within the previous 6 months, uncontrolled hypertension or diabetes); current suicidal ideation with a plan and strong intent, or recent serious suicide attempt; history of ECT in the previous 6 months; current use of medications with CNS activity that interferes with EEG activity or any other antidepressant treatment within 2 weeks before enrollment; history of poor response to treatment with desipramine or fluoxetine; illicit drug use or alcohol abuse in the previous 3 months; and current enrollment in counseling or psychotherapy treatment. In addition, women who were pregnant or lactating or were of childbearing age and not using contraception were excluded.
Recruitment and Outcome Measures
Participants were recruited by advertisements in bilingual newspapers, radio, and television. Informed consent forms, questionnaires, and assessment scales were given in their preferred language (English or Spanish). In addition, clinical staff also participated in health fairs, conferences, and cable network programs through which they recruited participants, and some participants were referred by regional outpatient community clinics.
The presence of a current major depressive episode was determined by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (25) (mean kappa score for sensitivity and specificity among raters, 0.84–0.85), and diagnoses were confirmed by a research psychiatrist. Symptom severity was rated by experienced bilingual clinical personnel using Spanish or English versions of the HAM-D (24), the Hamilton Anxiety Rating Scale (HAM-A) (26), the Global Assessment Scale (GAS) (27), the Beck Depression Inventory (BDI) (28), and the Center for Epidemiologic Studies Depression (CES-D) Scale (29). Participants also completed an acculturation questionnaire to determine their language preference, education level, and generation status.
Interventions and Treatment
All participants received 1 week of single-blind placebo to minimize the effect of placebo response, which was defined as a decrease of 25% or more in HAM-D score compared with the screening visit or a HAM-D score <18. Those who did not show a placebo response were randomly assigned in a 1:1 ratio to receive either fluoxetine or desipramine for an 8-week double-blind phase. Participants initially received 10 mg/day of fluoxetine or 50 mg/day of desipramine, which increased at week 2 to 20 mg/day of fluoxetine or 100 mg/day of desipramine. At week 4, for participants who had less than a 25% decrease in their HAM-D score, dosages were increased to 30 mg/day of fluoxetine or 150 mg/day of desipramine. At week 6, for participants whose HAM-D score was >12, dosages were increased to 40 mg/day of fluoxetine or 200 mg/day of desipramine. The staff and participants were aware of dosage escalation, which occurred only if the previous dosage was well tolerated, but they were blind to the drug. At the end of the study, participants were referred to a psychiatric clinic of their choice for follow-up treatment. Random antidepressant blood levels were collected to ascertain medication adherence but not to obtain therapeutic levels.
Statistical Analysis
Analyses were performed using SAS, version 9.1.3 (SAS Institute, Cary, N.C.), and included all participants who received at least 1 week of study drug. The primary outcome measure was change in HAM-D score from week 0 to week 8. The secondary outcomes of interest were change in HAM-A, BDI, CES-D Scale, and GAS scores. Remission was defined as a HAM-D score <8, response was defined as a reduction of ≥50% in HAM-D score, and nonresponse was defined as a reduction of <50% in HAM-D score. Remission and response were compared between treatment groups. Student’s t test was used to compare the mean values for age, acculturation score, and baseline clinical measurements, and chi-square or Fisher’s exact test was used to compare the percentages of demographic characteristics and side effect events.
For repeated continuous outcome measure analyses, the likelihood-based mixed-effects model as the primary analysis of efficacy was used to assess differences between treatment groups in changes from baseline across 8 weeks. The model included the categorical effects of treatment, treatment week, treatment-by-week interaction, and gender, as well as continuous covariates of baseline score and age. Mixed-model repeated-measures analyses of changes from baseline were conducted using PROC MIXED in SAS. A macro was written in SAS for covariance structure selection by comparing Akaike’s information criterion and Schwarz’s Bayesian criterion using compound symmetry, unstructured, first-order autoregressive (AR[1]), and Huynh-Feldt covariance structures based on the “smaller is better” criterion. For each outcome comparison, the covariance structure was used if the sum of Akaike’s information criterion and Schwarz’s Bayesian criterion was smallest.
For repeated analyses of dichotomous outcomes (remission and response), the modified Poisson regression model with robust error variance estimated by the generalized estimating equation approach (30) was performed with PROC GENMOD in SAS to estimate the adjusted relative risk. To compare the time to response or remission between the two treatment groups, Cox regression analysis was performed with PROC TPHREG in SAS using the DISCRETE option to handle ties in event time. Gender, age, and baseline scores were included as covariates in all models. For all analyses, the threshold for significance was a p value ≤0.05 (two-sided).
Pharmacogenetics Procedures
Whole-exome genotyping.
Genomic DNA of 65 participants who completed the 8-week treatment course (36 of whom had remitted at week 8 and 29 of whom had not responded) was subjected to whole-exome genotyping, performed by the Australian Genome Research Facility (Melbourne), an Illumina Certified Service Provider for the Infinium Genotyping Service. We used the Illumina HumanExome-12v1_A BeadChip, which covers putative functional exonic variants selected from over 12,000 individuals. The exonic content consists of >250,000 markers representing diverse populations (including European, African, Chinese, and Hispanic individuals) and a range of common conditions, such as type 2 diabetes, cancer, metabolic disorders, and psychiatric disorders. Samples with calls below the Illumina-expected 99% SNP call rate were excluded. To test genotyping reliability and quality, an individual was duplicated. The identity by descent between all pairs of individuals was estimated and used for quality control.
Quality control and filtering.
GenomeStudio data were imported to SVS, version 7.6.7 (Golden Helix, Bozeman, Mont.; http://www.goldenhelix.com), an integrated collection of analytic tools for managing, analyzing, and visualizing multifaceted genomic and phenotypic data.
Parameters for excluding markers from analyses included 1) deviations from the Hardy-Weinberg equilibrium with p<2×10−7 (0.05/250.000 markers) in both case and control subjects (this avoids the exclusion of major effect causal variants); 2) a genotype call rate <90%; 3) more than two alleles; and 4) monoallelism. Genotype and allelic frequencies were estimated by maximum likelihood.
Genetic stratification analysis.
We estimated the inbreeding coefficient in order to detect the presence of hidden biological relatives in the sample, which might reduce the independence of the data. The fixation index between pairs of subpopulations, case subjects, and control subjects was estimated to evaluate the potential presence of genotype stratification (microdifferentiation), a common cause of spurious associations. Independently, an additional correction of putative population stratification was applied with 10 principal-components analyses to normalize genotypic data by its actual standard deviation.
Exome-wide association analysis.
The genotypic (additive model) and allelic tests of association were applied. Multiple test correction to determine exome-wide significance was performed using the false-discovery-rate approach. Mixed linear models were applied as a tool to include in the analyses fixed factors (sex, age, treatment) and random effects (family or population structure) and to contrast with other analytical tools, different from principal-components analysis, the effects of potential inbreeding (by inclusion of the kinship matrix as defined by identity by descent). We applied the single-locus mixed model (which assumes that all loci have a small effect on the trait) and the multilocus mixed model (which assumes that several loci have a large effect on the trait) as implemented in SVS. Linkage disequilibrium analysis was also implemented using SVS.
Advance recursive partitioning (tree-based) approach (ARPA).
Rao has suggested that recursive partitioning techniques should be highly recommended for genetic dissection of complex traits (31). ARPA is widely used in predictive analyses, as it accounts for nonlinear and interaction effects, offers fast solutions to reveal hidden complex substructures, and provides truly nonbiased statistically significant analyses of seemingly unrelated high-dimension data (14).
ARPA accounts for the effect of hidden interactions better than alternative methods and is independent of the type of data (categorical, continuous, ordinal, etc.) and data distribution type (normal or nonnormal) (31). Furthermore, results supplied by tree-based analytics are easy to interpret visually and logically (14). Therefore, to generate the most comprehensive and parsimonious classificatory model to predict remission of major depression at end of treatment, we applied ARPA using a set of different modules implemented in the Salford Predictive Modeler (SPM) software package, namely, CART, random forest, and Tree-Net (http://www.salford-systems.com). SPM is a highly accurate and ultra-fast analytics and data-mining platform for creating predictive, descriptive, and analytical models from databases of any size, complexity, or organization. One important advantage of SPM compared with other available software is that it can use raw data with sparse or empty cells (a common problem when dealing with genetic data).
CART is a nonparametric approach in which a series of recursive subdivisions separates the data by dichotomization (32). The aim is to identify, at each partition step, the best predictive variable and its best corresponding splitting value while optimizing a splitting statistical criterion so that the data set is successfully divided into increasingly homogeneous subgroups (32). We used a battery of different statistical criteria as splitting rules (e.g., Gini index, entropy, and twoing) to determine the splitting rule that decreased the relative cost of the tree the most while increasing the prediction accuracy of target variable categories (32). The best split at each dichotomous node was chosen by either a measure of between-node dissimilarity or iterative hypothesis testing of all possible splits to find the most homogeneous split (lowest impurity) (32). Similarly, we used a wide range of empirical probabilities (priors) to model numerous scenarios recreating the distribution of the targeted variable categories in the population (32). Following this iterative process, each terminal node was assigned to a class outcome (remitter or nonresponder).
To avoid finishing with an overfitted CART predictive model (a common problem in CART analysis) and to ensure that the final splits were well substantiated, we applied tree pruning (32). During this procedure, predictor variables that were close competitors (surrogate predictors with comparable overall classification error to the optimal predictors) were pruned to eliminate redundant commonalities among variables, so the most parsimonious tree would have the lowest misclassification rate for an individual not included in the original data (32).
Furthermore, to exactly identify the most important set of variables predicting remission of major depression, we applied the random forest method using a bagging strategy (33). The random forest strategy differs from CART in the use of a limited number of variables to derive each node while creating 100 to 1000 trees (33). This strategy has proved to be immune to the overfitting generated by CART (33). In the random forest strategy, variables that appeared repeatedly as predictors in the trees were identified. The misclassification rate was recorded for each approach.
The TreeNet strategy was used to complement the CART and random forest analyses because it reaches a level of accuracy that is usually not attainable by single models (CART) or by ensembles such as bagging (random forest) (34). The TreeNet algorithm generates thousands of small decision trees built in a sequential error-correcting process converging on an accurate model (34).
To obtain honest assessments of the derived models and have a better view of their performance on future unseen data, we applied a cross-validation strategy in which both training with all the data and then indirect testing with all the data were performed. To do so, we randomly divided the data into 10 separate partitions (folds). This strategy allowed us to conduct a cross-validation and review the stability of results across multiple replications (32). Cross-validation is a model validation technique for assessing how the results of a statistical analysis will generalize to an independent data set; the n-fold cross-validation technique is designed to get the most out of data sets that are too small to accommodate a holdout or test sample. For our specific problem, we used a maximization algorithm that allowed us to validate the entire original results of our genome analysis by considering n-fold subsamples of the original set. So we used cross-validation to be able to both train with all the data and then indirectly test with all the data as well.
We also applied a categorical approach to link the set of genotypes of the associated marker (rs1321744) by using latent class analysis to identify unobservable subgroups within the subset of completers who were genotyped (remitters and nonresponders).
Results
Disposition and Baseline Characteristics of Patients
After assessing 4,323 people for eligibility by telephone, we scheduled 1,223 structured clinical interviews (a diagram of participant flow in the study is presented in Figure S1 in the data supplement that accompanies the online edition of this article). Of these, 885 individuals either were disqualified for the study by the SCID results or declined to participate, and a total of 338 participants were enrolled. Of those enrolled, 232 received at least one dose of study drug (112 in the desipramine group and 120 in the fluoxetine group) and were included in the intent-to-treat analysis; 166 participants completed 8 weeks of treatment and 66 did not complete the study because they dropped out, were nonadherent, or were terminated (34% and 23% in the desipramine and fluoxetine groups, respectively, not a statistically significant difference). No significant differences were found in mean retention time in noncompleters or in baseline demographic and clinical characteristics between treatment groups (see Table S1 in the online data supplement). Our participants were primarily (60%) first-generation Spanish-speaking-only Mexican Americans with 6–12 years of education (see Table S1).
Primary Outcome Measure
Table 1 summarizes the mean HAM-D score changes (least squares means after adjustment for age, gender, and baseline score). Mixed-model repeated-measures analysis revealed a between-subject effect of treatment (F=4.45, df=1, 227, p=0.036; least squares mean change difference=1.0, 95% CI=0.1–1.9), within-subject effect of treatment time (F=76.38, df=1, 1312, p<0.001), and no interaction between treatment and time. At endpoint (week 8), fluoxetine showed an advantage over desipramine of 2.6 points on the HAM-D score (−14.6 compared with −12.0) from baseline after adjustment for age, gender, and baseline score (95% CI=1.1–4.2; F=10.71, df=1, 131, p=0.001). No gender-specific effect was found on HAM-D score reductions, but significant effects were observed in baseline HAM-D scores (F values ≥23.27, df=1, 227, p values <0.001). Mixed-model repeated-measures analysis revealed an age effect on reduction in HAM-D score (coefficient=−0.1; F=5.89, df=1, 227, p=0.016).
| HAM-D | HAM-A | BDI | CES-D Scale | GAS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week and Treatment | N | LSM | SE | N | LSM | SE | N | LSM | SE | N | LSM | SE | N | LSM | SE |
| Week 1 | |||||||||||||||
| Desipramine | 111 | –1.5 | 0.5 | 107 | –0.8 | 0.6 | 100 | –1.9 | 0.6 | 109 | –1.0 | 0.8 | |||
| Fluoxetine | 120 | –2.0 | 0.5 | 116 | –1.3 | 0.6 | 111 | –1.6 | 0.6 | 115 | –1.8 | 0.8 | |||
| Week 2 | |||||||||||||||
| Desipramine | 105 | –4.2 | 0.5 | 102 | 0.2 | 1.5 | 96 | –4.9 | 0.7 | 100 | –3.1 | 0.8 | |||
| Fluoxetine | 113 | –4.3 | 0.5 | 111 | –3.9 | 1.5 | 104 | –3.4 | 0.7 | 106 | –4.1 | 0.8 | |||
| Week 3 | |||||||||||||||
| Desipramine | 99 | –6.2 | 0.5 | 98 | –2.8 | 0.6 | 92 | –6.8 | 0.8 | 95 | –5.7 | 0.8 | |||
| Fluoxetine | 110 | –6.0 | 0.5 | 105 | –4.4 | 0.6 | 97 | –6.5 | 0.8 | 104 | –6.1 | 0.8 | |||
| Week 4 | |||||||||||||||
| Desipramine | 97 | –7.3 | 0.5 | 95 | –3.8 | 0.6 | 88 | –8.5 | 0.9 | 92 | –7.0 | 0.8 | 96 | 7.7 | 0.9 |
| Fluoxetine | 106 | –7.6 | 0.5 | 101 | –5.1 | 0.6 | 96 | –7.8 | 0.9 | 103 | –7.1 | 0.8 | 103 | 8.7 | 0.9 |
| Week 5 | |||||||||||||||
| Desipramine | 86 | –9.3 | 0.6 | 88 | –5.7 | 0.7 | 84 | –10.1 | 0.9 | 82 | –8.5b | 0.9 | |||
| Fluoxetine | 100 | –9.8 | 0.5 | 97 | –7.5 | 0.7 | 95 | –10.3 | 0.9 | 91 | –11.1b | 0.8 | |||
| Week 6 | |||||||||||||||
| Desipramine | 82 | –10.0 | 0.6 | 81 | –5.8b | 0.7 | 77 | –9.7b | 0.9 | 75 | –8.6c | 0.9 | |||
| Fluoxetine | 95 | –11.1 | 0.5 | 89 | –8.2b | 0.7 | 88 | –12.1b | 0.8 | 86 | –11.9c | 0.8 | |||
| Week 7 | |||||||||||||||
| Desipramine | 75 | –10.5d | 0.6 | 74 | –6.1d | 0.7 | 75 | –11.8b | 0.8 | 71 | –10.2c | 0.9 | |||
| Fluoxetine | 93 | –13.2d | 0.5 | 88 | –9.7d | 0.7 | 83 | –14.2b | 0.8 | 86 | –13.7c | 0.9 | |||
| Week 8 | |||||||||||||||
| Desipramine | 74 | –12.0d | 0.6 | 72 | –7.4d | 0.7 | 71 | –12.2b | 0.9 | 72 | –11.1 | 0.9 | 64 | 18.5c | 1.0 |
| Fluoxetine | 92 | –14.6d | 0.5 | 90 | –10.6d | 0.6 | 85 | –15.3b | 0.9 | 81 | –13.4 | 0.9 | 83 | 22.9c | 0.9 |
TABLE 1. Least Squares Mean of Changes From Baseline in Mexican American Patients With Major Depression Who Received Desipramine or Fluoxetinea
Table 2 presents the response and remission rates after adjusting for age, sex, and baseline HAM-D score. At endpoint, response and remission rates were significantly 1.3–1.4 times higher in the fluoxetine group than in the desipramine group. Survival analysis (using a Cox regression model including all participants with response or remission or right-censoring time) revealed that fluoxetine was associated with a shorter time to response (hazard ratio=2.01, 95% CI=1.40–3.13; χ2=13.08, df=1, p<0.001) and a shorter time to remission (hazard ratio=1.57, 95% CI=0.98–2.51; χ2=3.54, df=1, p=0.060) than desipramine (Figure 1).
| Remission | Response | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week and Treatment | N | Rate (%) | Relative Risk | 95% CI | p | Rate % | Relative Risk | 95% CI | p |
| Week 1 | |||||||||
| Desipramine | 111 | 0.9 | Reference | 1.7 | Reference | ||||
| Fluoxetine | 120 | 1.7 | 1.90 | 0.17–21.08 | 0.600 | 3.3 | 1.87 | 0.35–10.10 | 0.466 |
| Week 2 | |||||||||
| Desipramine | 105 | 3.7 | Reference | 11.9 | Reference | ||||
| Fluoxetine | 113 | 3.5 | 0.95 | 0.24–3.72 | 0.936 | 9.4 | 0.79 | 0.37–1.68 | 0.534 |
| Week 3 | |||||||||
| Desipramine | 99 | 3.9 | Reference | 15.2 | Reference | ||||
| Fluoxetine | 110 | 5.5 | 1.40 | 0.40–4.84 | 0.598 | 14.9 | 0.98 | 0.52–1.83 | 0.945 |
| Week 4 | |||||||||
| Desipramine | 97 | 4.4 | Reference | 21.6 | Reference | ||||
| Fluoxetine | 106 | 10.2 | 2.33 | 0.81–6.69 | 0.117 | 30.7 | 1.42 | 0.90–2.26 | 0.135 |
| Week 5 | |||||||||
| Desipramine | 86 | 11.9 | Reference | 36.0 | Reference | ||||
| Fluoxetine | 100 | 19.9 | 1.67 | 0.84–3.33 | 0.143 | 50.2 | 1.39 | 1.00–1.94 | 0.049 |
| Week 6 | |||||||||
| Desipramine | 82 | 21.4 | Reference | 44.0 | Reference | ||||
| Fluoxetine | 95 | 25.0 | 1.17 | 0.68–2.00 | 0.575 | 59.4 | 1.35 | 1.02–1.79 | 0.036 |
| Week 7 | |||||||||
| Desipramine | 75 | 31.3 | Reference | 59.0 | Reference | ||||
| Fluoxetine | 93 | 51.0 | 1.63 | 1.11–2.39 | 0.012 | 75.1 | 1.27 | 1.04–1.56 | 0.020 |
| Week 8 | |||||||||
| Desipramine | 74 | 43.1 | Reference | 60.7 | Reference | ||||
| Fluoxetine | 92 | 59.1 | 1.37 | 1.01–1.87 | 0.046 | 79.7 | 1.31 | 1.09–1.59 | 0.005 |
TABLE 2. Remission and Response Rates in Mexican American Patients With Major Depression Who Received Desipramine or Fluoxetinea
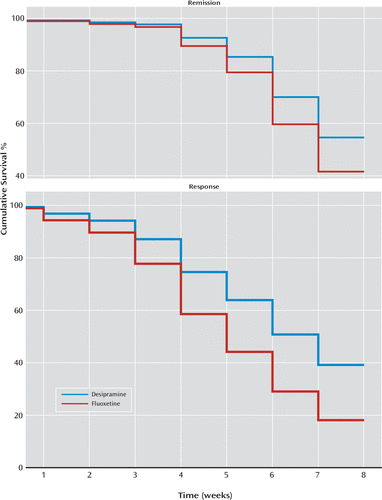
FIGURE 1. Survival Curves Showing Time to Remission or Response for Mexican American Patients With Major Depression Who Received Desipramine (N=112) or Fluoxetine (N=120)
Secondary Outcome Measures
The results of mixed-model repeated-measures analysis revealed a significant between-subject effect of treatment on the difference in mean change in HAM-A, BDI, and GAS scores (Table 1). The fluoxetine group showed greater improvement on HAM-A, BDI, CES-D Scale, and GAS scores than the desipramine group. Significant interactive effects of treatment and time were observed in BDI scores (F=2.09, df=7, 199, p=0.005) and GAS scores (F=4.92, df=7, 135, p=0.028). The fluoxetine group had a smaller reduction in BDI score in the first 4 weeks and a greater reduction in the last 3 weeks. CES-D Scale scores showed a treatment effect (F=5.30, df=1, 211, p=0.022) at weeks 5–7, but not at week 8. Baseline scores showed significant effects on specific score changes (p<0.05). No gender effect was observed, and age showed a significant effect only on increase in GAS score (mixed-model repeated-measures analysis: F=4.59, df=1, 190, p=0.033; last observation carried forward model: F=10.39, df=1, 198, p=0.002).
Adverse Drug Reactions
The data on adverse drug reactions indicated that 78% (182/232) of participants had one or more side effects (see Table S2 in the online data supplement). There was no significant difference in the overall occurrence of adverse drug reactions between treatment groups (90/112 in the desipramine group and 92/120 in the fluoxetine group). However, compared by category, patients treated with desipramine had significantly higher rates of anticholinergic adverse drug reactions (χ2=4.96, df=1, p=0.026), cardiovascular adverse drug reactions (χ2=7.22, df=1, p=0.007), perspiration, tingling/paresthesias, blurred vision, orthostatic hypotension (by pulse), and palpitations (p values <0.05).
Exome-Wide Association Analysis
After we filtered out markers that did not meet either quality control criteria or variability requirements, 52,103 variants remained for further analysis (Figure 2). The estimation of the fixation index coefficient reported a value of 0.001 that according to Price et al. (35) may be corrected by principal-components analysis using 50,000 SNPs. After principal-components analysis correction, we found that a single SNP marker located on chromosome 6 (exm-rs1321744) achieved exome-wide significance (p=1.98×10−6; false-discovery-rate-corrected p=0.05) (see Figure 3 and Table 3). This marker is located in a methylated DNA immunoprecipitation sequencing site (MeDIP-seq raw data) obtained using postmortem human frontal cortex gray matter (UCSC Genome Browser; http://genome.ucsc.edu), which suggests that it is involved in epigenetic regulation of neuronal gene expression. Interestingly, other top markers, which did not attain exome-wide significance, are also located in methylated DNA sites (Table 3). Linkage disequilibrium block harboring exm-rs1321744 does not harbor any gene; however, there are three genes surrounding exm-rs1321744: TBX18, NT5E, and SNX14.
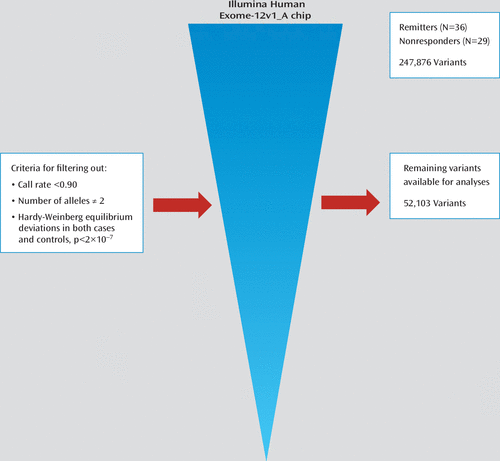
FIGURE 2. Process Used to Filter Out Single-Nucleotide Polymorphism (SNP) Exomic Variants in Mexican American Patients With Major Depression Who Received Desipramine or Fluoxetine, to Evaluate Pharmacogenetic Response as Remitters (N=36) and Nonresponders (N=29)a
a From 247,876 SNPs, 195,773 were discarded because they were monoallelic, because they had more than two alleles, because they had a call rate <90%, or because their genotype proportions deviated from the expected ones as defined by the Hardy-Weinberg equilibrium theorem in both case subjects (remitters) and control subjects (nonresponders) at a p value <2×10−7. The remaining 52,103 SNPs were used for exome-wide association analysis.
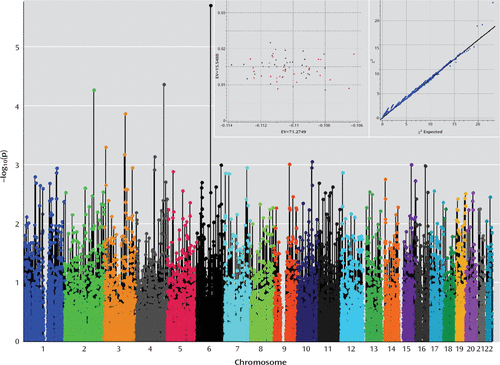
FIGURE 3. Manhattan Plot to Evaluate Pharmacogenetic Response in Mexican American Patients With Major Depression Who Received Desipramine or Fluoxetine, as Remitters (N=36) and Nonresponders (N=29)a
a Genotype model (additive) after correcting stratification by principal-components analysis was used. A unique significant peak at chromosome 6 remained significant after correction for multiple comparisons by false discovery rate. In the inset, the principal-components analysis shows absence of stratification between remitters and nonresponders (left box), and a Q-Q plot depicts the fitting of chi-square against the chi-square expected distribution (right box). EV=eigenvalue.
| Marker | Chr | Position | Reference/Alternate Allele | Major Allele | p (Fisher’s Exact Test) | FDR-Corrected p (Fisher’s Exact Test) | Allele Frequency (Cases) | Allele Frequency (Controls) | Classification | Gene | HGVS Coding | HGVS Protein |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| exm-rs1321744b,c | 6 | 85,891,744 | T/C | C | 1.98E–06 | 0.05 | 0.19 | 0.45 | Intergenic | |||
| exm-rs16867321c | 2 | 181,362,379 | C/T | C | 5.37E–05 | 0.42 | 0.58 | 0.28 | Intergenic | |||
| exm433050 | 4 | 169,083,694 | A/C | A | 4.41E–05 | 0.51 | 0.50 | 0.38 | Coding nonsynonymous | ANXA10 | c.211A>C | p.Met71Leu |
| exm-rs6583826c | 10 | 94,347,830 | G/A | A | 8.85E–04 | 1.00 | 0.57 | 0.24 | Intergenic | |||
| exm2259477c | 9 | 93,072,009 | C/T | C | 9.88E–04 | 1.00 | 0.57 | 0.28 | Intergenic | |||
| exm2265531c | 3 | 174,131,106 | A/G | A | 1.13E–03 | 1.00 | 0.39 | 0.43 | Intergenic | |||
| exm660951c | 7 | 138,455,988 | A/G | G | 1.13E–03 | 1.00 | 0.39 | 0.43 | Coding | ATP6V0A4 | c.5C>T | p.Ala2Val |
| exm345346c | 3 | 124,731,689 | T/A | T | 6.76E–04 | 1.00 | 0.31 | 0.33 | Coding nonsynonymous | HEG1 | c.2734A>T | p.Thr912Ser |
| exm2270591 | 7 | 27,223,563 | G/T | T | 1.43E–03 | 1.00 | 0.49 | 0.48 | Intronic | HOXA11 | ||
| exm-rs3729931 | 3 | 12,626,516 | G/A | A | 5.04E–04 | 1.00 | 0.54 | 0.26 | Intronic | RAF1 |
TABLE 3. Exome-Wide Association Analysis Resultsa
Advance Recursive Partitioning (Tree-Based) Approach (ARPA)
The ARPA analysis generated a classification tree in which the three main splitter variants were exm-rs1321744, exm-rs350035, and exm-rs7679 (Figure 4A). Both left and right terminal nodes have a conspicuous power to discriminate between remitters (category 1) and nonresponders (category 0). The receiver operating characteristic (ROC) integral to evaluate the predictive accuracy of this derived model is 0.9454, which describes the high sensitivity and specificity of the tree in discriminating between remitters and nonresponders (Figure 4B). The TreeNet and random forest analyses (see Figure S2 in the online data supplement) show that after 150 simulated trees, the classification error is lower than 10% for both the training and the testing sample, and the ROC curve converges to values higher than 90% for both the training and the testing samples. Furthermore, the random forest and TreeNet analyses disclosed the same set of variables as the main predictors of remission and also discarded the possibility of model overfitting described for the CART analyses.
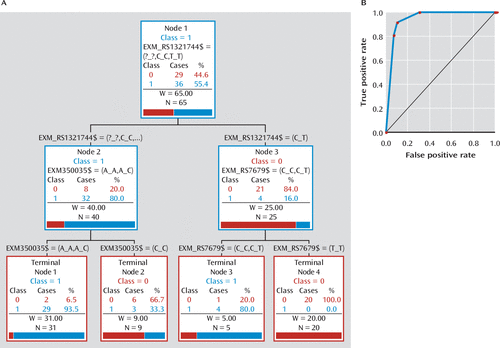
FIGURE 4. Results of Advance Recursive Partitioning (Tree-Based) Approach (ARPA) Analysisa
a In panel A, a reconstructed classificatory tree shows those variants involved in the process of branching. Nonresponders (class 0) are indicated in red, and remitters (class 1) are indicated in blue. In panel B, the receiver operating characteristic (ROC) integral=0.9454.
Finally, we applied a categorical approach to link the set of genotypes of the associated marker (exm-rs1321744) using latent class analysis, which shows how the patterns of remission, as depicted by HAM-D scores, split the set of patients into two significantly different clusters associated with the three genotypes CC, CT, and TT (Figure 5). Cluster 1, constituted by remitters, is associated with the CC genotype, exm-rs1321744. Cluster 2, constituted by nonresponders, is associated with the CT genotype, exm-rs1321744. Treatment with either desipramine or fluoxetine did not produce any significant additional splitting of these two clusters.
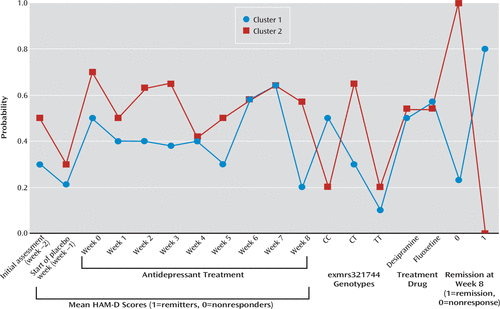
FIGURE 5. Patterns of Hamilton Depression Rating Scale (HAM-D) Scores Indicating Two Significantly Different Clusters Associated With the exm-rs1321744 Genotypesa
a Cluster 1 is constituted by remitters and is associated with the CC genotype. Cluster 2 is constituted by nonresponders and is associated with the CT genotype. Treatment with either desipramine or fluoxetine did not produce any significant additional splitting of these two clusters.
Discussion
In this single-site double-blind antidepressant trial of predominantly first-generation Mexican American patients, we found that both fluoxetine and desipramine were effective; however, patients treated with fluoxetine had significantly better scores at endpoint on the HAM-D, HAM-A, BDI, and GAS than desipramine-treated patients across all analytical approaches. The advantage of fluoxetine over desipramine was also evidenced by a shorter time to response in the survival analysis, a lower occurrence of anticholinergic and cardiovascular adverse drug reactions, and a lower dropout rate. These clinical outcomes contrast with previous studies showing similarities in efficacy between SSRIs and tricyclic antidepressants (36) and no difference in the response rate between SSRIs and tricyclics in an intent-to-treat analysis (37).
Our results are in concordance with data showing higher dropout rates among patients treated with tricyclic antidepressants because of lack of efficacy or adverse reactions (30.0% and 24.7%, respectively) (37). Tricyclic antidepressants have historically been found to be associated with moderate to severe adverse drug reactions (38); SSRIs are associated with milder adverse drug reactions that may diminish as treatment continues, and they also have lower toxicity and lower lethality when taken in an overdose situation (39). The overall comparability we observed in tolerability between desipramine and fluoxetine could be related to the close monitoring of our patients, a practice that improves outcomes of treatment with tricyclic antidepressants in depression (40). Our data supported a higher dropout rate and occurrence of anticholinergic and cardiovascular side effects in desipramine-treated patients. Previous studies have indicated that Africans are more sensitive to tricyclic antidepressants (41, 42), and populations with higher African genetic admixture rates, such as Puerto Rican Americans, have elevated adverse drug reaction rates and dropout rates with tricyclic antidepressants (43). This could also have contributed to a greater sensitivity to tricyclic antidepressants in our Hispanic subgroup with an African genetic admixture.
To our knowledge, this is the first randomized double-blind placebo lead-in trial conducted in the United States comparing the clinical efficacy and tolerability of antidepressants in depressed individuals of Mexican descent. The reasons for the lack of prospective randomized clinical trials in ethnic minorities are multiple and include difficulties in recruitment and retention of appropriate subjects and significantly less adherence to antidepressant therapy during the initial 100-day period (44). Considerable resources were needed for our study. Recruitment was challenging despite the fact that over 8 million persons of Mexican descent live in California (1), including 3 million in Los Angeles County. We conducted more than 4,300 telephone interviews and scheduled 1,223 screening visits in order to obtain 166 study completers.
A World Health Organization study (45) found that treatment with tricyclic antidepressants was more cost-effective than treatment with SSRIs in 14 different populations (including Mexico and the United States), particularly in lower-income subregions. However, in our study, fluoxetine treatment produced a better and faster response than desipramine in first-generation Mexican Americans with mild to moderate major depressive disorder, which suggests that fluoxetine may constitute a better drug of first choice for patients of Mexican descent.
Our whole-exome genotyping approach identified one functional intergenic SNP in chromosome 6 with exome-wide association with remission (p=1.98×10−6; false-discovery-rate-corrected p=0.05), which is remarkable given the small number of remitters (N=36) and nonresponders (N=29). Mexicans are an admixture of Europeans, Native Americans, and Africans, but a considerable proportion of their ancestry is Caucasian (46). For European-descendent populations, a p value ≤7.2×10−8 is regarded as compellingly significant for a genome-wide effect (47), but this assumption is appropriate for hypotheses that are tested on a genome scale (48). Our results strongly support the involvement of common functional variants in antidepressant drug response, specifically of brain methylation sites. Not much is known about the function of the intergenic exm-rs1321744, but growing clinical and preclinical evidence has implicated brain epigenetic changes in stress, depression, and antidepressant action (15–19); however, that body of work has focused mainly on regulation of the hypothalamic-pituitary-adrenal axis.
Even though linkage disequilibrium block harboring exm-rs1321744 does not harbor any gene, there are three genes surrounding the significant associated peak: TBX18, NT5E, and SNX14. TBX18 encodes a member of an evolutionary conserved family of transcription factors that plays a crucial role in embryonic development. NT5E encodes a plasma protein membrane that catalyzes the conversion of extracellular nucleotides to membrane-permeable nucleosides. SNX14 encodes a member of the sorting nexin family that is involved in intracellular trafficking. The encoded protein also contains a regulator of G protein signaling domains that act as GTPase activating proteins for G alpha subunits of heterotrimeric G proteins.
Our ARPA results suggest that the phenotype for antidepressant response is polygenic, as tree analysis showed that three common functional SNPs could predict remitter versus nonresponder status with 94% accuracy in our population. The top main splitter, exm-rs1321744, was discussed above, but, intriguingly, the other main splitter variants are located in regions relevant to lipoprotein function; rs7679 (in the PCIF1 gene, chromosome 20) has genome-wide association with blood lipoprotein concentrations (49), and intergenic exm-rs350035 on chromosome 5 is 10 nucleotides away from rs351629, a genome-wide SNP associated with triglycerides phenotype (50) (source, dbGaP; http://www.ncbi.nlm.nih.gov/gap).
Our study had several limitations. One is that the genotyping was done in only 65 of 166 participants who completed 8 weeks of treatment and 232 intent-to-treat subjects. We found no differences in week 8 HAM-D scores between completers who were genotyped and those who were not (see Table S3 in the online data supplement), which supports the absence of any ascertainment bias. A second limitation is that antidepressant blood levels were not included as a covariate, as they were randomly collected at different times of the day; some patients could come for follow-ups only in the evening. The absence of placebo is another important limitation. However, as the efficacies of desipramine or fluoxetine were well known when we designed our study, it would be ethically problematic to justify a placebo arm. To minimize placebo response in our study, patients received placebo during the first week of their treatment. Placebo was given single-blinded; patients knew that they were going to receive 1 week of placebo at some point in their treatment course, but staff knew that this would occur in the first week of treatment. In our study, we determined a priori that placebo response was a reason to remove participants from the study, and therefore we believe that the SNPs we identified are likely to predict response to antidepressant treatment and not merely improvement.
1
2 : Hispanics and Mental Health: A Framework for Research. Malabar, Fla, Robert E Krieger, 1989Google Scholar
3 : Research and treatment approaches to depression. Nat Rev Neurosci 2001; 2:343–351Crossref, Medline, Google Scholar
4 : Lifetime prevalence of DSM-III-R psychiatric disorders among urban and rural Mexican Americans in California. Arch Gen Psychiatry 1998; 55:771–778Crossref, Medline, Google Scholar
5 : The Hispanic response to psychotropic medications, in Ethnicity and Psychopharmacology. Edited by Ruiz P. Washington, DC, American Psychiatric Press, 2000, pp 55–89Google Scholar
6 : Pharmacotherapy of Hispanic depressed patients: clinical observations. Am J Psychother 1982; 36:505–512Crossref, Medline, Google Scholar
7 : Open trial of nefazodone among Hispanics with major depression: efficacy, tolerability, and adherence issues. Depress Anxiety 2001; 13:118–124Crossref, Medline, Google Scholar
8 : An open-label study of SSRI treatment in depressed Hispanic and non-Hispanic women. J Clin Psychiatry 1997; 58:31Crossref, Medline, Google Scholar
9 : Citalopram treatment of major depressive disorder in Hispanic HIV and AIDS patients: a prospective study. Psychosomatics 2004; 45:210–216Crossref, Medline, Google Scholar
10 : Pharmacogenetics of antidepressant drugs: an update after almost 20 years of research. Am J Med Genet B Neuropsychiatr Genet 2013; 162B:487–520Crossref, Medline, Google Scholar
11 : Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:183–194Crossref, Medline, Google Scholar
12
13 : Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry 2009; 66:488–497Crossref, Medline, Google Scholar
14 : Prediction of susceptibility to major depression by a model of interactions of multiple functional genetic variants and environmental factors. Mol Psychiatry 2012; 17:624–633Crossref, Medline, Google Scholar
15 : Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci 2010; 13:1351–1353Crossref, Medline, Google Scholar
16 : Antidepressants inhibit DNA methyltransferase 1 through reducing G9a levels. Biochem J 2012; 448:93–102Crossref, Medline, Google Scholar
17 : Epigenetics, depression, and antidepressant treatment. Curr Pharm Des 2012; 18:5879–5889Crossref, Medline, Google Scholar
18 : Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos Trans R Soc Lond B Biol Sci 2012; 367:2485–2494Crossref, Medline, Google Scholar
19 : Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 2013; 53:59–87Crossref, Medline, Google Scholar
20 : Placebo-controlled studies in depression: necessary, ethical, and feasible. Eur Arch Psychiatry Clin Neurosci 2003; 253:22–28Crossref, Medline, Google Scholar
21 : Burdens and benefits of placebos in antidepressant clinical trials: a decision and cost-effectiveness analysis. Am J Psychiatry 2003; 160:1272–1276Link, Google Scholar
22 : Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry 2004; 9:1075–1082Crossref, Medline, Google Scholar
23 : A comparison of three indicators for identifying Mexican Americans in epidemiologic research: methodological findings from the San Antonio Heart Study. Am J Epidemiol 1986; 123:96–112Crossref, Medline, Google Scholar
24 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
25 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1994Google Scholar
26 : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
27 : The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
28 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
29 : Reliability of the CES-D scale in different ethnic contexts. Psychiatry Res 1980; 2:125–134Crossref, Medline, Google Scholar
30 : A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706Crossref, Medline, Google Scholar
31 : CAT scans, PET scans, and genomic scans. Genet Epidemiol 1998; 15:1–18Crossref, Medline, Google Scholar
32 : Classification and Regression Trees. Belmont, Calif, Wadsworth International Group, 1984Google Scholar
33 : Random forests. Mach Learn 2001; 45:5–32Crossref, Google Scholar
34 : Greedy function approximation: a gradient boosting machine. Ann Stat 2001; 29:1189–1232Crossref, Google Scholar
35 : Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38:904–909Crossref, Medline, Google Scholar
36 : Efficacy and tolerability of selective serotonin reuptake inhibitors compared with tricyclic antidepressants in depression treated in primary care: systematic review and meta-analysis. BMJ 2003; 326:1014Crossref, Medline, Google Scholar
37 : Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety 1997; 6:10–18Crossref, Medline, Google Scholar
38 : Adverse drug reactions to first- and second-generation antidepressants: a critical evaluation of drug surveillance data. Br J Psychiatry 1986; 148:38–43Crossref, Medline, Google Scholar
39 : Safety and tolerability considerations: tricyclic antidepressants vs selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl 2000; 403:17–25Crossref, Medline, Google Scholar
40 : Collaborative management to achieve treatment guidelines: impact on depression in primary care. JAMA 1995; 273:1026–1031Crossref, Medline, Google Scholar
41 : Determination of appropriate clomipramine dosage among depressed African outpatients in Dar es Salaam, Tanzania. Cent Afr J Med 1994; 40:178–182Medline, Google Scholar
42 : Tricyclic plasma levels: effect of age, race, sex, and smoking. JAMA 1977; 238:2167–2169Crossref, Medline, Google Scholar
43 : Origins of US Hispanics: implications for diabetes. Diabetes Care 1991; 14:618–627Crossref, Medline, Google Scholar
44 : Hispanic ethnicity, physician-patient communication, and antidepressant adherence. Compr Psychiatry 2003; 44:198–204Crossref, Medline, Google Scholar
45 : Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br J Psychiatry 2004; 184:393–403Crossref, Medline, Google Scholar
46 : Population structure of Hispanics in the United States: the multi-ethnic study of atherosclerosis. PLoS Genet 2012; 8:e1002640Crossref, Medline, Google Scholar
47 : Estimation of significance thresholds for genomewide association scans. Genet Epidemiol 2008; 32:227–234Crossref, Medline, Google Scholar
48 : Chapter 11: Genome-wide association studies. PLoS Comput Biol 2012; 8:e1002822Crossref, Medline, Google Scholar
49 : Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 2009; 41:56–65Crossref, Medline, Google Scholar
50 : A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet 2007; 8(suppl 1):S17Crossref, Medline, Google Scholar



