The Hotel Study: Multimorbidity in a Community Sample Living in Marginal Housing
Abstract
Objective
The health of people living in marginal housing is not well characterized, particularly from the perspective of multimorbid illness. The authors investigated this population in a community sample.
Method
A prospective community sample (N=293) of adults living in single-room occupancy hotels was followed for a median of 23.7 months. Assessment included psychiatric and neurological evaluation, multimodal MRI, and viral testing.
Results
Previous homelessness was described in 66.6% of participants. Fifteen deaths occurred during 552 person-years of follow-up. The standardized mortality ratio was 4.83 (95% CI=2.91–8.01). Substance dependence was ubiquitous (95.2%), with 61.7% injection drug use. Psychosis was the most common mental illness (47.4%). A neurological disorder was present in 45.8% of participants, with definite MRI findings in 28.0%. HIV serology was positive in 18.4% of participants, and hepatitis C virus serology in 70.3%. The median number of multimorbid illnesses (from a list of 12) was three. Burden of multimorbidity was significantly correlated with lower role functioning score. Comorbid addiction or physical illness significantly decreased the likelihood of treatment for psychosis but not the likelihood of treatment for opioid dependence or HIV disease. Participants who died during follow-up appeared to have profiles of multimorbidity similar to those of the overall sample.
Conclusions
This marginally housed cohort had greater than expected mortality and high levels of multimorbidity with adverse associations with role function and likelihood of treatment for psychosis. These findings may guide the development of effective health care delivery in the setting of marginal housing.
Enclaves of marginal or substandard urban housing with low-income tenants are associated with substance dependence, mental illness, and infectious diseases (1, 2). This is the case in both resource-rich and resource-limited settings (2). In the Downtown Eastside neighborhood of Vancouver, British Columbia, single-room occupancy hotels have low barriers to tenancy and are often the only alternative to homelessness. In response to an epidemic of overdose deaths in this neighborhood, a supervised injecting facility was established (3). Specific initiatives were also deployed to facilitate access to highly active antiretroviral therapy (4). These initiatives limited overdose deaths (3) and decreased HIV-/AIDS-related morbidity and mortality as well as new HIV diagnoses (4). However, knowledge concerning the general health of people living in marginal housing is poor (5). Street homelessness, or living in shelters, is associated with high mortality and is linked to psychiatric disorders, including substance abuse (6, 7). Living in marginal housing may be associated with mortality similar to that associated with homelessness (8).
Increasing emphasis is being placed on the role of multimorbidity in determining health outcomes (9). In aging populations, physical illness may predispose to psychiatric disorders (10). For the relatively younger residents of single-room occupancy hotels, physical illness may be a consequence of substance dependence and mental illness (11). The implications of injected opioid dependence for infectious disease are relatively well defined (12). Less is known about risks related to stimulant drugs, particularly crack cocaine and methamphetamine, which are often used nonparenterally (13–15). Neurological disorders in the homeless include traumatic brain injury (TBI) (16) and cognitive impairment (17). The prevalence of other neurological disorders, such as seizures, movement disorders, and stroke, is unknown. The relationships between neurological illness, substance dependence, and mental illness are unclear, as are the implications for psychosocial function and the ability to access medical care. This information is important to inform efforts to control HIV and hepatitis C virus (HCV) through the implementation of “seek, test, treat, and retain” strategies (18, 19). To begin to establish an evidence base addressing these issues, we initiated a longitudinal cohort study of multimorbidity in residents of single-room occupancy hotels. We report the baseline findings and mortality for the initial 2-year phase of follow-up.
Method
Study Enrollment and Design
The study was carried out in Vancouver, British Columbia, with staggered recruitment from single-room occupancy hotels located in a low-income neighborhood and managed by a not-for-profit housing agency. In Canada, below-standard housing is defined as falling short in at least one of the following criteria: adequacy (not in need of repairs, according to residents), affordability (costs <30% of before-tax household income), or suitability (makeup of bedrooms and household). In many single-room occupancy hotels, the need for repairs is obvious even from casual observation. Rents generally range from 40% to 65% of the income provided by social service benefits. The single-room occupancy hotels typically comprise single rooms of 80 to 120 square feet (8–12 m2), with a sink and possibly a hotplate. Toilet and shower facilities, located at the end of hallways, are shared by 10 to 15 tenants. All single-room occupancy hotels housing study participants were over 75 years old and had evidence of bedbug, cockroach, and mouse infestation.
Following the baseline assessment (see Table 1 for instruments used [20–41]), the study design included monthly follow-up for up to 5 years. Inclusion criteria were living in one of four single-room occupancy hotels and ability to communicate in English. Inability to provide informed consent was the sole exclusion criterion. Informed consent was obtained to communicate clinically significant findings to the participants’ physicians. Medical care was provided free of charge through the Canadian health system. Participants received a modest honorarium. The protocol was approved by the ethics board of the University of British Columbia.
| Variable and Assessment Measure |
|---|
| Sociodemographic data |
| Standard interview incorporates questions from the Canadian Community Health Survey (20). (Administered by a research assistant.) |
| Substance use |
| Initial interview records lifetime history of use, age of first exposure, and periods of heavy use for alcohol and illicit drugs. (Administered by a research assistant.) |
| Fagerström Test for Nicotine Dependence (21). (Administered by a research assistant.) |
| Maudsley Addiction Profile (22): assesses drug use, related mental and physical symptoms, and risk behaviors for the past 30 days. Includes a rating of frequency of thoughts of ending life, scored on a scale of 0 to 4, with 2 representing “sometimes.” (Administered by a research assistant.) |
| Time-Line Follow-Back (23): records alcohol and drug use (prescribed and illicit, types, amounts, and pattern) over the previous 4 weeks, as well as money spent on alcohol and illicit drugs. (Administered by a research assistant.) |
| Urine drug screen: detects amphetamines, methamphetamine, barbiturates, benzodiazepines, cocaine (crack), marijuana, methadone, 3,4-methylenedioxymethamphetamine (Ecstasy), opiates, and tricyclic antidepressants. (Administered by a research assistant.) |
| Mental illness |
| Mini-International Neuropsychiatric Interview (24, 25): a semistructured clinical interview used to collect information allowing a diagnosis of DSM-IV axis I disorders, validated in substance-using and general medical samples. (Administered by a research assistant.) |
| International Personality Disorder Examination, Screener (26): a screening instrument for DSM-IV personality disorders. (Administered by a research assistant.) |
| Positive and Negative Syndrome Scale (27): a 30-item scale rated after an interview and mental status examination by a psychiatrist, used to assess the severity of a range of symptoms of psychosis and general mental health. (Administered by a psychiatrist.) |
| Beck Depression Inventory (28): a self-report measure of depression, including an assessment of suicidal ideation, scored on a scale of 0 to 3, with 1 representing thoughts of killing self, without intent. (Administered by a research assistant.) |
| Trauma History Questionnaire (29): measures exposure to traumatic life events and records frequency and age of exposure. (Administered by a research assistant.) |
| Best Estimate Clinical Evaluation and Diagnosis (30): information obtained from all assessments and from hospital records is used to make DSM-IV diagnoses of substance dependence and mental illness. (Psychiatrist assessment.) |
| Cognitive functioning |
| Wechsler Test of Adult Reading (31): provides an index of premorbid intellectual ability. (Administered by a research assistant/neuropsychologist interpretation.) |
| Stroop color and word test (32): measures the ability of the individual to separate word and color naming stimuli; this requires sustained attention and inhibition of a dominant response set. (Administered by a research assistant/neuropsychologist interpretation.) |
| Intradimensional-extradimensional shift task from the Cambridge Neuropsychological Automated Test Battery (33): evaluates attentional shifting to attributes of a complex stimulus array. (Administered by a research assistant/neuropsychologist interpretation.) |
| Rapid Visual Information Processing Task from the Cambridge Neuropsychological Automated Test Battery (34): a test that requires monitoring and responding to specific digit sequences and inhibiting responses to distracters. (Administered by a research assistant/neuropsychologist interpretation.) |
| Hopkins Verbal Learning Test, Revised (35): a brief assessment of memory, which includes many of the elements also found in detailed tests, such as the California Verbal Learning Test. (Administered by a research assistant/neuropsychologist interpretation.) |
| Iowa gambling task (36): assesses decision making in response to differential incentive conditions, sensitive to orbitofrontal functioning, and used to evaluate decision making. (Administered by a research assistant/neuropsychologist interpretation.) |
| Neurological illness |
| Traumatic brain injury: inquiry into serious head or facial injury, the event causing the injury, the extent of the injury, duration of loss of consciousness, need for hospitalization, duration of symptoms of dizziness, blurred vision, and confusion or memory loss. (Administered by a research assistant/neuropsychologist interpretation.) |
| Extrapyramidal Symptom Rating Scale (37): rated after a movement disorders examination. (Administered by a psychiatrist or neurologist.) |
| Barnes Akathisia Rating Scale (38): rated after a movement disorders examination. (Administered by a psychiatrist or neurologist.) |
| Cambridge Neurological Inventory (39): a focused neurological examination for motor coordination and sensory integration soft signs, including anomia. (Administered by a psychiatrist or neurologist.) |
| Medical illness |
| Serology for HIV, hepatitis B virus, and hepatitis C virus, qualitative polymerase chain reaction for hepatitis C virus; blood samples were drawn for testing at the British Columbia Centre for Disease Control. |
| CBC and differential, platelet count, AST, ALT. |
| Psychosocial functioning |
| Role Functioning Scale (40): a rating of daily functioning in four domains (work productivity, independent living, and immediate and extended social network relationships; each rated on a scale of 1 to 7). Higher scores represent better function. (Administered by a research assistant.) |
| Social and Occupational Functioning Assessment Scale (41): rated on a scale of 0 to 100, with higher scores representing better functioning. (Administered by a research assistant.) |
Assessment of Mortality
Mortality is the only outcome reported from follow-up. For participants who died, hospital records were obtained from the year before death; health care providers were interviewed; and coroner’s reports were requested.
Assessment of Substance Dependence
A lifetime review of substance exposure was obtained, and drug-dependence-related sections of the Mini-International Neuropsychiatric Interview were completed. A description, on a week-by-week basis, of all alcohol and prescription, licit, and illicit drugs used over the previous 4 weeks was recorded, as well as scores for the Maudsley Addiction Profile for the same period. A urine drug screen also was obtained.
Assessment and Diagnosis of Mental Illness
Records of hospitalization for mental illness were obtained, dating as far back as 50 years. The Mini-International Neuropsychiatric Interview was administered, and it was supplemented by a clinical interview and mental status examination carried out by a psychiatrist. All available clinical information (see Table 1) was used to make psychiatric and substance dependence diagnoses using procedures from the Best Estimate Clinical Evaluation and Diagnosis form (30), as previously used for genetic studies (42) and adapted in this study to DSM-IV criteria rather than DSM-III-R criteria. Previous reports of these diagnostic procedures indicated between-rater reliabilities of 0.53 to 0.91 for major mental disorders (43). The two diagnosticians in this study (F.V.R. and W.G.H.) used this diagnostic process independently for 98 participants. For the major mental illness categories reported, the kappa values were 0.77 for psychosis, 0.60 for mood disorders, and 0.61 for anxiety disorders. For substance dependence, the kappa values were 0.81 for alcohol, 0.74 for methamphetamine, 0.71 for cocaine, and 0.73 for opioids. Level of psychosocial functioning was rated using the Role Functioning Scale (40) and the Social and Occupational Functioning Assessment Scale in DSM-IV (41).
Assessment and Diagnosis of Physical Illness
Medical history was reviewed with a structured interview. Inquiry into neurological symptoms included history of seizures (most recent and treatment) and TBI, including duration of loss of consciousness, confusion or memory loss, dizziness, headache or blurred vision, and need for hospitalization. A screening neurological examination was carried out by a psychiatrist or a neurologist, and ratings were completed. Cognitive disorders were diagnosed on the basis of clinical findings from the history, the neurological examination, and neuropsychological testing, according to DSM-IV criteria.
An MRI scan was obtained using a Philips 3.0-T Achieva scanner (Philips Healthcare, Amsterdam). Sequences included a full-brain three-dimensional spoiled gradient echo, fluid attenuated inversion recovery, three-dimensional venous blood-oxygen-level-dependent imaging, and MR angiography. All MRI scans were reviewed by a neuroradiologist, and findings were reported according to standardized definitions (44).
Blood samples drawn for testing at the British Columbia Centre for Disease Control included serology for HIV, hepatitis B virus (HBV), and HCV, as well as qualitative polymerase chain reaction for HCV. A CBC and differential with platelet count was conducted, and AST and ALT levels were determined.
Statistical Analysis
The standardized mortality ratio was calculated (by H.W.). This was the ratio of the observed number of deaths to the number of deaths expected if the study cohort experienced the age- and sex-specific death rates seen in the 2009 Canadian general population. The Boice-Monson method was used to calculate the 95% confidence interval.
The likelihood-ratio chi-square test was used to compare the prevalence of seizures and of cognitive impairment in those with and without a history of TBI (this and subsequent analyses were conducted by W.G.H.). A similar approach was used to compare risk behavior in the past 30 days between participants who were infectious (with HIV, HCV, or HBV) and those who were not.
To examine the consequences of multimorbidity for psychosocial function, we selected 12 illnesses (psychosis; alcohol, stimulant, or opioid dependence; movement disorder; TBI; seizures; cognitive impairment; brain infarction; and active HIV, HCV, or HBV infection). Each participant was assigned a multimorbidity score representing the sum of illnesses present, with a range of 0–12. If an illness, such as stroke, was not assessed because of contraindications for MRI or serology for an illness was missing, that illness was scored as absent. Spearman correlation was performed between the multimorbidity score and the total score for the Role Functioning Scale, as well as the score for the Social and Occupational Functioning Assessment Scale.
To investigate the possible effects of multimorbidity on likelihood of treatment, we examined treatment history regarding psychosis (antipsychotic drug treatment prescribed), opioid dependence (methadone prescribed), and HIV (highly active antiretroviral therapy prescribed). In the group of participants with psychosis, we used the likelihood-ratio chi-square statistic to compare the use of antipsychotic drugs among participants with psychosis only with the use among participants with psychosis and comorbid opioid dependence or HIV. Similar comparisons were performed in the opioid-dependent group, using methadone treatment as the outcome, and in the HIV-infected group, using highly active antiretroviral therapy as the outcome.
Results
Participants
Participants were enrolled in a staggered fashion between November 13, 2008, and July 31, 2011. On a hotel-by-hotel basis, all tenants were approached to participate, and 293/406 (72.2%) agreed and met inclusion criteria. Table 2 summarizes participants’ demographic characteristics. At enrollment, most participants had lived in their current single-room occupancy hotel for over a year. Two-thirds had a history of homelessness. By the end of the period of observation, 150/292 (51.4%) participants were living in the same hotels as at enrollment. Most others were living nearby in different hotels, and only 15/292 (5.1%) had become homeless.
| Characteristic | |||
|---|---|---|---|
| N | Median | Interquartile Range | |
| Age (years) | 293 | 44.1 | 37.1–50.9 |
| Monthly income (Canadian dollars) | 286 | 870 | 610–1,100 |
| Months in current hotel at baseline | 292 | 16 | 2–52 |
| Months since last homeless | 195 | 38 | 8–93 |
| Total N | N | % | |
| Female | 293 | 68 | 23.2 |
| Current marital status | |||
| Married or common-law | 293 | 50 | 17.1 |
| Separated or divorced | 293 | 67 | 22.9 |
| Single | 293 | 176 | 60.1 |
| Ethnicity | |||
| White | 293 | 172 | 58.7 |
| Black | 293 | 7 | 2.4 |
| Asian | 293 | 8 | 2.7 |
| Aboriginal | 293 | 83 | 28.3 |
| Mixed/other | 293 | 23 | 7.8 |
| Education | |||
| Did not complete high school | 293 | 168 | 57.3 |
| Completed high school | 293 | 113 | 38.6 |
| Completed a college or university program | 293 | 12 | 4.1 |
| Earned income in addition to benefits | 291 | 23 | 7.6 |
| Homeless in the past | 293 | 195 | 66.6 |
| Jailed in the past | 293 | 71 | 24.2 |
Mortality
As a consequence of the staggered enrollment to allow completion of baseline assessments, participants had a variable period of follow-up or months at risk, ending January 31, 2012 (minimum for all participants, 6 months; 31/293 (11%) were lost to follow-up before the sixth monthly assessment). The median period of risk was 23.7 months, taken into account as part of the calculation of standardized mortality ratio. During 552 person-years of observation, 15/293 (5.1%) participants died. Coroner’s reports were obtained for the seven who died outside hospital settings. No deaths were attributed to suicide, 10 were a consequence of physical illness, and five were drug overdose-related (see Table S1 in the data supplement that accompanies the online edition of this article). The standardized mortality ratio was 4.83 (95% confidence interval=2.91–8.01) compared with age- and sex-matched Canadian population data.
Substance Dependence and Mental Illness
Substance dependence affected nearly all participants (Table 3). In the previous year, 179/290 (61.7%) participants had injected drugs, and 241/292 (82.5%) participants reported ever having injected. Mental illness affected the majority of participants, most commonly psychosis. Current suicidal ideation (defined as a score ≥1 on the suicidal ideation item of the Beck Depression Inventory [1=thoughts of suicide but would not carry it out] and a score ≥2 on the suicidal ideation item of the Maudsley Addiction Profile [2=thoughts of ending life sometimes]) was present in 28/288 (9.7%) participants. Only a minority (30/293 [10.2%]) had a history of long-term psychiatric hospitalization, with a greater number reporting hospitalization for mental illness in a general hospital (105/293 [35.8%]). Similar proportions suffered from schizophrenia or other chronic form of psychosis or from substance-induced psychosis (see Table S2 in the online data supplement).
| Clinical Characteristic | Total N | Baseline | Lifetime | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Substance dependence, any (nicotine excluded)a | 293 | 279 | 95.2 | 287 | 98.0 |
| Stimulant use (cocaine and/or methamphetamine) | 293 | 240 | 81.9 | 257 | 87.7 |
| Opioid use (heroin or other) | 293 | 115 | 39.2 | 179 | 61.9 |
| Alcohol dependence | 293 | 56 | 19.1 | 140 | 47.8 |
| Tobacco use (daily) | 289 | 240 | 83.0 | 260 | 90.0 |
| Mental illness, anyb | 293 | 218 | 74.4 | 250 | 85.3 |
| Psychotic illness, any | 293 | 139 | 47.4 | 172 | 58.7 |
| Mood disorder, any | 293 | 87 | 29.7 | 155 | 52.9 |
| Anxiety disorder, any | 293 | 70 | 23.5 | 92 | 31.4 |
| Neurological illness (active and/or current treatment)c | 273 | 125 | 45.8 | ||
| Movement disorderd | 269 | 52 | 19.3 | ||
| Brain infarction on MRI, any | 232 | 26 | 11.2 | ||
| Aneurysm on MR angiography | 232 | 20 | 8.6 | ||
| Traumatic brain injury (definite)e | 293 | 31 | 10.6 | ||
| Seizures in past year and/or current treatment | 292 | 26 | 8.9 | ||
| Clinical cognitive impairment (according to DSM-IV criteria) | 293 | 19 | 6.5 | ||
| Other neurological illnessf | 293 | 4 | 1.4 | ||
| Other MRI findingsg | 232 | 7 | 3.0 | ||
| Infection | |||||
| Anti-HIV positive | 283 | 52 | 18.4 | ||
| Anti-hepatitis C virus positive | 283 | 199 | 70.3 | ||
| Hepatitis C viremia (hepatitis C virus seropositive only) | 190 | 145 | 76.3 | ||
| AST:platelet ratio index (hepatitis C virus seropositive only)h | |||||
| 0–0.7 | 191 | 139 | 72.8 | ||
| >0.7 | 191 | 52 | 27.2 | ||
| >2 | 191 | 11 | 5.8 | ||
| Hepatitis B virus surface antigen positive | 283 | 3 | 1.1 | ||
Neurological Illness and Viral Exposure
Movement disorders were the most common neurological finding and were often associated with exposure to stimulant or antipsychotic drugs or both (Table 3; see also Table S3 in the data supplement). Of those with a movement disorder, the frequencies of the most common syndromes were as follows: parkinsonism, N=11/52 (21.2%); dyskinesia, N=23/52 (44.2%); and akathisia, N=34/52 (65.4%).
Pathological findings on MRI were found in 65/232 (28.0%) participants; brain infarction was the most common finding. The prevalence rate was 13/143 (9.1%) for those 30–49 years old, 11/57 (19.3%) for those 50–59 years old, and 2/11 (18.2%) for those 60–67 years old. For those with brain infarction, rates of seizures, movement disorder, or clinically significant cognitive impairment did not differ significantly from those of participants with no brain infarction. Of the 20 aneurysms detected on MR angiography, all were 7 mm or less in size, and one each was located in the anterior or posterior communicating artery. A participant who died from a subarachnoid hemorrhage did not have an aneurysm detected on the earlier MRI.
A history of serious head or facial injury was endorsed by 186/292 (63.7%) participants; more narrowly defined TBI was less frequent (Table 3). Examples of MRI findings related to TBI are presented in Figure S1 in the online data supplement. Seizures were more common in those with definite TBI (N=15/31 [48.4%]) than in those without (N=11/262 [4.2%] p<0.001). Similarly, clinical diagnoses of cognitive impairment were more frequent among participants with definite TBI (N=10/31 [32.3%]) compared with those without (N=9/262 [3.4%] p<0.001).
Positive serology for HCV and for HIV was common. Nine new cases of HCV, two new carriers of HBV, and one new case of HIV were detected at study entry. The AST:platelet ratio index was above a threshold of 2.0, suggestive of hepatic cirrhosis, in 5.8% of participants (Table 3; see also Table S4 in the data supplement). Only 10 participants reported previous interferon-based treatment for HCV. In contrast, nearly all those with positive HIV serology had received antiretroviral therapy, with 42/47 (89.4%) of those with available data having virologic suppression in the past (see Table S5 in the data supplement). Behaviors known to increase the risk of viral transmission, including penetrative sex without a condom, injection drug use, needle sharing, and crack pipe sharing, were reported by 207/270 (76.7%) members of the cohort (see Table S5 in the data supplement). Injection drug use was more prevalent in those at risk of spreading HCV, HIV, or HBV than those not at risk (p<0.001). Of those who had injected in the previous month, 109/146 (74.7%) reported using the supervised injecting facility.
Multimorbidity
Of the 12 illnesses evaluated in greater detail, the median multimorbidity score (the sum of illnesses present) was 3 (Figure 1), with an interquartile range of 2–4. The median multimorbidity burden was 3 for both male and female participants and was not correlated with age (Spearman ra=0.06). Greater multimorbidity was correlated with lower scores on the Role Functioning Scale (ra=−0.21, p<0.001; N=289) and the Social and Occupational Functioning Assessment Scale (ra=−0.20, p<0.001; N=290). This finding was similar when the sample was limited to those with complete data for all 12 multimorbidity assessments, including MRI and serology (Role Functioning Scale: ra=−0.22, p=0.001; Social and Occupational Functioning Assessment Scale: ra=−0.23, p<0.001; N=215).
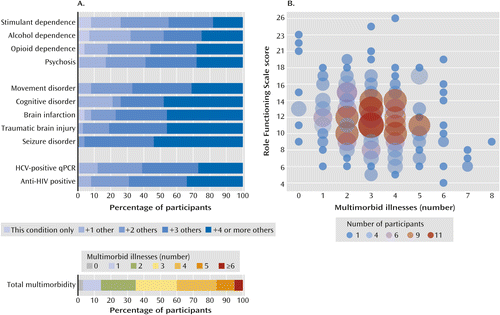
a Panel A shows the distribution of total multimorbid illnesses (0–12) in the cohort. The lower part of the panel shows the percentage of participants with increasing multimorbidity for each of the 12 conditions assessed. Since only three participants had persistent HBV infection, there is no bar to represent this group. Two of these participants scored +3, and one scored +4. Panel B shows the relationship between multimorbidity (0–12), scores on the Role Functioning Scale, and the number of participants at each intersection of multimorbidity number and level of function. The Role Functioning Scale comprises four items (work productivity, independent living [self-care], immediate social network relationships, and extended social network relationships); each item is scored on a scale of 1 to 7, with higher scores indicating better functioning. HCV=hepatitis C virus; qPCR=quantitative polymerase chain reaction.
The prevalence of treatment of three illnesses—psychosis (32.6%), opioid dependence (49.6%), and AIDS (61.5%)—was suboptimal (Table 4). Participants with psychosis with multimorbidity (opioid dependence and/or HIV/AIDS) were less than half as likely to have their psychosis treated than those with psychosis alone (p=0.003). In contrast, the presence of multimorbidity did not influence the likelihood of treatment for opioid addiction or HIV/AIDS.
| Treatment Provided | N | % |
|---|---|---|
| Antipsychotic medication | ||
| Of total number with psychosis (N=135) | 44 | 32.6 |
| Without opioid dependence or HIV/AIDS multimorbidity (N=64) | 29 | 45.3 |
| With opioid dependence or HIV/AIDS multimorbidity (N=71) | 15 | 21.1a |
| Methadone | ||
| Of total number with opioid dependence (N=113) | 56 | 49.6 |
| Without psychosis or HIV/AIDS multimorbidity (N=52) | 24 | 46.0 |
| With psychosis or HIV/AIDS multimorbidity (N=61) | 32 | 52.5 |
| Antiretroviral medication | ||
| Of total number with HIV/AIDS treatment indicated (N=52) | 32 | 61.5 |
| Without opioid dependence or psychosis multimorbidity (N=16) | 9 | 56.3 |
| With opioid dependence or psychosis multimorbidity (N=36) | 23 | 63.9 |
At baseline, participants who subsequently died had a greater multimorbidity score (median=4) than those who were alive at follow-up (median=3); however, this difference was not statistically significant.
Discussion
In our sample, participants with a high prevalence of previous homelessness and considerable social disadvantage living in marginal housing had a high level of mortality. Substance dependence, mental and neurological illnesses, and infectious diseases were common. Drug dependence played a direct role in one-third of deaths; most others represented complex multimorbidity. Greater multimorbidity was associated with poorer psychosocial functioning. The high level of directly observed mortality among persons living in single-room occupancy hotels is consistent with findings from analyses of administrative data sets comprised of persons living in similar circumstances in Canada (8) and is similar to data reported for those living in literal homelessness in the United States and Scandinavia (6, 7, 45).
In the community setting of our sample, there are approximately 7,100 single-room occupancy hotel rooms, and there are an estimated 1,600 homeless persons living on the street or in shelters (46). A recent study of street and shelter homelessness in Vancouver found an ethnic and educational background similar to that in our sample, with a slightly younger mean age (38.0 years compared with 44.1 years) and a larger proportion of women (37.2% compared with 23.3%) (47). Two-thirds of our sample had a history of homelessness. We cautiously suggest that many of the present findings may be relevant to the Vancouver homeless population and perhaps other homeless populations. However, obtaining the comprehensive evaluations reported, including detailed review of medical and psychiatric records, neurological examination, MRI, and neuropsychological testing, as well as serology and liver function testing to allow diagnosis of multimorbidity, makes replication of our study challenging.
Substance dependence was nearly universal. Dependence on cocaine or methamphetamine has long been associated with psychotic symptoms (48, 49). The estimated prevalence of psychosis in our sample (47.4%) was higher than the estimated prevalence in a meta-analysis of studies of the homeless (12.7%) (11). However, our estimate of the prevalence of schizophrenia and schizoaffective disorder was 12.6%, consistent with the meta-analysis and with a recent study using the Mini-International Neuropsychiatric Interview for diagnosis in a sample of homeless persons in three cities in our province (47). The findings from urine drug screens were consistent with the high prevalence of substance-induced psychosis in our sample, contributing to the high overall prevalence of psychosis. Although the prevalence of schizophrenia and related psychotic illness was still high in absolute terms, only a minority of those with psychosis represented mentally ill patients who had previously been cared for in an asylum or similar institutional setting.
Neurological illness was also common. The high frequency of movement disorders is likely related to stimulant drug use as well as exposure to antipsychotic drugs (50, 51). However, most participants with stimulant dependence in our study did not have movement disorders. The prevalence of brain infarctions that we observed appears to be similar to reported rates for healthy persons ages 30–49 (9.1% in the present study compared with 4%−9% in other reports) but higher for those ages 50–59 (19.3% compared with <9%) and 60–69 (18.2% compared with <12%) (44, 52). The high prevalence of crack cocaine use in our cohort may have contributed to risk for brain infarction (53, 54). Those with MRI evidence of infarction did not have elevated rates of seizures, movement disorders, or clinically obvious cognitive impairment. The relatively high rate of aneurysms may be related to stimulant dependence (55, 56). Most aneurysms were not in a size range or location associated with risk of rupture (57); however, ongoing use of stimulants could modify the predictive value of anatomical risk factors. The broadest definition of TBI yielded a high prevalence, similar to that reported for people living in homeless shelters (16). More narrowly defined TBI was more likely to be associated with ongoing symptoms, such as seizures or cognitive impairment, in contrast to brain infarctions that appeared relatively silent.
The very high rates of HIV and HCV in our cohort were similar to those reported in a previous study of people living in the same neighborhood (58). The high rate of previous successful treatment for HIV/AIDS confirms that with appropriately deployed strategies, patients with HIV/AIDS are amenable to therapeutic intervention (4). However, the rates of ongoing treatment were disappointing, although not as low as those for treatment of opioid dependence and psychosis. Infection with HCV was least likely to be treated (58), even in the presence of relatively high rates of participants with elevated biomarkers suggesting fibrosis or cirrhosis. This finding is consistent with findings from other reports indicating that social disadvantage, poor health literacy, and disengagement from the health care system are risk factors for low likelihood of treatment of HCV (19). Of additional concern, behaviors increasing the risk of spreading infection were common. As oral drug treatments for HCV become available, a greater emphasis should be placed on the challenges of delivering this care in a population with multimorbid illness (19). In particular, the high prevalence of stimulant use, the absence of substitution treatment analogous to methadone, and the potential difficulties accessing care in the face of ongoing psychosis and other mental illness will require the development of comprehensive strategies, perhaps modified from those proposed for opioid addiction and HIV infection (59).
Multimorbidity was highly prevalent, with co-occurring substance dependence, mental and neurological illnesses, and infectious diseases. Multimorbidity is reported to increase in association with greater socioeconomic deprivation (10). Even within the narrowed range of severe social deprivation in our cohort of persons living in single-room occupancy hotels, greater multimorbidity was associated with poorer psychosocial function. Multimorbidity was also associated with a lower likelihood of treatment of psychosis but not opioid dependence or HIV. Internationally, in the overall population, more severe mental illnesses, such as bipolar disorder, are more likely to be treated than less severe illnesses (60). This relationship may break down in the face of multimorbidity between mental illness, substance dependence, and physical illness.
The opportunity to investigate a reasonably large cohort of persons living with social disadvantage in single-room occupancy hotels was the unique feature of our study. Other investigators have described the challenges of gaining access to single-room occupancy hotels and other types of marginal housing, resulting in a paucity of information on the health status of tenants (5). While we cannot be certain that our observations generalize to other settings, many clinicians in urban practice are familiar with smaller numbers of individuals in public clinics with similar multimorbidity. Local assessment of specific health needs in marginally housed populations may be as important as locally based assessment in the homeless (11). Although we attempted to be thorough and detailed with our assessment and analysis strategy, undoubtedly other illnesses were missed, and the psychiatric diagnoses could change over time. Our sample of women was likely too small to permit informative sex-based analyses. Finally, our assessment of mortality had at least two limitations. Although only 11% of participants could not be followed up for at least 6 months, we were unable to systematically search death records or coroner’s reports to determine whether these individuals had died. If some of those lost to follow-up had died, our standardized mortality rate might be too conservative. Second, although none of the outside-of-hospital deaths in our sample were attributed to suicide, this cause of death may be underestimated. Four of the seven participants who died outside hospital settings were seen at least 1 month before death, and none expressed suicidal ideation according to the previously described criteria. However, since five of these seven deaths were attributed to overdoses, excluding suicide as a cause is difficult.
In conclusion, mortality was high in this cohort of persons living in marginal housing. Multimorbidity was common, and provision of treatment was inadequate. Collaborative care strategies may have a role in improving the health of persons living in these circumstances and needs to be investigated (61, 62).
1 : Urbanization: an emerging humanitarian disaster. N Engl J Med 2009; 361:741–743Crossref, Medline, Google Scholar
2 : Neighborhood effects on the long-term well-being of low-income adults. Science 2012; 337:1505–1510Crossref, Medline, Google Scholar
3 : Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. Lancet 2011; 377:1429–1437Crossref, Medline, Google Scholar
4 : Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–539Crossref, Medline, Google Scholar
5 : The health and housing in transition study: a longitudinal study of the health of homeless and vulnerably housed adults in three Canadian cities. Int J Public Health 2011; 56:609–623Crossref, Medline, Google Scholar
6 : Psychiatric disorders and mortality among people in homeless shelters in Denmark: a nationwide register-based cohort study. Lancet 2011; 377:2205–2214Crossref, Medline, Google Scholar
7 : Mortality and causes of death among homeless women and men in Stockholm. Scand J Public Health 2011; 39:121–127Crossref, Medline, Google Scholar
8 : Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow-up study. BMJ 2009; 339:b4036Crossref, Medline, Google Scholar
9 : Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858Crossref, Medline, Google Scholar
10 : Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380:37–43Crossref, Medline, Google Scholar
11 : The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med 2008; 5:e225Crossref, Medline, Google Scholar
12 : HIV and risk environment for injecting drug users: the past, present, and future. Lancet 2010; 376:268–284Crossref, Medline, Google Scholar
13 : Smoking of crack cocaine as a risk factor for HIV infection among people who use injection drugs. CMAJ 2009; 181:585–589Crossref, Medline, Google Scholar
14 : Amphetamine-group substances and HIV. Lancet 2010; 376:458–474Crossref, Medline, Google Scholar
15 ;
16 : The effect of traumatic brain injury on the health of homeless people. CMAJ 2008; 179:779–784Crossref, Medline, Google Scholar
17 : A systematic review of cognitive deficits in homeless adults: implications for service delivery. Can J Psychiatry 2009; 54:123–133Crossref, Medline, Google Scholar
18 : Enhanced HIV testing, treatment, and support for HIV-infected substance users. JAMA 2010; 303:1423–1424Crossref, Medline, Google Scholar
19 : Overcoming barriers to care for hepatitis C. N Engl J Med 2012; 366:2436–2438Crossref, Medline, Google Scholar
20 Statistics Canada: Canadian Community Health Survey, Cycle 1.2: Mental Health and Well-Being. Ottawa, Statistics Canada, 2003 (http://www23.statcan.gc.ca/imdb-bmdi/document/3226_DLI_D1_T22_V2-eng.pdf)Google Scholar
21 : Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 1978; 3:235–241Crossref, Medline, Google Scholar
22 : The Maudsley Addiction Profile (MAP): a brief instrument for assessing treatment outcome. Addiction 1998; 93:1857–1867Crossref, Medline, Google Scholar
23 : Utility of the Time-Line Follow-Back to assess substance use among homeless adults. J Nerv Ment Dis 2003; 191:145–153Crossref, Medline, Google Scholar
24 : DSM-III-R psychotic disorders: procedural validity of the Mini International Neuropsychiatric interview (MINI): concordance and causes for discordance with the CIDI. Eur Psychiatry 1998; 13:26–34Crossref, Medline, Google Scholar
25 : WHO Multi-Site Project on Methamphetamine-Induced Psychosis: a descriptive report of findings from participating countries. Parkside, South Australia, Drug & Alcohol Services South Australia, 2006Google Scholar
26 : Detecting personality disorders in a nonclinical population: application of a 2-stage procedure for case identification. Arch Gen Psychiatry 1997; 54:345–351Crossref, Medline, Google Scholar
27 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Google Scholar
28 : Validity of the BPRS, the BDI, and the BAI in dual diagnosis patients. Addict Behav 2008; 33:292–300Crossref, Medline, Google Scholar
29 : Psychometric evaluation of trauma and posttraumatic stress disorder assessments in persons with severe mental illness. Psychol Assess 2001; 13:110–117Crossref, Medline, Google Scholar
30 : Best Estimate Clinical Evaluation and Diagnosis Form (BECED). New York, Department of Research Assessment and Training, New York State Psychiatric Institute, 1988Google Scholar
31 : Wechsler Test of Adult Reading. New York, The Psychological Corporation, 2001Google Scholar
32 : Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res 2002; 111:65–74Crossref, Medline, Google Scholar
33 : Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 2000; 23:113–126Crossref, Medline, Google Scholar
34 : Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology (Berl) 1984; 82:147–150Crossref, Medline, Google Scholar
35 : Hopkins Verbal Learning Test, Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998; 12:43–55Crossref, Google Scholar
36 : Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia 2002; 40:1675–1689Crossref, Medline, Google Scholar
37 : Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res 2005; 76:247–265Crossref, Medline, Google Scholar
38 : A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
39 : The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res 1995; 56:183–204Crossref, Medline, Google Scholar
40 : Assessing levels of adaptive functioning: the Role Functioning Scale. Community Ment Health J 1993; 29:119–131Crossref, Medline, Google Scholar
41
42 : Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 2000; 288:678–682Crossref, Medline, Google Scholar
43 : Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry 1982; 39:879–883Crossref, Medline, Google Scholar
44 : Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357:1821–1828Crossref, Medline, Google Scholar
45 : Mortality in a cohort of homeless adults in Philadelphia. N Engl J Med 1994; 331:304–309Crossref, Medline, Google Scholar
46 : 6th Homeless Count in City of Vancouver, March 2012: Significant Changes Since 2005. Vancouver, Eberle Planning and Research, 2012Google Scholar
47 : Mental disorder, service use, and barriers to care among 500 homeless people in 3 different urban settings. Soc Psychiatry Psychiatr Epidemiol (Epub ahead of print, Jan 9, 2013)Google Scholar
48 : Amphetamine Psychosis. London, Chapman & Hall, 1958Google Scholar
49 : Cocaine psychoses: a continuum model. Am J Psychiatry 1975; 132:225–231Link, Google Scholar
50 : Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 2012; 120:35–40Crossref, Medline, Google Scholar
51 : Substance abuse and movement disorders. Mov Disord 2010; 25:2010–2020Crossref, Medline, Google Scholar
52 : Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 2008; 39:2929–2935Crossref, Medline, Google Scholar
53 : Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry 2012; 17:1017–1025Crossref, Medline, Google Scholar
54 : Cerebrovascular complications of the use of the “crack” form of alkaloidal cocaine. N Engl J Med 1990; 323:699–704Crossref, Medline, Google Scholar
55 : The deleterious effects of methamphetamine use on initial presentation and clinical outcomes in aneurysmal subarachnoid hemorrhage. J Neurosurg 2012; 117:781–786 (Epub ahead of print Aug 24, 2012)Crossref, Medline, Google Scholar
56 : Influence of cocaine on ruptured intracranial aneurysms: a case control study of poor prognostic indicators. J Neurosurg 2008; 108:470–476Crossref, Medline, Google Scholar
57 ;
58 : Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat 2009; 16:352–358Crossref, Medline, Google Scholar
59 : Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet 2010; 376:551–563Crossref, Medline, Google Scholar
60 : Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet 2007; 370:841–850Crossref, Medline, Google Scholar
61 : Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010; 363:2611–2620Crossref, Medline, Google Scholar
62 : Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012; 345:e5205Crossref, Medline, Google Scholar



