l-Methylfolate as Adjunctive Therapy for SSRI-Resistant Major Depression: Results of Two Randomized, Double-Blind, Parallel-Sequential Trials
Abstract
Objective
The authors conducted two multicenter sequential parallel comparison design trials to investigate the effect of l-methylfolate augmentation in the treatment of major depressive disorder in patients who had a partial response or no response to selective serotonin reuptake inhibitors (SSRIs).
Method
In the first trial, 148 outpatients with SSRI-resistant major depressive disorder were enrolled in a 60-day study divided into two 30-day periods. Patients were randomly assigned, in a 2:3:3 ratio, to receive l-methylfolate for 60 days (7.5 mg/day for 30 days followed by 15 mg/day for 30 days), placebo for 30 days followed by l-methylfolate (7.5 mg/day) for 30 days, or placebo for 60 days. SSRI dosages were kept constant throughout the study. In the second trial, with 75 patients, the design was identical to the first, except that the l-methylfolate dosage was 15 mg/day during both 30-day periods.
Results
In the first trial, no significant difference was observed in outcomes between the treatment groups. In the second trial, adjunctive l-methylfolate at 15 mg/day showed significantly greater efficacy compared with continued SSRI therapy plus placebo on both primary outcome measures (response rate and degree of change in depression symptom score) and two secondary outcome measures of symptom severity. The number needed to treat for response was approximately six in favor of adjunctive l-methylfolate at 15 mg/day. l-Methylfolate was well tolerated, with rates of adverse events no different from those reported with placebo.
Conclusions
Adjunctive l-methylfolate at 15 mg/day may constitute an effective, safe, and relatively well tolerated treatment strategy for patients with major depressive disorder who have a partial response or no response to SSRIs.
Evidence began to accumulate for an association between folate-deficiency states and depression soon after clinically reliable assays for folate became widely available in the 1960s (1–9). In addition to studies suggesting a relationship between low folate levels and an elevated risk for major depressive disorder, there is also accumulating evidence to suggest that low folate levels in patients with major depression may predict poorer prognosis during treatment (3, 10–15). These studies have in turn attracted the interest of the research community regarding the use of folate as a potential treatment for major depression. To date, several clinical studies have examined the use of folic acid or various folic acid metabolites (16–23) as monotherapy or as adjunctive therapy for major depression.
Despite the progressive development of dozens of antidepressant agents, more than half of all patients treated with antidepressant monotherapy will fail to experience a remission of their major depressive episode (24). Thus, developing safe, well-tolerated, and effective treatments that would help bring about remission in patients with antidepressant-resistant major depression is of paramount importance. In light of studies suggesting the potential efficacy, safety, and tolerability of l-methylfolate as an adjunct to standard antidepressants (19, 20), it may represent a unique opportunity for novel treatment development in major depression. l-Methylfolate is the biologically active form of folate and the only form of folate that crosses the blood-brain barrier. l-Methylfolate also regulates the formation of a critical cofactor for neurotransmitter synthesis, tetrahydrobiopterin (BH4). BH4 in turn activates tryptophan hydroxylase and tyrosine hydroxylase, which are necessary and rate-limiting enzymes for the synthesis of three monoamines: serotonin, dopamine, and norepinephrine. Because l-methylfolate indirectly regulates monoamine levels, low CNS levels of l-methylfolate could lead to monoamine deficiency. Until recently, randomized, double-blind, placebo-controlled trials focusing on the use of l-methylfolate as adjunctive therapy for antidepressant-resistant major depression were lacking. Here we report on the outcome of two separate trials of l-methylfolate as an adjunct to a selective serotonin reuptake inhibitor (SSRI), identical in design except for differences in dosing. In order to enhance our study’s statistical power to detect a difference in antidepressant effect between drug and placebo, the sequential parallel comparison design (25) was selected as the study design for our clinical trials.
Method
Trial 1
Study design.
The first trial was a 60-day, multicenter (11 clinical sites in the United States), randomized, double-blind, sequential parallel comparison trial of adjunctive l-methylfolate for SSRI-resistant major depressive disorder. Institutional review board-approved written informed consent was obtained from all study patients before any study procedures were conducted. Eligibility was assessed primarily during the screening visit and secondarily during the baseline visit, which occurred 14 days after the screening visit. Patient inclusion and exclusion criteria were as follows: being 18–65 years of age; meeting DSM-IV criteria for current major depressive disorder during the screening and baseline visit, as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders (26); having a score ≥12 on the Quick Inventory of Depressive Symptomatology–Self-Rated (QIDS-SR) (24) at both screening and baseline visits; having received treatment with an SSRI at adequate dosages (defined as ≥20 mg/day of fluoxetine, citalopram, or paroxetine; ≥10 mg/day of escitalopram; or ≥50 mg/day of sertraline) during the current episode for at least 8 weeks, as assessed historically using the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (which measures historical, not prospective antidepressant use); and having been on a stable dose of SSRI for the past 4 weeks at the baseline visit. Exclusion criteria were breastfeeding, pregnancy, or being of child-bearing age and not using a medically accepted means of contraception; demonstrating >25% decrease in depressive symptom severity as reflected by the QIDS-SR total score between screening and baseline; having a serious suicide or homicide risk or an unstable medical illness as assessed by the evaluating clinician; having an active substance use disorder within the past 6 months; having a history of mania, hypomania (including antidepressant-induced), psychotic symptoms, or seizure disorder; showing clinical evidence of untreated hypothyroidism; having failed to experience sufficient symptom improvement after more than two antidepressant trials during the current major depressive episode; and taking vitamins or dietary supplements containing >400 μg of folate or >6 μg of vitamin B12.
Study procedures.
Patients who were found eligible during the baseline visit were enrolled in the study according to a format of the sequential parallel comparison design (25), and eligible patients were randomized to one of three treatment groups in a 2:3:3 ratio. The study was divided into two 30-day phases (phases 1 and 2). One group of patients (randomization probability 3:8) received two dummy pills identical to l-methylfolate in appearance during phases 1 and 2 (placebo-placebo group). The second group (randomization probability 3:8) received two dummy pills during phase 1 and one dummy pill and one 7.5-mg l-methylfolate pill during phase 2 (placebo-drug group). The third group (randomization probability 2:8) received one dummy pill and one 7.5-mg l-methylfolate pill during phase 1 and two 7.5-mg l-methylfolate pills during phase 2 (drug-drug group). This format of the sequential parallel comparison design was selected instead of the one (25) involving a randomization to drug and placebo in phase 1 and a re-randomization of placebo nonresponders to drug or placebo in phase 2.
Postbaseline study visits occurred every 10 days. SSRI dosages remained constant during phases 1 and 2 of the study. Participants unable to tolerate the study medications as per protocol were withdrawn from the study.
The 17-item Hamilton Depression Rating Scale (HAM-D) (27), QIDS-SR, and Clinical Global Impressions (CGI) severity and improvement scales (28) were administered during all postscreen visits.
Trial 2
The results of the first trial were available to the investigators and sponsor prior to, and were informative in the development of the design of, the second trial. In the first trial, 7.5 mg/day of adjunctive l-methylfolate did not appear to result in superior treatment outcome (efficacy) than continued antidepressant therapy plus placebo. However, among patients who did not respond to 7.5 mg/day of l-methylfolate in phase 1 but whose dosage of l-methylfolate was increased to 15 mg/day in phase 2, the response rates in phase 2 were greater than in patients who continued on antidepressant monotherapy and adjunctive placebo (24.0% compared with 9%), although the difference fell short of statistical significance (p=0.1). Given the possibility of a better response with a longer trial of l-methylfolate, the target dose for the second trial was set at 15 mg/day.
The design of the second trial was identical to the first except that the dosing of l-methylfolate was 15 mg throughout the trial for all patients receiving it (those assigned to the placebo-drug group and to the drug-drug group). Six clinical sites participated in the second trial, and all were selected from the 11 sites that participated in the first trial based on their demonstrated ability to enroll the target patients in a reasonable time frame during the first trial. Institutional review board-approved written informed consent was obtained from all study patients before any study procedures were conducted.
General Statistical Considerations
The two primary outcome measures for both studies were defined as the difference in response rates according to the HAM-D and in degree of improvement in HAM-D score between the two treatment groups. Response according to the HAM-D was defined as a reduction of ≥50% in HAM-D score during treatment (or a final score of ≤7). The Hochberg-Benjamini approach (29) was used to control for multiple testing. According to this approach, the trial can be declared a success if one of the two primary outcome measures is significant at the 0.025 level or if both are significant at the 0.05 level. The sample sizes of the two studies were selected based on power calculations with specific assumptions about response rates in the two phases, according to the analytical method described in Fava et al. (25) for the sequential parallel comparison design. Secondary outcome measures included continuous change in scores on the QIDS-SR and CGI severity scale during treatment. Additional secondary outcome measures included the proportion of patients who met response criteria according to the QIDS (a reduction of ≥50% in QIDS score during treatment or a final score ≤5), remission status according to the QIDS (a final score ≤5), or remission status according to the HAM-D (a final score ≤7).
Sequential Parallel Comparison Design Analytic Model
The traditional analytical approach for standard antidepressant clinical trials has been to compare the difference in symptom improvement during treatment with a drug and with placebo for all patients randomized to treatment (intent-to-treat approach), defining endpoint symptom severity for patients who prematurely discontinue treatment as the last available depression severity score (last observation carried forward).
The format of the sequential parallel comparison design approach used in this study, however, was conducted as follows:
| 1. | A standard intent-to-treat/last-observation-carried-forward approach was employed for patients treated with drug during phase 1. | ||||
| 2. | The phase 2 data set of interest was limited to patients treated with placebo during phase 1 who completed phase 1 without experiencing a clinical response according to the HAM-D and entered phase 2. | ||||
| 3. | The last-observation-carried-forward approach was applied to the sequential parallel comparison design analysis data set for phase 2, with the final visit of phase 1/first visit of phase 2 serving as the new baseline visit. Drug was compared with placebo in phase 2 for this patient subset alone. | ||||
| 4. | The intent-to-treat/last-observation-carried-forward data comparing drug and placebo during phase 1 (see step 1 above) was combined with the data comparing drug and placebo according to the sequential parallel comparison design model for phase 2 (steps 2 and 3 above) and analyzed using the statistical model described by Fava et al. (25) using a weight and a randomization fraction chosen to maximize the power of the test. When calculating the pooled treatment effect from treatment effects obtained in phases 1 and 2, equal weights were given for each phase. | ||||
In the first trial, only the 7.5-mg/day arms were designed to be comparable to placebo. Thus, the 15-mg/day arm was not pooled with the 7.5-mg/day arms when conducting efficacy analyses.
Dichotomous measures were analyzed according to the sequential parallel comparison design method for dichotomous outcomes as described by Fava et al. (25), while the “seemingly unrelated regressions model,” controlling for baseline scores, was employed for the comparison of continuous outcomes according to the method of Tamura and Huang (30). Equal weights of 0.5 for each phase were selected before the analyses were conducted to avoid more arbitrary or data-driven choices. For the comparison of adverse events between groups, the chi-square test was used. All tests were two-tailed, with alpha set at 0.05 (no adjustments for multiple comparisons). Safety and tolerability analyses were conducted based on all data available (all patients randomized, all study visits).
Power Calculations
For the first trial, assuming a difference in response rates in drug versus placebo of 0.375 versus 0.20 in phase 1 and 0.25 versus 0.10 in phase 2, 148 subjects with a phase 1 allocation of 3:3:2 to placebo:placebo:drug were required to attain 80% power with the two-sided test of the hypothesis at the 0.05 level. Participant retention between phases was assumed to be 85%.
For the second trial, assuming a difference of –2.0 in HAM-D score change between the treatment and placebo groups in both phases and standard deviations of reductions equal to 4.0 in both treatment arms and both phases (approximate pooled effect size of 0.35), 75 subjects with a phase 1 allocation of 3:3:2 to placebo:placebo:drug were required to attain 80% power with the two-sided test of the hypothesis at the 0.05 level. Placebo response in phase 1 was expected to be 25% and participant retention between phases 85%.
Results
Trial 1
In the first trial, 148 patients (103 [69.5%] of them women) were randomly assigned to treatment. The participants’ mean age was 47.9 years (SD=11.6). Their mean baseline HAM-D score was 19.7 (SD=4.7). A total of 36 patients were assigned to the drug-drug sequence (7.5 mg/day of l-methylfolate in phase 1 and 15 mg/day in phase 2), 58 to the placebo-drug sequence (placebo in phase 1 and 7.5 mg/day of l-methylfolate in phase 2), and 54 to the placebo-placebo sequence (placebo in both phases 1 and 2). A total of 119 patients (80.0%) completed the study. All patients were taking an SSRI at the time of randomization and continued throughout the trial; 36 were on sertraline, 35 on escitalopram, 35 on fluoxetine, 32 on citalopram, and 10 on paroxetine. Efficacy analyses of phases 1 and 2 are reported in Table 1. Pooling of phases 1 and 2 for the two primary outcome measures is depicted in Figures 1 and 2.
| Phase 1 (30 Days) | Phase 2 (30 days)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurea | MTHF, 7.5 mg/day (N=36) | Placebo (N=112) | MTHF, 7.5 or 15 mg/day (N=35) | Placebo (N=33) | Pooled MTHFc | Pooled Placeboc | pd | ||||
| N | % | N | % | N | % | N | % | % | % | ||
| Completed treatment | 33 | 91.6 | 98 | 87.5 | 30 | 85.7 | 30 | 90.9 | |||
| HAM-D | |||||||||||
| Response | 7 | 19.4 | 32 | 28.5 | 6 | 17.1 | 3 | 9.0 | 18.3 | 18.8 | 0.92 |
| Remission | 4 | 11.1 | 20 | 17.8 | 5 | 14.2 | 2 | 6.0 | 12.7 | 11.9 | 0.15 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | ||
| Baseline score | 18.8 | 4.2 | 19.9 | 4.8 | 16.2 | 3.4 | 16.8 | 4.7 | 17.5 | 18.4 | |
| Score reduction | −4.3 | 5.0 | −6.3 | 6.6 | −3.1 | 4.2 | −2.1 | 4.9 | −3.70 | −4.19 | 0.87 |
| QIDS-SR | N | % | N | % | N | % | N | % | % | % | |
| Response | 9 | 25.0 | 28 | 28.5 | 3 | 8.5 | 3 | 9.0 | 16.8 | 18.8 | 0.70 |
| Remission | 0 | 0.0 | 10 | 8.9 | 3 | 8.5 | 1 | 3.0 | 4.3 | 6.0 | 0.58 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | ||
| Baseline score | 20.7 | 4.7 | 21.0 | 5.0 | 15.5 | 5.9 | 17.0 | 5.5 | 18.1 | 19.0 | |
| Score reduction | −4.6 | 5.1 | −6.9 | 6.2 | −1.9 | 4.1 | −2.5 | 4.1 | −3.25 | −4.73 | 0.07 |
| CGI severity scale | |||||||||||
| Baseline score | 4.1 | 0.5 | 4.2 | 0.6 | 3.8 | 0.7 | 3.9 | 0.7 | 4.0 | 4.1 | |
| Score reduction | −0.5 | 0.9 | −0.7 | 1.0 | −0.5 | 0.7 | −0.4 | 0.8 | −0.53 | −0.59 | 0.96 |
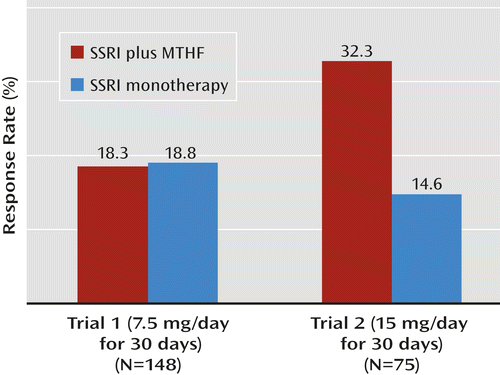
a Response was defined as a reduction of ≥50% in Hamilton Depression Rating Scale score during treatment or a final score of ≤7. Significant difference between groups in trial 2 (p=0.04). The pooled analysis was conducted as described in Fava et al. (25).
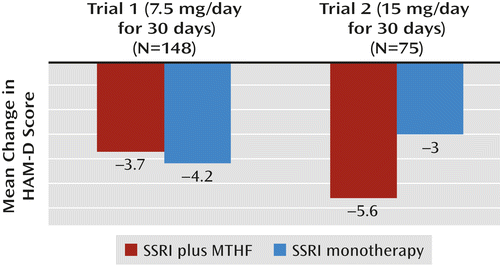
a Significant difference between groups in trial 2 (p=0.05). The pooled analysis was conducted as described in Tamura and Huang (30).
As noted above, 7.5 mg/day of adjunctive l-methylfolate did not appear to result in a treatment outcome (efficacy) superior to continued SSRI therapy plus placebo in either phase of the study. However, patients who underwent an increase in dosage to 15 mg/day in phase 2 had a greater response rate than those who continued on SSRI therapy plus adjunctive placebo (24.0% compared with 9%), although the difference fell short of statistical significance (p=0.1).
Adverse events are reported in Table 2. There was no statistically significant difference in the change in weight, supine and standing heart rate, or supine and standing diastolic and systolic blood pressure between the l-methylfolate and placebo groups in phase 1 or 2.
| Placebo (N=112)a | MTHF, 7.5 mg/day (N=94)a | MTHF, 15 mg/day (N=30)a | |||||
|---|---|---|---|---|---|---|---|
| Side Effect Category | N | % | N | % | N | % | p |
| Gastrointestinal | 23 | 20.1 | 9 | 9.6 | 3 | 10.0 | 0.06 |
| Sleep | 12 | 10.7 | 3 | 3.2 | 2 | 6.7 | 0.11 |
| Psychological | 12 | 10.7 | 2 | 2.1 | 2 | 6.7 | 0.05 |
| Somatic | 22 | 19.6 | 9 | 9.6 | 3 | 10.0 | 0.09 |
| Infectious | 13 | 11.6 | 6 | 6.4 | 2 | 6.7 | 0.38 |
| Cardiovascular | 4 | 3.6 | 0 | 0.0 | 2 | 6.7 | 0.08 |
| Sexual | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.99 |
| Miscellaneous | 3 | 2.7 | 1 | 1.1 | 2 | 6.7 | 0.24 |
Trial 2
Given the results in trial 1, the second trial used only 15 mg/day of l-methylfolate in the drug-drug and placebo-drug sequences. Seventy-five patients (53 [70.6%] of them women) were assigned to treatment as follows: 19 to the drug-drug sequence (15 mg/day of l-methylfolate in both phases 1 and 2), 28 to the placebo-drug sequence (placebo in phase 1 and 15 mg/day of l-methylfolate in phase 2), and 28 to the placebo-placebo sequence (placebo in both phases 1 and 2). The participants’ mean age was 48.4 years (SD=12.1), and their mean baseline HAM-D score was 21.2 (SD=3.9). A total of 61 patients (81.3%) completed the study. Again, all participants were taking an SSRI at baseline and continued throughout the trial. Efficacy analyses of phases 1 and 2 are presented in Table 3. Pooling of phases 1 and 2 on the two primary outcome measures is depicted in Figures 1 and 2.
| Phase 1 (30 days) | Phase 2 (30 days)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurea | MTHF, 15 mg/day (N=19) | Placebo (N=56) | MTHF, 15 mg/day (N=18) | Placebo (N=21) | Pooled MTHFc | Pooled Placeboc | pd | ||||
| N | % | N | % | N | % | N | % | % | % | ||
| Completed treatment | 17 | 89.4 | 50 | 89.2 | 15 | 83.3 | 19 | 90.4 | |||
| HAM-D | |||||||||||
| Response | 7 | 36.8 | 11 | 19.6 | 5 | 27.7 | 2 | 9.5 | 32.3 | 14.6 | 0.04 |
| Remission | 3 | 15.7 | 7 | 12.5 | 2 | 11.1 | 1 | 4.7 | 13.4 | 8.6 | 0.45 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | ||
| Baseline score | 21.2 | 4.1 | 21.2 | 3.2 | 19.5 | 3.8 | 17.6 | 4.5 | 20.4 | 19.4 | |
| Score reduction | −7.5 | 5.5 | −4.4 | 5.8 | −3.8 | 6.2 | −1.7 | 4.7 | −5.58 | −3.04 | 0.05 |
| QIDS-SR | N | % | N | % | N | % | N | % | % | % | |
| Response | 7 | 36.8 | 12 | 21.4 | 2 | 11.1 | 1 | 4.7 | 23.9 | 13.1 | 0.15 |
| Remission | 4 | 21.0 | 1 | 1.7 | 1 | 5.5 | 1 | 4.7 | 13.3 | 3.3 | 0.09 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Mean | ||
| Baseline score | 15.7 | 5.8 | 17.2 | 5.5 | 17.3 | 4.8 | 17.1 | 5.6 | 16.5 | 17.2 | |
| Score reduction | −8.1 | 5.3 | −5.7 | 5.6 | −1.3 | 4.9 | −0.5 | 4.9 | −4.7 | −2.62 | 0.04 |
| CGI severity scale | |||||||||||
| Baseline score | 4.6 | 0.6 | 4.4 | 0.6 | 4.0 | 0.6 | 3.9 | 0.8 | 4.4 | 4.2 | |
| Score reduction | −1.3 | 0.9 | −0.6 | 1.0 | −0.5 | 1.0 | −0.1 | 0.6 | −0.92 | −0.34 | 0.01 |
In the second trial, 15 mg/day of adjunctive l-methylfolate appeared to result in a treatment outcome (efficacy) superior to continued SSRI therapy plus placebo in both primary outcome measures, achieving statistical significance at the 0.05 level both in the difference in response rates (32.3% compared with 14.6%, p=0.04) and in the difference in degree of improvement (–5.58 compared with –3.04, p=0.05) on the HAM-D. The results also suggested superior efficacy for l-methylfolate on two of the secondary outcome measures—change in score on the QIDS-SR (–4.7 compared with –2.62, p=0.04) and on the CGI severity scale (–0.92 compared with –0.34, p=0.01).
Adverse events are reported in Table 4. There was no statistically significant difference in the change in weight, supine and standing heart rate, or supine and standing diastolic and systolic blood pressure between the l-methylfolate and placebo groups in phase 1 or 2. One patient in the l-methylfolate group was withdrawn from the trial because of the development of manic symptoms.
| Placebo (N=54)a | MTHF, 15 mg/day (N=42)a | ||||
|---|---|---|---|---|---|
| Side Effect Category | N | % | N | % | p |
| Gastrointestinal | 8 | 14.8 | 7 | 16.7 | 0.98 |
| Sleep | 3 | 5.5 | 1 | 2.4 | 0.80 |
| Psychological | 9 | 16.7 | 4 | 9.5 | 0.47 |
| Somatic | 16 | 29.6 | 6 | 14.3 | 0.13 |
| Infectious | 7 | 13.0 | 5 | 11.9 | 0.66 |
| Cardiovascular | 0 | 0.0 | 0 | 0.0 | 0.99 |
| Sexual | 0 | 0.0 | 1 | 2.4 | 0.90 |
| Miscellaneous | 5 | 9.3 | 1 | 2.4 | 0.34 |
Discussion
This is the first report of randomized, double-blind, placebo-controlled trials involving the adjunctive use of l-methylfolate (at 7.5 mg/day and 15 mg/day) for patients with major depression who have responded only partially or not at all to SSRIs. To enhance statistical power, both studies used a novel sequential parallel comparison design (25). Results of both studies were informative about the use of l-methylfolate as adjunctive therapy in major depression. In the first trial, we found no difference between placebo and adjunctive l-methylfolate at 7.5 mg/day (administered for up to 30 days); however, among patients who experienced no response with 7.5 mg/day, the response rate and change in depression symptom score were greater than among those who continued on SSRI therapy plus placebo, although the difference did not reach statistical significance. The latter finding informed our design of the second trial, which was identical to the first trial except that all patients treated with adjunctive l-methylfolate received 15 mg/day.
The results of the second trial indicated greater efficacy for 15 mg/day of adjunctive l-methylfolate (administered for up to 30 days) with continued SSRI therapy compared with continued SSRI therapy plus placebo on both primary outcome measures. These results suggest that 15 mg/day of l-methylfolate can be a useful adjunctive treatment strategy for patients with major depression who have not responded to SSRIs. The number needed to treat for response in the second trial was approximately six in favor of adjunctive l-methylfolate compared with placebo, which is comparable to results reported for other augmentation strategies in major depression, such as use of atypical antipsychotics (31) and lithium (32). In both trials, l-methylfolate appeared to be relatively well tolerated, with approximately 80% of patients completing 60 days of double-blind treatment. No statistically significant difference was observed in physiological variables (supine and standing blood pressure and heart rate) between the two groups, and side effects reported for the adjunctive l-methylfolate group were comparable in type and frequency to those reported for the placebo group.
Although we observed no differences between groups in remission rates in this study, it must be kept in mind that the evaluable treatment segments in phases 1 and 2 were at most 30 days in duration, which is quite brief for detecting remission. In the first phase of the Sequenced Treatment Alternatives for Depression study (STAR*D), for instance, the overall remission rate following 14 weeks of therapy with citalopram was approximately 33%, with the vast majority of remissions being achieved after week 4 (33). In addition, further skewing toward late remission would be expected in our sample compared with that of phase 1 of STAR*D given that the latter sample was of patients with less refractory depression than ours (especially our phase 2 sample, since the STAR*D sample consisted of patients who were treatment-naive during their current episode).
Several limitations should be taken into account when interpreting our findings. First, these trials exclusively involved the use of SSRIs as augmented agents. Whether 15 mg/day of l-methylfolate would be an effective adjunctive therapy for other classes of antidepressants remains to be determined. In addition, our studies examined the use of 7.5 mg/day and 15 mg/day of l-methylfolate. Whether higher dosages of l-methylfolate would result in similar or different outcomes than 15 mg/day is unclear. Furthermore, these trials excluded certain populations of patients (children and adolescents; patients with bipolar disorder, psychotic major depression, active substance use disorders, or unstable axis III comorbid illness; and women with perinatal depression). Further studies are needed to examine whether 15 mg/day of l-methylfolate would be efficacious in these patient populations as well. A final limitation concerns the fact that these trials did not utilize a standard parallel comparison design, but instead used the sequential parallel comparison design, a novel study design that enhances statistical power in randomized clinical trials (34, 35). However, the effects noted with the sequential parallel comparison design are certainly consistent with those noted in phase 1 of the study and therefore typical of a standard design.
In summary, our results suggest that 15 mg/day, but not 7.5 mg/day, of adjunctive l-methylfolate may constitute an effective, safe, and relatively well tolerated augmentation strategy for patients with major depression who have had no response or a partial response to SSRIs. Replication of these results in an independent cohort is needed, as well as additional research to further clarify the antidepressant role of l-methylfolate and other elements of the one-carbon cycle.
1 : Serum and red blood cell folate in depression. Acta Psychiatr Scand 1989; 80:78–82Crossref, Medline, Google Scholar
2 : Serum folate values in 423 psychiatric patients. BMJ 1967; 4:512–516Crossref, Medline, Google Scholar
3 : Folate deficiency in depressive illness. Br J Psychiatry 1970; 117:287–292Crossref, Medline, Google Scholar
4 : Antiepileptic therapy, folate deficiency, and psychiatric morbidity: a general practice survey. Epilepsia 1985; 26:434–440Crossref, Medline, Google Scholar
5 : The neuropsychiatry of megaloblastic anemia. Br Med J 1980; 281:1036–1038Crossref, Medline, Google Scholar
6 : Folic acid deficiency and depression. Psychosomatics 1980; 21:926–929Crossref, Medline, Google Scholar
7 : Folate deficiency, biopterin, and monoamine metabolism in depression. Psychol Med 1992; 22:871–876Crossref, Medline, Google Scholar
8 : 5-Methyltetrahydrofolate level in the serum of depressed subjects and its relationship to the outcome of ECT. J Affect Disord 1994; 32:163–168Crossref, Medline, Google Scholar
9 : Change in folate status with antidepressant treatment. Psychiatry Res 1994; 53:313–322Crossref, Medline, Google Scholar
10 : Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997; 154:426–428Link, Google Scholar
11 : Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol 2003; 23:309–313Crossref, Medline, Google Scholar
12 : Serum folate, vitamin B12, and homocysteine in major depressive disorder, part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry 2004; 65:1090–1095Crossref, Medline, Google Scholar
13 : Serum folate, vitamin B12, and homocysteine in major depressive disorder, part 2: predictors of relapse during the continuation phase of pharmacotherapy. J Clin Psychiatry 2004; 65:1096–1098Crossref, Medline, Google Scholar
14 : The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Int J Neuropsychopharmacol 2005; 8:523–528Crossref, Medline, Google Scholar
15 : Brain MRI white matter hyperintensities, cardiovascular risk factors, and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder (part II). Psychiatry Res 2005; 140:301–307Crossref, Medline, Google Scholar
16 : Folic acid enhances lithium prophylaxis. J Affect Disord 1986; 10:9–13Crossref, Medline, Google Scholar
17 : Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord 2000; 60:121–130Crossref, Medline, Google Scholar
18 : Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry 2002; 14:33–38Crossref, Medline, Google Scholar
19 : Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990; 336:392–395Crossref, Medline, Google Scholar
20 : Oral 5-methyltetrahydrofolate (MTHF) in depression association with senile organic mental disorders (OMDs): a double-blind, multicenter study vs trazodone (TRZ). Aging (Milano) 1993; 5:63–71Medline, Google Scholar
21 : An open trial of methyltetrahydrofolate in elderly depressed patients. Ann Clin Psychiatry 1993; 5:101–105Crossref, Medline, Google Scholar
22 : S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol 2004; 24:661–664Crossref, Medline, Google Scholar
23 : S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry 2010; 167:942–948Link, Google Scholar
24 : The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med 2004; 34:73–82Crossref, Medline, Google Scholar
25 : The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom 2003; 72:115–127Crossref, Medline, Google Scholar
26 : Structured Clinical Interview for DSM-IV Axis I Disorders With Psychotic Screen (SCID-I/P w/ Psychotic Screen) Version 2.0. New York, NY, Biometrics Research Department, 1995Google Scholar
27 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
28 Guy W, ed: ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
29 Benjamini Y, Hochberg Y: Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57 289–300Google Scholar
30 : An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clin Trials 2007; 4:309–317Crossref, Medline, Google Scholar
31 : Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 2009; 166:980–991Link, Google Scholar
32 : Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999; 19:427–434Crossref, Medline, Google Scholar
33 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
34 : A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 2012; 81:87–97Crossref, Medline, Google Scholar
35 : Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemp Clin Trials 2011; 32:592–604Crossref, Medline, Google Scholar



